
Jian-Pi-Yi-Qi-Fang ameliorates chronic atrophic gastritis in rats through promoting the proliferation and differentiation of gastric stem cells
Introduction
Chronic atrophic gastritis (CAG) is a common intractable digestive system disease with various etiologies, manifested as inflammation and reduction or atrophy of gastric glands and thinning of gastric mucosa, with or without intestinal and/or pseudo-pyloric metaplasia (1). It is closely related to the occurrence and development of gastric cancer (GC) (2), which represents a huge health and psychological burden for patients. Although the current treatment measures, such as eradication of Helicobacter pylori (Hp), vitamin supplement, gastric mucosal protection, acid suppression, and motility stimulation, cannot fundamentally reverse the process of gastric mucosal atrophy, and have limitations and side effects (3), many studies have shown that traditional Chinese medicine (TCM) is very effective for CAG (4,5).
According to TCM, the pathogenesis of CAG is characterized by “deficiency in origin and excess in superficiality”. “Deficiency in origin” is dominated by weakness of the spleen and the stomach and “excess in superficiality” refers to stagnated Qi, damp-heat, and blood stasis. The basic pathogenesis of CAG is weakness of the spleen and the stomach, and the main aim of treatment is to strengthen the spleen and replenish Qi (6). Jian-Pi-Yi-Qi-Fang (JPYQF) is derived from “Xiang Sha Liu Jun Tang (XSLJT)” in Luo Mei’s “Gu Jin Ming Yi Fang Lun” of the Qing Dynasty. We modified the original formula and added Curcuma phaeocaulis Valeton and Radix Curcumae to invigorate blood circulation, eliminate stagnation, move Qi, and relieve pain. Adding muscovite can strengthen the spleen, benefit the qi, remove blood stasis, and generate new blood. Hedyotis diffusa is added to reduce inflammation and for its anti-tumor and anti-cancer properties. The composition of this formula strengthens the spleen, benefiting Qi, invigorating blood, and moving Qi (7). Our previous randomized, double-blind, multi-center clinical trial showed that the formula could significantly improve the clinical symptoms of CAG, and the gastroscopic and pathological signs of gastric mucosal atrophy (8). Therefore, it is potential to be the agent for treating CAG. Through in vivo studies, we found that the formula could alleviate gastric mucosal atrophy by affecting the expression levels of genes and proteins related to the Wnt/β-catenin, PI3K-Akt, and Notch pathways (7,9,10), which partially explains the therapeutic mechanisms.
Recently, stem cell biology studies have shown that the gastric mucosal epithelium is maintained by gastric stem cells in and below the isthmus, and the proliferation and differentiation of gastric stem cells can supplement various gastric mucosal epithelial cells (11,12). Aberrant proliferation and differentiation of gastric stem cells may be one of the pathogeneses of CAG (13). In addition, the microenvironment of gastric stem cells is largely regulated by Wnt signaling (14). The gradient expression of Wnt signaling in gastric glands can not only maintain the self-renewal and proliferation of gastric stem cells, but also induce gastric stem cells to differentiate (15,16). It is speculated that the pathogenesis of CAG is related to the changes in the proliferation and differentiation state of gastric stem cells caused by aberrant Wnt signaling. The formula JPYQF may play a therapeutic role by affecting the expression of Wnt signaling-related proteins and genes to promote the proliferation and differentiation of gastric stem cells. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3749/rc).
Methods
Animals
A total of 40 male Sprague-Dawley (SD) rats, weighing 220±10 g, were purchased from Nantong University (Jiangsu, China) [animal quality certificate no. SCXK (Su) 2019-0001], and were adaptively reared for 1 week in the specific-pathogen-free (SPF) Laboratory Animal Center of Jiangsu Province Institute of Traditional Chinese Medicine with a 12/12 h light/dark cycle at (21±1) ℃ and 55%±5% humidity. Animal experiments were approved by the Ethics Committee of Jiangsu Province Integrated Chinese and Western Medicine Hospital (No. AEWC-20160810-12), in compliance with institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Reagents
We purchased N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Ranitidine hydrochloride was purchased from Shanghai Hengshan Pharmaceutical Co., Ltd. (Shanghai, China). Teprenone was purchased from Eisai China Inc. (Shanghai, China). Folate was purchased from Tianjin Lisheng Pharmaceutical Co., Ltd. (Tianjin, China). Hematoxylin and eosin (H&E) stain kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The Lgr5, Sox2, Rspo1, and Wnt3A antibodies were purchased from Beijing Bioss Biotechnology Co., Ltd. (Beijing, China). The Ki67 antibody was purchased from Abcam Technology, Inc. (Cambridge, MA, USA). The Muc5AC antibody, TRIzol® reagent, reverse transcription polymerase chain reaction (RT-PCR) kit, and quantitative PCR (qPCR) kit were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The GAPDH and PCNA antibodies, the goat anti-rabbit, and rabbit anti-mouse IgG antibodies were purchased from Cell Signaling Technology, Inc. (CST; Danvers, MA, USA). The radioimmunoprecipitation assay (RIPA) lysis buffer, bicinchoninic acid (BCA) protein concentration assay kit, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) preparation kit, and polyvinylidene fluoride (PVDF) membranes were purchased from Shanghai Beyotime Biotech Co., Ltd. (Shanghai, China). The enhanced chemiluminescence (ECL) kit was purchased from Beijing Labgic Technology Co., Ltd. (Beijing, China). The reverse transcription-quantitative (RT-q) PCR primers for Lgr5, Muc5AC, Muc6, Wnt3A, Rspo1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were produced by Nanjing Genscript Biological Technology Co., Ltd. (Nanjing, China).
Preparation of JPYQF water extract
All the medicinal herbs in JPYQF were obtained from Jiangsu Province Integrated Chinese and Western Medicine Hospital, and identified by the deputy chief Chinese pharmacist Dai Ying. Each formula consisted of Dangshen [Codonopsis pilosula (Franch.) Nannf.] 15 g, Baizhu (Atractylodes macrocephala Koidz.) 10 g, Fuling [Poria cocos (Schw.) Wolf] 10 g, Gancao (Radix Glycyrrhizae preparata) 3 g, Chenpi (Citrus reticulata Blanco) 6 g, Banxia (Pinellia ternata [Thunb] Breit.) 6 g, Muxiang (Aucklandia lappa Decne) 10 g, Sharen (Amomum villosum Lour) 6 g, Ezhu (Curcuma phaeocaulis Val.) 10 g, Baihuasheshecao [Oldenlandia diffusa (willd.) Roxb] 30 g, Yujin (Curcuma wenyujin) 10 g and Yunmushi (Mica) 30 g, comprising a total of 146 g. A round-bottomed flask containing 146 g of herbals was filled with 1.46 L of water and allowed to soak for 0.5 h. The herbs were immersed in water that was continually cooked for 0.5 h. The water extract was filtered and gathered for the first time. The round-bottomed flask was then filled with 0.73 L of water, which was then constantly boiled for 0.5 h. The water extract was filtered and gathered for the second time. Utilizing a vacuum rotary evaporator, the entire amount of water extract gathered was concentrated to 48.7 mL (3 g/mL crude drug) and kept at −80 ℃ in an ultra-low temperature refrigerator.
Establishment of CAG rat model and administration of JPYQF
After 1 week of habituation, the 40 rats were randomly divided into the control group, model group, positive drug group (2.7 mg/kg folate and 13.5 mg/kg teprenone), low-dose group of JPYQF (JPYQF-L, 13.2 g/kg crude drug), middle-dose group of JPYQF (JPYQF-M, 26.4 g/kg crude drug), and high-dose group of JPYQF (JPYQF-H, 52.8 g/kg crude drug) using a random number table method, with 5 rats/group (7,9). The control group had free access to typical food and water. The CAG model rats were provided with abundant drink water containing 150 µg/mL MNNG and food containing 0.03 % ranitidine (alternate day fasting, 1 day with sufficient food, and 1 day with fasting) according to the literature (7,17). The construction of the CAG rat model lasted 24 weeks.
Then, rats in drug-treated groups were administrated with drugs via gavage daily. At the same time, rats in the control and model group were given sterile distilled water. The experiment was terminated, and the number of rats remained unchanged for 8 weeks after the end of treatment. All rats were anesthetized by 30 mg/kg (body weight) sodium pentobarbital intraperitoneal injection. Gastric tissues were harvested for the assays of markers associated with gastric stem cell proliferation and differentiation.
Histopathological observation
The 4% formalin-fixed rat gastric tissues (25 ℃ for 24 h) underwent routine alcohol dehydration and paraffin embedding. The embedded tissues were cut into 5 µm slices and mounted on slides. After dewaxing and rehydrating, the slices were stained with H&E. The stained slices were dehydrated with graded ethanol and xylene. The glass slides were mounted with neutral balsam and covered with coverslips. The rat gastric tissues were imaged using the Olympus CKX-41 fluorescence inverted microscope (Olympus, Inc., Tokyo, Japan). The atrophy and inflammatory of gastric glands were scored by 2 pathologists according to the visual analog scale of the new Sydney system (18,19).
Immunohistochemistry
The paraffin-embedded gastric tissues were cut into 4 µm slices. The slides were submersed in citrate unmasking solution for antigen retrieval at high temperature, and then incubated with hydrogen peroxide for 15 min and blocked with blocking solution for 1 hour at room temperature. The slides were coated with Lgr5 (1:300 dilution; cat. no. bs-20747R; Bioss), Sox2 (1:300 dilution; cat no. bs-23176R; Bioss), Ki67 (1:300 dilution; cat no. ab-15580; Abcam), PCNA (1:300 dilution; cat no. 13110P; CST), Muc5AC (1:300 dilution; cat no. MA5-12178; Thermo Fisher), Wnt3A (1:300 dilution; cat no. bs-1700R; Bioss), Rspo1 (1:300 dilution; cat no. bs-21386R; Bioss), and GAPDH (1:300 dilution; cat no. 5174S; CST) primary antibodies and incubated overnight at 4 ℃. The next day, the slices were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:500 dilution; cat no. 7074; CST) for 1 hour at room temperature, and then were incubated with 3,3'-diaminobenzidine (DAB) solution for 3 min and counterstained with hematoxylin for 30 s. After dehydrating with graded ethanol and xylene, the slides were mounted with neutral balsam mounting medium and covered using coverslips. The Olympus CKX-41 inverted microscope (Olympus Corp.) were used to image the rat gastric tissues at ×100 magnification. Staining of the immunohistochemistry (IHC) sections was determined using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Western blotting
Gastric tissues of rats in each group were lysed with RIPA buffer, and the protein concentrations were measured through a BCA protein kit. The proteins were loaded and separated through 5% and 10% SDS-PAGE and then transferred onto PVDF membranes. The membranes were washed and blocked using a 5% skimmed milk powder blocking solution for 1 hour. The membranes were then incubated for 8 hours with appropriate elementary antibodies, anti-GAPDH (1:1,000 dilution), anti-Lgr5 (1:1,000 dilution), anti-Sox2 (1:1,000 dilution), anti-Ki67 (1:1,000 dilution), anti-PCNA (1:1,000 dilution), anti-Wnt3A (1:1,000 dilution), and anti-Rspo1 (1:1,000 dilution) at 4 ℃. After washing 4 times with tris-buffered saline with Tween 20 (TBST) for 15 min each time, the membranes were incubated again with secondary antibodies (goat anti-rabbit or rabbit anti-mouse IgG, 1:10,000 dilution) for 1 h at room temperature. Immunoreactivity was investigated through the ECL method. The images were scanned using an automatic chemiluminescence image analysis system (Tanon 4200; Abclonal, Wuhan, China). The data was analyzed with Image J software (Version 1.4.3.67; National Institutes of Health, Bethesda, MD, USA).
RT-qPCR
The total RNA was extracted from rat gastric tissues using TRlzol reagent, following the manufacturer’s instructions. A reverse transcription kit was adopted for the reverse transcription of extracted RNA into complementary DNA (cDNA). The RT-qPCR analysis was performed using StepOnePlus Real-Time PCR System (Thermo Fisher Scientific Inc., USA). The procedure was as follows: pre-denaturation at 95 ℃ for 2 min, denaturation at 95 ℃ for 5 s, and annealing and elongation at 60 ℃ for 10 s, with 40 cycles. The sequences of primers used for RT-qPCR are as follows: GAPDH: F: 5'-GGCACAGTCAAGGCTGAGAATG-3', R: 5'-ATGGTGGTGAAGACGCCAGTA-3'; Lgr5: F: 5'-ACTGTCACTGTGAGCTGGATGG-3', R: 5'-GCATTTCCAGCAAGACGCAAC'; Muc5AC: F: 5'-CCGTTGTTTCTGCACCATGT-3', R: 5'-AGTAACAGTGGCCGTCAAGG'; Muc6: F: 5'-TTACTCGCACTCAGAGACCTCCTTAG-3', R: 5'-TCTGCCTGAAGATGGTGATGTTGTG-3'; Wnt3A: F: 5'-GTTCTTCTCTGGTCCTTGGCTGTG-3', R: 5'-GGCATGATCTCCACGTAGTTCCTG-3'; Rspo1: F: 5'-CAAACCAACTGCTCAGACACCAAAG-3', R: 5'-CTGGCTCCTTGCTGTTCTTCCTG-3'. The cycle threshold (Ct) value obtained was normalized with GADPH as the internal reference and finally calculated with 2−∆∆Ct to obtain the relative expression of the gene to be tested.
Statistical analysis
The data were presented as the mean ± SD. The difference between groups was analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. A P value <0.05 suggested a statistically significant difference. The data was analyzed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA), and graphs were plotted using GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, CA, USA). The highest and lowest values are removed from the statistics. All experiments were repeated three times under the identical settings.
Results
Effect of JPYQF on body weight, gastric morphology, and pathology of rats with CAG
As shown in the Table 1, there was no significant difference in the initial body weight between the rats in the control and the model group. After 24 weeks, compared with the control group rats, those in the model group developed symptoms, such as yellow and dull hair, passive activity, decreased eating, and reduced body weight. After 8 weeks of drug intervention, the above symptoms were improved by positive drugs and JPYQF. Meanwhile, the body weight in all drug intervention groups increased significantly, which was more obvious in the JPYQF-H group. It is suggested that JPYQF can relieve the symptoms in CAG model rats.
Table 1
| Groups | Initial body weight, g | Body weight after 24-week modeling, g | Body weight after 8-week drug intervention, g |
|---|---|---|---|
| Control | 208.900±11.709 | 661.100±14.391 | 668.000±27.630 |
| Model | 210.650±10.589 | 544.300±25.048** | 484.700±57.028 |
| Positive drug | – | 550.700±33.782** | 557.400±33.637## |
| JPYQF-L | – | 544.300±36.873** | 556.600±28.061## |
| JPYQF-M | – | 557.400±31.919** | 569.100±50.580## |
| JPYQF-H | – | 535.800±20.257** | 585.000±32.289## |
Values were expressed as mean ± SD. Compared with the same stage of control group, **, P<0.01; compared with the same stage of model group, ##, P<0.01. JPYQF-L, Jian-Pi-Yi-Qi-Fang low-dose group; JPYQF-M, Jian-Pi-Yi-Qi-Fang middle-dose group; JPYQF-H, Jian-Pi-Yi-Qi-Fang high-dose group.
During the administration of MNNG and ranitidine, no rats died and there were no significant changes in health status, and only the model rats showed a decrease in appetite. At the 8th, 12th, 16th, 20th, and 24th weeks of modeling, 2 model rats were randomly sacrificed to examine the gastric mucosa’s macroscopic and microscopic pathological state. All rat stomachs were cut along the side of the greater curvature. The 5 animals in each group were included in each analysis and the highest and lowest values were removed from the statistics. As shown in Figure 1A, in the control group, the macroscopic manifestations of the gastric mucosa of the rats was light orange-red, shiny, and the mucosal folds were obvious, tight, and regular. Compared with the control group, the gastric mucosa in the model group was dark red with poor gloss, and the mucosal folds were significantly reduced or had even disappeared. Compared with the model group, the gastric mucosal redness and swelling were improved, the gloss was better, and the mucosal folds increased and tended to be regular in each JPYQF and the positive drug group.

As shown in Figure 1B, in the control group, the structure of gastric mucosal epithelium was clear with little inflammatory cell infiltration, and the glands were normal in number and shape and were tightly and neatly arranged. Compared with the control group, the gastric mucosal epithelium of the rats in the model group was significantly thinner, with goblet cells and more inflammatory cell infiltration, and the glands were significantly reduced with loose and disorderly arrangement, indicating that the CAG model had been successfully established. After JPYQF and positive drugs treatment, the preceding symptoms were improved to varying degrees, manifested as thickening of the mucosal epithelium, reduced infiltration of inflammatory cells, increased and normal glands. In contrast, the improvement of the gastric mucosa of the rats in JPYQF-H group was greater than that in the positive drug group.
Effect of JPYQF on the expression of gastric stem cell markers in rats with CAG
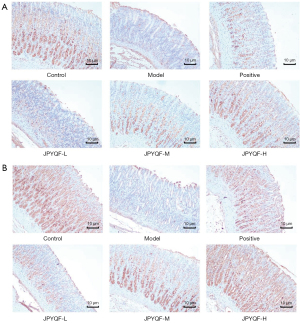
The in situ protein expression of Lgr5 and Sox2 in gastric tissues were detected via IHC. The results indicated that in the control group, Lgr5 was mainly expressed in the base of the gland, and Sox2 was expressed in the middle and lower part of the gland. Compared with the control group, the expression areas of Lgr5 and Sox2 were less, and the staining was lighter in the model group. Compared with the model group, the expression areas of Lgr5 and Sox2 in each drug intervention group were increased, and the staining was deepened. Among the intervention groups, the expression area and staining depth of Lgr5 and Sox2 in the JPYQF-H group were similar to those in the control group (Figure 2).
The protein expression levels of Lgr5 and Sox2 were further detected by WB. The results indicated that compared with the control group, the expression levels of Lgr5 and Sox2 were significantly decreased in the gastric tissue of the rats in the model group. Compared with the model group, the expression levels of Lgr5 and Sox2 were significantly increased in the JPYQF-H and positive drug groups (Figure 3A-3C).
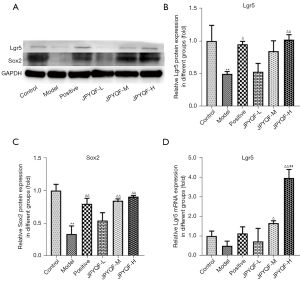
The relative messenger RNA (mRNA) expression level of Lgr5 in rat gastric tissues were detected via RT-qPCR. As shown in Figure 3D, compared with the control group, the expression level of Lgr5 showed a downward trend in the gastric tissue of the rats in the model group. Compared with the model and positive drug group, the expression level of Lgr5 was significantly increased in the JPYQF-H group. Therefore, based on the Western blot (WB) and RT-qPCR experiments, it was suggested that the expression levels of gastric stem cells in the gastric tissue of rats with CAG was reduced and that JPYQF could increase them.
Effects of JPYQF on the proliferation of gastric stem cells in rats with CAG
As shown in Figure 4, in the control group, Ki67 was expressed in the isthmus and base of the gland, and PCNA was expressed in the isthmus. Compared with the control group, the expression areas of Ki67 and PCNA were reduced and lightly stained in the model group. In addition, the expression area of PCNA was mostly concentrated in the base of the gland. Compared with the model group, Ki67 and PCNA staining were significantly increased in each drug intervention group. In the JPYQF-M, JPYQF-H, and the positive drug groups, the staining areas and depth tended to be consistent with the control group.
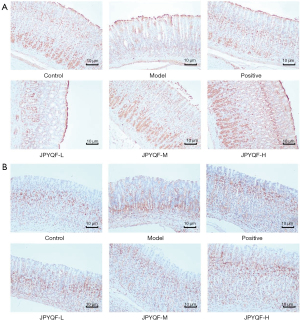
The average optical density (OD) of the PCNA-expressing region was analyzed using Image-Pro Plus 6.0 software. The results indicated that compared with the control group, the expression levels of PCNA in the isthmus of gastric glands were significantly decreased and those in the base were increased in the model group. After treatment with JPYQF and positive drugs, the expression levels of PCNA in the isthmus were increased and those in the base were significantly decreased (Table 2).
Table 2
| Groups | Isthmus | Base |
|---|---|---|
| Control | 0.010±0.001 | 0.005±0.002 |
| Model | 0.004±0.000** | 0.016±0.002** |
| Positive drug | 0.009±0.000# | 0.005±0.002## |
| JPYQF-L | 0.011±0.001## | 0.005±0.001## |
| JPYQF-M | 0.011±0.003## | 0.005±0.003## |
| JPYQF-H | 0.012±0.001## | 0.005±0.001## |
Values were expressed as mean ± SD. Compared with the same area of control group, **, P<0.01; compared with the same area of model group, #, P<0.05, ##, P<0.01. All experiments were repeated 3 times. JPYQF-L, Jian-Pi-Yi-Qi-Fang low-dose group; JPYQF-M, Jian-Pi-Yi-Qi-Fang middle-dose group; JPYQF-H, Jian-Pi-Yi-Qi-Fang high-dose group.
As detected by WB, the expression levels of PCNA were significantly decreased in the model group compared with those in the control group. After treatment with JPYQF and positive drugs, the expression levels of PCNA were significantly increased. There were no significant differences in the expression levels of Ki67 among the groups, but the expression levels in the JPYQF-H group tended to be consistent with the control group, as shown in Figure 5. These results suggested that there was aberrant proliferation of gastric stem cells in rats with CAG, and JPYQF could promote the proliferation of gastric stem cells in a certain gland area.

Effects of JPYQF on the differentiation of gastric stem cells in rats with CAG
The results of IHC and OD values showed that in the control group, the positive expression of Muc5AC was tan in colour and expressed in gastric pits on the mucosal surface. Compared with the control group, the staining of Muc5AC was lighter and the expression level was decreased in the model group. Compared with the model group, the positive expression levels of Muc5AC were increased to varying degrees in each drug intervention group, among which, those in the JPYQF-H group were increased significantly, as shown in Figure 6 and Table 3.
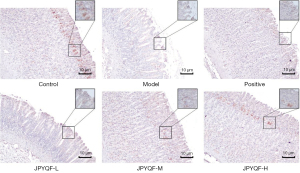
Table 3
| Groups | Average OD |
|---|---|
| Control | 0.017±0.005 |
| Model | 0.001±0.001* |
| Positive drug | 0.007±0.004 |
| JPYQF-L | 0.003±0.003 |
| JPYQF-M | 0.008±0.005 |
| JPYQF-H | 0.014±0.004# |
Values were expressed as mean ± SD. Compared with the same area of control group, *, P<0.05; compared with the same area of model group, #, P<0.05. All experiments were repeated 3 times. OD, optical density; JPYQF-L, Jian-Pi-Yi-Qi-Fang low-dose group; JPYQF-M, Jian-Pi-Yi-Qi-Fang middle-dose group; JPYQF-H, Jian-Pi-Yi-Qi-Fang high-dose group.
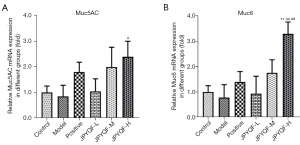
As shown in Figure 7, compared to the control group, the relative mRNA expression levels of Muc5AC and Muc6 in rat gastric tissues showed a downward trend in the model group, but the difference was not statistically significant. After treatment with JPYQF-H, the expression levels of Muc5AC and Muc6 were significantly increased. In addition, compared with the positive drug group, the expression level of Muc6 was significantly increased in the JPYQF-H group. Therefore, based on the IHC and RT-qPCR experiments, it was suggested that the differentiation of gastric stem cells was reduced in rats with CAG, and JPYQF could promote the differentiation of gastric stem cells into gastric epithelial cells.
Effects of JPYQF on Wnt signaling initiation and enhancement factors in rats with CAG
Compared with the control group, the positive expression regions of Wnt3A and Rspo1 in the base of the gastric gland were significantly reduced in the model group. After administration with JPYQF and positive drugs, the positive expression areas were increased and the brown staining was deepened. Besides, compared with the positive drug group, the positive expression areas of Wnt3A and Rspo1 were increased and the staining deepened in the JPYQF-M and JPYQF-H groups (Figure 8).

Based on the WB experiment, it was suggested that the expression levels of Wnt3A and Rspo1 were significantly decreased in the model group compared with the control group. Compared with the model group, the expression levels of Wnt3A and Rspo1 were significantly increased in the JPYQF-M and JPYQF-H groups (Figure 9A-9C).
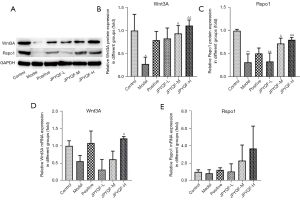
The RT-qPCR examination showed that compared with the control group, the relative mRNA expression levels of Wnt3A and Rspo1 in the gastric tissue of the rats had a downward trend in the model group. Compared with the model group, the relative mRNA expression level of Rspo1 showed an upward trend and Wnt3A increased significantly in the JPYQF-H group (Figure 9D,9E). These results suggested that Wnt signaling in gastric tissue of CAG rats may be in a low activation state, and JPYQF could activate and enhance Wnt signaling.
Discussion
We found that the protein expression levels of Lgr5, Sox2, PCNA, and Ki67, and the relative mRNA expression level of Lgr5 were decreased in the gastric tissue of CAG model rats, suggesting that CAG may have gastric stem cell proliferation retardation. After administration with JPYQF, the expression levels of the above proteins and gene were all increased, suggesting that JPYQF could promote the proliferation of CAG gastric stem cells. Both Lgr5 and Sox2 are currently recognized gastric stem cell markers. The Lgr5+ and Sox2+ stem cells are located at the bottom of pyloric glands and are capable of long-term renewal and generation of all lineages of the stomach epithelium, which are responsible for maintaining homeostasis of gastric mucosal epithelium (11,12). Both Ki67 and PCNA are widely used proliferation indicators, and their combined detection can reflect cell proliferation status more accurately. Similar to our results, previous studies have demonstrated that the expression of Sox2 and Ki67 were decreased in CAG (20,21). Therefore, we speculated that the pathogenesis of CAG may be related to the blocked proliferation of Lgr5+ and Sox2+ gastric stem cells, which cannot replenish gastric gland cells, resulting in reduced glands and mucosal atrophy. In contrast to our study, some researchers believe that the Lgr5+ stem cells are the origin of GC, and that CAG is a kind of precancerous disease caused by the aberrant proliferation of the Lgr5+ stem cells with the relation to Hp infection, chronic inflammation, and other factors (22,23). Studies found that TCM could not only promote the proliferation of gastric stem cells, but also inhibit the aberrant proliferation of gastric epithelial cells to prevent CAG from deteriorating into gastric cancer (GC) (24,25). Interestingly, our study found that compared with the model group, JPYQF increased the expression levels of PCNA and Ki67 and changed their expression areas. Virtually consistent with the control group, the expression regions of PCNA and Ki67 were concentrated in the gastric gland isthmus in the JPYQF-H group. It is suggested that JPYQF could not only promote the proliferation of gastric stem cells, but also prevent the aberrant expansion of the stem cell niche, thereby avoiding the aberrant proliferation of gastric stem cells and carcinogenesis.
In this study, we found that the differentiated cells of the gastric epithelium were reduced in CAG while the administration with JPYQF could promote the differentiation of gastric stem cells and increase mature gastric epithelial cells. Muc5AC and Muc6 are the main secreted mucins of normal gastric antrum mucosa, playing an important role in protecting the mucosal epithelium (26). In the studies of pluripotent stem cell and adult stem cell culture, cells expressing Muc5AC and Muc6 were taken as indicators of specific differentiation and formation of mature gastric epithelial cells (27,28) and reduced Muc5AC expression was associated with reduction of gastric differentiation (29). Mounting evidence supports that the aberrant differentiation of gastric stem cells exerts crucial effects on the progression of CAG and CG. In recent years, several studies have shown that Hp infection and chronic inflammation may alter the differentiation direction, causing gastric stem/progenitor or mature cells to differentiate or transdifferentiation into intestinal metaplasia or spasmolytic polypeptide-expressing metaplasia (SPEM) (30,31). The metaplastic epithelium of intestine and pyloric gland replaces normal gastric epithelium, resulting in CAG or neoplasia. Similar to our results, studies have found that the differentiation of gastric stem cells was disordered in the gastric mucosa of CAG and TCM could affect the differentiation direction of cells (32,33). The active ingredients in JPYQF, such as costunolide and extraction of Atractylodes rhizoma and Codonopsis radix, were found to induce the differentiation of adult stem cells into mature cells of corresponding organs (34,35). Besides, studies have also indicated that Hedyotis diffusa and curcuma in JPYQF could inhibit the proliferation and differentiation of tumor stem cells (36,37). Therefore, it is speculated that JPYQF could promote the differentiation of gastric stem cells into normal gastric mucosa epithelial cells to relieve atrophy, and could also inhibit the differentiation into cancer cells to prevent GC.
Further, we found that the proteins and genes expression levels of Wnt3A and Rspo1 were decreased in the gastric tissue of rats with CAG. After treatment with JPYQF, the proteins and genes expression levels were increased. It is suggested that there was inhibition or low activation of Wnt signaling in the gastric mucosa of CAG and that JPYQF could activate and enhance the signaling. These results were consistent with previous report (38). Wnt3A is an important member of the Wnt family and activates the canonical Wnt signaling to exert its biological functions (39). Rspo1 is one of the 4 vertebrate R-spondin proteins that are secreted agonists of the canonical Wnt/β-catenin signaling pathway and are potent stimulators of adult stem cell proliferation in vivo and in vitro (40). In addition, R-spondins uniquely synergize with Wnt proteins to play an important role in the activation of Wnt signaling on the cell membrane (40). In contrast with our study, some studies have demonstrated that the aberrant activation of Wnt signaling is one of the pathogeneses of CAG (41), and TCM exerts its therapeutic effect on CAG by inhibiting the Wnt signaling pathway (42). However, the Wnt signaling pathway has a crucial role in regulating stem cell expansion and differentiation and is required for self-renewal and maintenance of tissue homeostasis and repair (15,43). A remarkably balanced setting for Wnt signaling is essential for the regeneration of damaged tissues, such as gastrointestinal mucosa (43). In addition, recent research has shown that senescent cells are abundant in atrophic mucosa; inflammation and Hp promoted gastric mucosa senescence and atrophy (44). Therefore, repairing damaged gastric mucosa and reducing mucosal inflammation promptly and effectively may be able to alleviate CAG and delay further precancerous lesion progression; the low activation or inhibition of Wnt signaling may fail to regulate the proliferation and differentiation of gastric stem cells to repair mucosal damage.
Nevertheless, CAG is mostly managed symptomatically, as atrophy-alleviating or reversing therapies are not known to exist. According to the Chinese consensus on chronic gastritis, Teprenone and folic acid were chosen for this study as positive control agents. The consensus pointed that selenium and certain vitamins may lower the risk of stomach carcinogenesis. As a vitamin, folic acid may lessen the development of gastric cancer by improving the pathological tissue state of CAG. As a gastric mucosal protective agent, Teprenone can strengthen the gastric mucosal barrier and encourage the repair of gastric mucosal damage. Compared with positive drugs, the composition of formula JPYQF has the above-mentioned similar effects on CAG. In addition, the formula is based on the traditional efficacy, combined with the use of drugs for identifying diseases. Therefore, it has multiple effects, such as relieving pain, anti-inflammatory, anti-tumor and potentially promoting the proliferation and differentiation of gastric stem cell.
There were several limitations to this study. For example, no transgenic or knockout CAG rat models were employed to further investigate the effect of JPYQF on Wnt signaling and the proliferation and differentiation of gastric stem cells.
In this study, a CAG rat model was employed to investigate the state of gastric stem cell proliferation and differentiation, and to preliminarily explore the effect of JPYQF on these cells and its mechanism. Our results demonstrated that JPYQF may promote the restricted proliferation and normal differentiation of gastric stem cells to replenish gastric gland cells and repair mucosal damage, in part, through initiating and enhancing the low activated Wnt signaling, which provides evidence to support the clinical use of JPYQF in treating CAG.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81804071, 81573966).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3749/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3749/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3749/coif). All authors report that this work was supported by the National Natural Science Foundation of China (Nos. 81804071, 81573966). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were approved by the Ethics Committee of Jiangsu Province Integrated Chinese and Western Medicine Hospital (No. AEWC-20160810-12), in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fang JY, Du YQ, Liu WZ, et al. Chinese consensus on chronic gastritis (2017, Shanghai). J Dig Dis 2018;19:182-203. [Crossref] [PubMed]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735-40. [PubMed]
- den Hollander WJ, Kuipers EJ. Current pharmacotherapy options for gastritis. Expert Opin Pharmacother 2012;13:2625-36. [Crossref] [PubMed]
- Yang L, Hu Z, Zhu J, et al. Effects of weifuchun tablet for chronic atrophic gastritis: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e20374. [Crossref] [PubMed]
- Dai YK, Zhang YZ, Li DY, et al. The efficacy of Jianpi Yiqi therapy for chronic atrophic gastritis: A systematic review and meta-analysis. PLoS One 2017;12:e0181906. [Crossref] [PubMed]
- Tian G, Wu C, Li J, et al. Network pharmacology based investigation into the effect and mechanism of Modified Sijunzi Decoction against the subtypes of chronic atrophic gastritis. Pharmacol Res 2019;144:158-66. [Crossref] [PubMed]
- Yan Z, Xu T, Xu Y, et al. Jianpiyiqi formula ameliorates chronic atrophic gastritis in rats by modulating the Wnt/β-catenin signaling pathway. Exp Ther Med 2021;22:878. [Crossref] [PubMed]
- Su KL, Zhu FS, Shi T, et al. Clinical Observation on Treatment of 62 Cases of Chronic Atrophic Gastritis with Spleen Deficiency by Weiwei I Granule. Pharmacology and Clinics of Chinese Materia Medica 2013;29:154-6.
- Yan ZP, Xu TT, An ZT, et al. Effects of Jianpi Yiqi Formula on expression of PI3K-Akt signaling pathway in gastric tissue of rats with chronic atrophic gastritis. China Journal of Traditional Chinese Medicine and Pharmacy 2019;34:4800-4.
- Xu YX, Yan ZP, Xu TT, et al. Effects of Jianpi Yiqi Formula on gastric tissue of rats with chronic atrophic gastritis with syndrome of spleen deficiency. China Medical Herald 2021;18:4-7.
- Arnold K, Sarkar A, Yram MA, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011;9:317-29. [Crossref] [PubMed]
- Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25-36. [Crossref] [PubMed]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 1993;236:259-79. [Crossref] [PubMed]
- Sigal M, Logan CY, Kapalczynska M, et al. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 2017;548:451-5. [Crossref] [PubMed]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014;346:1248012. [Crossref] [PubMed]
- Sayols S, Klassek J, Werner C, et al. Signalling codes for the maintenance and lineage commitment of embryonic gastric epithelial progenitors. Development 2020;147:dev188839. [Crossref] [PubMed]
- Hao X, Liu Y, Zhou P, et al. Integrating Network Pharmacology and Experimental Validation to Investigate the Mechanisms of Huazhuojiedu Decoction to Treat Chronic Atrophic Gastritis. Evid Based Complement Alternat Med 2020;2020:2638362. [Crossref] [PubMed]
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-81. [Crossref] [PubMed]
- Crafa P, Russo M, Miraglia C, et al. From Sidney to OLGA: an overview of atrophic gastritis. Acta Biomed 2018;89:93-9. [PubMed]
- Tsukamoto T, Mizoshita T, Tatematsu M. Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization. Gastric Cancer 2006;9:156-66. [Crossref] [PubMed]
- Yang TT, Cao N, Zhang HH, et al. Helicobacter pylori infection-induced H3Ser10 phosphorylation in stepwise gastric carcinogenesis and its clinical implications. Helicobacter 2018;23:e12486. [Crossref] [PubMed]
- Fatehullah A, Terakado Y, Sagiraju S, et al. A tumour-resident Lgr5+ stem-cell-like pool drives the establishment and progression of advanced gastric cancers. Nat Cell Biol 2021;23:1299-313. [Crossref] [PubMed]
- Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016;150:64-78. [Crossref] [PubMed]
- Tian FL, Yang XJ, Chen WQ, et al. Effects of Yiwei Xiaoyu Granule on intestinal metaplasia of gastric mucosa in rats with atrophic gastritis. Journal of Basic Chinese Medicine 2020;26:1080-3.
- Zhao ZK, Qin ED, Zhang MH, et al. Compound of traditional Chinese medicine on chronic atrophic gastritis inhibit cell proliferation and PCNA, p53 expression. Journal of Logistics University of PAP 2009;18:388-91. (Medical Sciences).
- Muthupalani S, Ge Z, Joy J, et al. Muc5ac null mice are predisposed to spontaneous gastric antro-pyloric hyperplasia and adenomas coupled with attenuated H. pylori-induced corpus mucous metaplasia. Lab Invest 2019;99:1887-905. [Crossref] [PubMed]
- Boccellato F, Woelffling S, Imai-Matsushima A, et al. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut 2019;68:400-13. [Crossref] [PubMed]
- Ootani A, Toda S, Fujimoto K, et al. Foveolar differentiation of mouse gastric mucosa in vitro. Am J Pathol 2003;162:1905-12. [Crossref] [PubMed]
- Baldus SE, Mönig SP, Arkenau V, et al. Correlation of MUC5AC immunoreactivity with histopathological subtypes and prognosis of gastric carcinoma. Ann Surg Oncol 2002;9:887-93. [Crossref] [PubMed]
- Shibata W, Sue S, Tsumura S, et al. Helicobacter-induced gastric inflammation alters the properties of gastric tissue stem/progenitor cells. BMC Gastroenterol 2017;17:145. Erratum in: BMC Gastroenterol 2018;18:4. [Crossref] [PubMed]
- Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010;139:2028-2037.e9. [Crossref] [PubMed]
- Yuan DM. Absence of Slc26a9 results in the disorder of gastric epithelial cell differentiation of, which is the key event to development of chronic atrophic gastritis. Zunyi: Zunyi Medical University; 2020.
- Ma JR, Zhang N, Kuang SJ, et al. Effect of Hewei Decoction on regeneration of gastric mucosal epithelium in mice with chronic atrophic gastritis. Chinese Journal of Digestion and Medical Imageology 2014;4:83-7. (Electronic Edition).
- Ren L, Li HM, Gao YH, et al. Effect of Costunolide on Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in Rats Cultured in vitro. Medical & Pharmaceutical Journal of Chinese People's Liberation Army 2021;33:1-4.
- Zhang ZL, Wang JH, Shen XL, et al. Effects of extracts from Codonopsis pilosula and Atractylodes macrocephala on growth and differentiation of IEC-6 Cells. Chinese Pharmacological Bulletin 2002;18:444-7.
- Shi YR, Xu HB, Shi MY, et al. Hedyotis diffusa Willd inhibits the differentiation of colonic cancer stem cells through Wnt signaling pathway. Pharmacology and Clinics of Chinese Materia Medica 2015;31:133-6.
- Peng Z, Zhou W, Zhang C, et al. Curcumol Controls Choriocarcinoma Stem-Like Cells Self-Renewal via Repression of DNA Methyltransferase (DNMT)- and Histone Deacetylase (HDAC)-Mediated Epigenetic Regulation. Med Sci Monit 2018;24:461-72. [Crossref] [PubMed]
- He JJ. Effect of Rong Wei Li Qi Decoction Joint Geckoon Gastric Mucosa Repair and Wnt Signal Pathway about Model Rats of Chronic Atrophic Gastritis. Wuhan: Hubei University of Chinese Medicine, 2017.
- He S, Lu Y, Liu X, et al. Wnt3a: functions and implications in cancer. Chin J Cancer 2015;34:554-62. [Crossref] [PubMed]
- de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol 2012;13:242. [Crossref] [PubMed]
- Chu A, Yu X, Guo Q, et al. H. pylori slyD, a novel virulence factor, is associated with Wnt pathway protein expression during gastric disease progression. Microb Pathog 2020;148:104428. [Crossref] [PubMed]
- Li JH, Li XQ. Effect of Weiwei Decoction Combined with Cimetidine on Gastric Mucosal Repair and Wnt Pathway Expression in rats with Chronic Atrophic Gastritis. Journal of Sichuan of Traditional Chinese Medicine 2019;37:37-41.
- Zhao J, Kim KA, Abo A. Tipping the balance: modulating the Wnt pathway for tissue repair. Trends Biotechnol 2009;27:131-6. [Crossref] [PubMed]
- Cai Q, Shi P, Yuan Y, et al. Inflammation-Associated Senescence Promotes Helicobacter pylori-Induced Atrophic Gastritis. Cell Mol Gastroenterol Hepatol 2021;11:857-80. [Crossref] [PubMed]
(English Language Editor: J. Jones)


