
Protective role of muscones on astrocytes under a mechanical-chemical damage model
Introduction
At present, the incidence of spinal cord injury (SCI) is increasing worldwide (1). In the United States, the incidence of SCI ranges between 28 and 55 cases per million people, and there are now 10,000 new cases per year (2). Due to the non-renewability of neuronal cells, the treatment of SCI remains a major clinical challenge. Trauma is the leading cause of death and disability worldwide. Nearly all SCIs cause damage to both upper and lower motor neurons, as they involve both the gray-matter and descending white-matter tracts. The initial mechanical forces applied to the spinal cord at the time of injury is known as the primary injury. After the primary injury, ischemia and hypoxia induce cell death and autolysis, which are associated with the release of a group of inflammatory factors and biologically active substances, such as superoxide dismutase (SOD), malonaldehyde (MDA), lactate dehydrogenase (LDH), and tumor necrosis factor-α (TNF-α). These processes are called the secondary injury. These inflammatory factors activate the inflammation cascade and enhance cell apoptosis (2-4).
Glutamate (Glu) can damage neurons in vivo at the concentrations released upon SCI. Glu is the major excitatory neurotransmitter in the nervous system (5,6). In addition to surgical treatment, both gene and stem cell therapies have shown promising outcomes in the treatment of a variety of diseases (7). Spinal cord damage can be reduced by preventing, attenuating, and reversing the secondary injury. Studies have proposed interventions targeting the secondary injury with the aim of preserving and improving spinal cord neurological functions (3,5). Neuroglial cells refer to specialized cells found in the brain and spinal cord that support the neurons and their fibers (8,9). The glutamine-Glu cycle provides neurons with astrocyte-generated Glu/-aminobutyric acid and oxidizes Glu in astrocytes. Glu is the predominant excitatory neurotransmitter in the central nervous system. Glutamatergic transmission is critical for controlling neuronal activity (10,11). However, the inflammatory factors may stimulate the proliferation and activation of astrocytes (12,13). To repair the damaged tissue, astrocytes first proliferate, and then, wall off the wounded areas to form a glial scar that reduces the spread and persistence of inflammatory cells. The glial scar serves to repair the blood brain barrier, prevents an overwhelming inflammatory response, and decreases neuronal loss and demyelination; however, it also blocks axon guidance and inhibits neural regeneration. Thus, inhibitory treatments of glial scar have been considered useful in neuronal regeneration (14,15). The key way in which to attenuate the secondary injury is to protect the immune and inflammatory cells and reduce the proliferation of astrocytes.
Muscone is regarded as the most important component in musk, and is responsible for the main bioactivity. Recent research showed that muscone possesses an anti-inflammatory function similar to glucocorticoid. It can penetrate the blood-brain barrier, mediate the effects of hypoxia on the central nervous system, and reduce edema. Muscone protects PC12 cells against Glu-induced apoptosis by attenuating reactive oxygen species (ROS) generation and calcium ion influx (16). Muscone has also been shown to reduce neuronal apoptosis in the hippocampus of neonatal rats after ketamine anesthesia (17,18). Further, 4-methylcyclopentadecanone has been shown to protect against cerebral ischemia/reperfusion injury and is associated with anti-inflammatory actions though the inhibition of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway (4). Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through the inhibition of NF-κB and NLR family pyrin domain containing 3 (NLRP3) inflammasome (19). However, the effects of muscone on the spinal cord are still unknown.
Thus, in the present study, we established a primary astrocyte mechanical-chemical damage (MCD) model and used different concentrations of muscone to treat the cells. We also identified the levels of TNF-α, interleukin (IL)-1B, SOD, MDA, LDH, and intracellular calcium to investigate the protective effects of muscone on astrocytes. Additionally, we measured the Glu concentration to assess the ability of astrocytes to recycle vesicles. Finally, we measured the changes in the expression levels of excitatory amino acid transporters (EAATs) and glial fibrillary acidic proteins (GFAPs) to identify the possible mechanism by which muscone exerts a protective effect on astrocytes. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3848/rc).
Methods
Ethical statement
The study protocol was approved by the Ethics Review Board of the University-Town Hospital of Chongqing Medical University (No. LL-202133). The experimental procedures followed the national guidelines for the Care and Use of Laboratory Animals.
Isolation and culture of primary astrocytes
Neonatal (Sprague Dawley, female, specific-pathogen-free grade) rats (Kunming Institute of Zoology, Cas, China) were decapitated, and spinal cord samples were collected. The pia mater was pierced by blood vessels to the brain and spinal cord, and its capillaries nourished the brain. The spinal cord cells were harvested in Dulbecco’s Modified Eagle’s medium (GibcoTM, cat: 11965092, New York, NY, USA), containing 10% fetal bovine serum (Gibco™, cat: 10099141, New York, NY, USA) and 2 mmol/L of Gln (Sigma-Aldrich, cat: 1.00289, St. Louis, MO, USA). Then, the astrocytes, fibroblasts, neuron cells, oligodendroglia cells, and microglial cells were separated by centrifugation. The astrocytes were collected, blocked with bovine serum albumin (Jackson Immuno Research Laboratories Inc., cat: 001-000-161, West Grove, PA, USA), and incubated with rabbit anti-GFAP antibody (Sigma-Aldrich, cat: SAB5600060, St. Louis, MO, USA). The astrocytes store in a refrigerator at −80 ℃. Next, the astrocytes were stained with a 3,3'-diaminobenzidine (DAB) Staining Kit (ZSGB-BIO Co., Ltd., cat: ZLI-9018, Beijing; Standard Compound Microscope System, Olympus, Tokyo, Japan).
A primary astrocyte MCD model
The astrocytes were incubated, washed with phosphate-buffered saline (PBS; ZSGB-BIO, ZLI-9061, Beijing, China), and digested with trypsin (Sigma-Aldrich, cat: T4799, St. Louis, MO, USA). The density of the cell suspension was adjusted, and the astrocytes were seeded into 96-well plates. They were then cultivated for 24 h to assess their adherence capability. A mechanical damage model was established by syringe needle (0.45 mm diameter) administration into the astrocytes in vitro. Next, the medium was changed, and then astrocytes were allowed to adhere and grow overnight. Then, 500 µmol/L of Glu (Sigma-Aldrich, cat: 6106-04-3, St. Louis, MO, USA) was added to simulate the chemical damage. According to the study reported by Du et al. (4), different concentrations of muscone (Sigma-Aldrich, cat: PHL89737, St. Louis, MO, USA) were added according to the study design (Table 1).
Table 1
| Group | Mechanical damage | Concentration of Glu | Concentration of muscone |
|---|---|---|---|
| A1 | – | – | 0 |
| B1 | P | 500 μmol/L | 0 |
| C1 | P | 500 μmol/L | 3 μg/mL |
| D1 | P | 500 μmol/L | 6 μg/mL |
| E1 | P | 500 μmol/L | 12 μg/mL |
Glu, glutamate.
MTT assay
The cells were seeded into 6-well plates and then washed with PBS at 6, 12, 24, 48, and 72 h after the addition of the muscone. Next, 20 µmol of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reagent [Sigma-Aldrich, cat: CT02, Sigma-Aldrich (Shanghai) Trading Co., Ltd. Shanghai, China] was added into each well and incubated for an additional 6 h. Afterward, the medium was sucked out, and 150 µL of dimethyl sulfoxide (Sigma-Aldrich, cat: D 2650, St. Louis, MO, USA) was added into each well and shaken gently. The optical density was quantified using a spectrophotometer (Bio-Rad Laboratories Inc., Hercules, CA, USA) under a wavelength of 490 nm.
Biochemical analysis
The levels of MDA, SOD, TNF-α, and LDH were detected by commercial enzyme-linked immunosorbent assay (ELISA) kits (BioVision Inc., Milpitas, CA, USA) at different time points in accordance with the manufacturer’s instructions.
Detection of intracellular calcium concentration by flow cytometry
The fluo-4 AM working solution (Dojindo Molecular Technologies, Inc., Tokyo, Japan) was added, and the cells and working solution were incubated for 20 min at 37 ℃. The cells were rinsed with PBS twice and then incubated with Hank’s Balanced Salt solution (Sigma-Aldrich, cat: H6648, St. Louis, MO, USA) for 40 min at 37 ℃ in a humidified atmosphere containing 5% carbon dioxide. The fluorescence was monitored continuously at excitation and emission wavelengths of 494 and 516 nm, respectively.
ELISAs for the quantitative determination of Glu decarboxylase acid
In order to figure out the possible mechanism of the protective effect of muscone and compare it with MK801 (Sigma-Aldrich, cat: M107, St. Louis, MO, USA), the experiment groups were redesigned as listed in Table 2. After being treated with muscone at 3, 6, and 12 h, the culture medium (0.2 mL) in each group was sucked out and subjected to ELISA tests (Biovision, cat: E4962-100, St. Louis, MO, USA). Fresh medium was used a as a blank control and 50 µmol/L of Glu was used as the standard for the calibration.
Table 2
| Group | Mechanical damage | Concentration of Glu | Concentration of muscone | Concentration of MK801 |
|---|---|---|---|---|
| A2 | P | 500 μmol/L | 0 | 0 |
| B2 | P | 500 μmol/L | 6 μg/mL | 0 |
| C2 | P | 500 μmol/L | 0 | 50 μmol/L |
| D2 | p | 500 μmol/L | 6 μg/mL | 50 μmol/L |
Glu, glutamate.
Detection of intracellular calcium concentration
A2–D2 cells were collected at 3, 6 and 12 h after the muscone had been added. The intracellular calcium concentration was measured using flow cytometry technology. The experimental methods were the same as those described in Detection of intracellular calcium concentration by flow cytometry above. Different concentrations of muscone (Sigma-Aldrich, cat: PHL89737, St. Louis, MO, USA) were added according to the study design (Table 2).
qRT-PCR
The messenger ribonucleic acid (mRNA) levels of the EAATs and GFAPs in the astrocytes of each group were measured using the quantitative reverse transcription polymerase chain reaction (qRT-PCR) method. The 6-well plates were washed with PBS for groups A2–D2, and total RNA was then extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s protocol. Next, 5 µg of total RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using the ReadyScript® cDNA Synthesis Mix Kit (Sigma-Aldrich, cat: RDRT, St. Louis, MO, USA). β-actin (Sigma-Aldrich, cat: A1978, St. Louis, MO, USA) was used as an internal control. The q-PCR cycling conditions were as follows: 25 ℃ for 10 min, 42 ℃ for 50 min, and 85 ℃ for 5 min. The primer sequences used for q-PCR are listed in Table 3, and were purchased from Shanghai Chemicals Co., Ltd. (Shanghai, China). The differences in mRNA expression levels were calculated as the fold change using the 2-ΔΔCt method.
Table 3
| Genes | F/R | Primer sequence |
|---|---|---|
| EAAT1 | Forward | 5'-ATCGGAAACATGAAGGAGCAG-3' |
| Reverse | 5'-GAAGAGGATGCCCAGAGGTG-3' | |
| GFAP | Forward | 5'- CGGGAGTCGGCGAGTTAC-3' |
| Reverse | 5'-GGTGATGCGGTTTTCTTCG-3' | |
| Rat beta-actin | Forward | 5'- CCCATCTATGAGGGTTACGC-3' |
| Reverse | 5'- TTTAATGTCACGCACGATTTC-3 |
EAAT, excitatory amino acid transporter; GFAP, glial fibrillary acidic protein.
Western blot assays
The protein concentration was detected by the bicinchoninic acid (BCA) Protein Assay Kit (P0012S; Beyotime, Shanghai, China). Sodium dodecyl sulphate–polyacrylamide gel electrophoresis was used to separate the proteins, and the separated proteins were then transferred onto polyvinylidene fluoride (DuPo™, Tedlar, Wilmington, Delaware, USA) membranes. The membranes were incubated overnight with the following primary antibodies at 4 ℃: Histone Deacetylase 3 (HDAC3) (Abcam, cat: ab76295; dilution, 1:1,000; Cambridge, UK), (toll-like receptor 4) TLR4 (Abcam, cat: ab217274; dilution, 1:1,000; Cambridge, UK), NLR family pyrin domain containing 3 (NLRP3) (Abcam, cat: ab263899; dilution, 1:1,000; Cambridge, UK), caspase-1 (Abcam, cat: ab179515; dilution, 1:1,000; Cambridge, UK), and Interleukin 1β (IL-1β) (Abcam, cat: ab9722; dilution, 1:1,000; Cambridge, UK). Subsequently, the membranes were washed with Tris Buffered Saline with Tween (Sigma-Aldrich, TWEEN®20, cat: P1379, St. Louis, MO, USA) 3 times for 5 min each time, and incubated with goat anti-rabbit immunoglobulin G (IgG) (Abcam, cat: ab205718; dilution, 1:2,000; Cambridge, UK), and goat anti-mouse IgG (Abcam, cat: ab205719; dilution, 1:2,000; Cambridge, UK) antibodies as appropriate for 1 h at room temperature. The ChemiDoc XRS+ System (Bio-Rad Laboratories LLC, Hercules, CA, USA) was used for the chemiluminescence detection of the immunoreactive bands, and glyceraldehyde 3-phosphate dehydrogenase (Sigma-Aldrich, cat: G9545, St. Louis, MO, USA) was considered the internal control.
Statistical analysis
The statistical analysis was performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). All the experiments were performed in triplicate, each being repeated at least 3 times. The data are presented as the mean ± standard deviation, and a 1-way analysis of variance was applied to compare differences between the 2 groups. A P value <0.05 was considered statistically significant.
Results
High-purity astrocytes
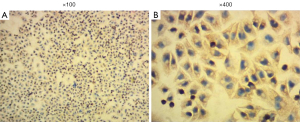
To acquire high-purity astrocytes, the purified astrocytes were cultured in 6-well plates for 6 days. Microscopic observation showed the typical morphology of astrocytes, and almost no microglia and oligodendrocytes were observed (Figure 1). Immunofluorescence staining was used to stain the astrocyte-specific GFAPs. The astrocytes were then counted under magnification of ×400 in 20 high-power fields, and the purity of the astrocytes was as high as 95%.
The protective role of muscone in astrocytes
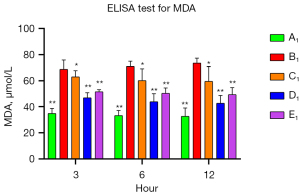
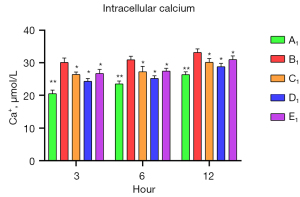
To verify the protective role of muscone in astrocytes, a rat model of mechanical and chemical damage to primary spinal astrocytes was established and treated with different concentrations of muscone (i.e., 3, 6, and 12 µg/mL). The results of the MTT assays showed that the number of live cells increased after treatment with muscone than B1 (Figure 2). The most significant protective effect was observed in the 6 µg/mL group of the treatment with muscone three groups (Figure 3). The peak value of cell viability appeared after 48 h in both treatment groups. The ELISA test results for LDH in the medium corresponded to the MTT test results. The LDH level of the non-treatment group significantly increased but decreased after treatment with muscone (the muscone group). A higher concentration of muscone was not positively effective at reducing the rate of cell apoptosis and decreasing the LDH level (Table 4). However, the MDA level after cell damage gradually increased, but significantly decreased after treatment with muscone (Figure 4). The minimum MDA level was detected in the 6 µg/mL group, indicating that lipid metabolic disorders and lipid peroxidation were effectively suppressed. The flow cytometry results showed that the intracellular calcium levels were progressively elevated after the injury, and the MCDs remarkably increased the intracellular calcium concentration in the astrocytes. In the C1–E1 groups, muscone significantly reduced the intracellular calcium concentration in the astrocytes (Figure 5).
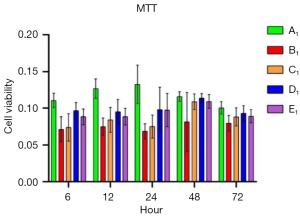
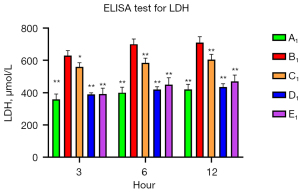
Table 4
| Group | Concentration of muscone | LDH (U/L) | Suppression ratio (%) |
|---|---|---|---|
| A1 | – | 357.76±3.47 | – |
| B1 | – | 628.83±3.97 | – |
| C1 | 3 μg/mL | 559.623±3.56 | 25.53±4.72 |
| D1 | 6 μg/mL | 388.55±1.88 | 88.27±3.76 |
| E1 | 12 μg/mL | 390.19±3.81 | 88.04±4.53 |
Suppression ratio: mean ± standard deviation. LDH, lactate dehydrogenase.
The anti-inflammatory effects of muscone on astrocytes
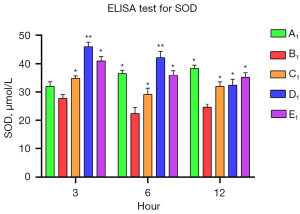
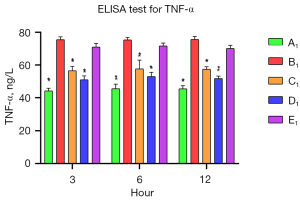
The levels of SOD and TNF-α in the A1–E1 groups were measured by commercial ELISA kits to assess the anti-inflammatory effects of muscone on the astrocytes. After the damage, the SOD level was significantly reduced in the B1 group, and it decreased progressively. The SOD levels of the muscone treatment groups (C1–E1) were significantly elevated compared to that of the B1 group. In contrast to the A1 group in which the SOD level gradually increased, the SOD levels progressively decreased in the D1 and B1 groups (Figure 6). The muscone treatment groups (C1–E1) showed statistically significant differences in the SOD levels compared to the non-treatment group (B1). The TNF-α concentration was 44.37 ng/L at 3 h after treatment. The abundance of the non-treatment group (B1) was significantly elevated to 75.73 ng/L. After treatment with 3 µg/mL of muscone (C1), the TNF-α level decreased to 51 ng/mL in the D1 group, but it did not significantly decrease in the E1 group (71.33 ng/mL). Compared to the non-treatment group (B1), a significant difference (P<0.05) was found in the TNF-α levels in the C1 and D1 groups. In contrast to the SOD level, the TNF-α level did not increase over time. The lowest amount of secretion was found in the non-treatment group (A1) (Figure 7).
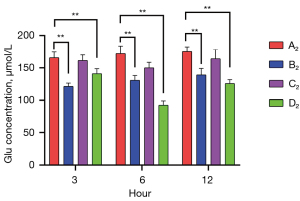
Muscone reduced Glu levels in damaged astrocytes
Commercial ELISA kits were used to measure the Glu levels of the A2–D2 groups at 3, 6, and 12 h after treatment. The highest Glu levels were detected in the non-treatment group (A2) [165,738 (3 h), 172,1604 (6 h), and 175,3126 µmol/L (12 h)]. In the muscone treatment group (B2), the Glu levels were 121.53 (3 h), 130.90 (6 h), and 139.30 µmol/L (12 h), which were not only lower than those in the A2 group, but were also significantly lower than those in the C2 group [161.45 (3 h), 150.01 (6 h), and 164.15 µmol/L (12 h)]. The lowest concentration of Glu was identified in the group treated with both muscone and MK801 (D2) [141.11 (3 h), 92.60 (6 h), and 125.84 µmol/L (12 h)]. There were no significant differences between the A2 and C2 groups at the time points of 3 and 12 h. However, significant differences were found after a comparison of other groups at any time points. The lowest Glu concentration over the majority of the time points was detected in the D2 group (Figure 8).
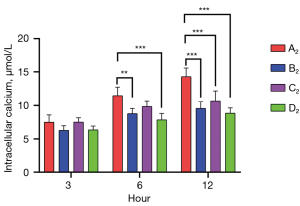
Muscone decreased the intracellular calcium levels in astrocytes
The intracellular calcium levels of the A2–D2 groups were measured by flow cytometry at 3, 6, and 12 h after treatment. The highest intracellular calcium concentration was detected in the non-treatment group 7.51 (3 h), 11.44 (6 h), and 14.29 µmol/L (12 h). After treatment with muscone (B2), the intracellular calcium concentration decreased to 6.28 (3 h), 8.79 (6 h), and 9.57 µmol/L (12 h). After treatment with MK801 (C2), the intracellular calcium concentration was slightly higher [7.52 (3 h), 9.85 (6 h), and 10.66 µmol/L (12 h)] than that after treatment with muscone, but it was still lower than that of the non-treatment group. As predicted, the group treated with muscone and MK801 had the lowest intracellular calcium level 6.36 (3 h), 7.86 (6 h), and 8.85 µmol/L (12 h). Both groups showed no significant differences at 3 h after treatment. After 6 h, there were significant differences between groups A2 and B2, groups A2 and D2, and groups C2 and D2. After 12 h, there were significant differences between the A2 and treatment groups (Figure 9).
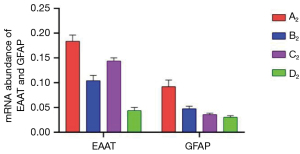
Muscone decreased the mRNA expression levels of EAAT and GFAP
The astrocytes of the A2–D2 group were collected for qRT-PCR test at 6 h after treatment. The mRNA expression level of EAAT in the A2 group was 0.1838, and after treatment with muscone (B2), it decreased to 0.1042. After treatment with MK801, the mRNA expression level of EAAT was slightly higher than that of the B2 group (0.144), but it was lower than that of the A2 group. The mRNA expression level of EAAT in the D2 group was 0.0438. The mRNA expression level of GFAP exhibited the same trend as that of EAAT. The mRNA expression level of GFAP reached its peak (0.0922) in A2, and reduced to 0.0474 after treatment with muscone (B2). However, compared to the EAAT, after treatment with MK801 (C2), the level value was lower in the B2 group (0.036). The mRNA expression level of GFAP in the D2 group was 0.0304 (Figure 10).
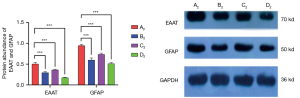
Muscone decreased the protein expression levels of EAAT and GFAP
The astrocytes of the A2–D2 groups were harvested for Western blotting. The protein expression levels of EAAT and GFAP were highly consistent with the mRNA expression levels of EAAT and GFAP. In the A2 group, the protein expression level of EAAT was 0.5092, and it decreased to 0.2971 in the B2 group. In the C2 group, the level was 0.3606. After treatment with muscone and MK801 simultaneously, the protein expression level of EAAT reduced rapidly to 0.1725. The protein expression level of GFAP in the A2 group was 0.9498, and it reduced to 0.6038 and 0.7386 after treatment with muscone (B2) and Mk801 (C2), respectively. After treatment with muscone and MK801 simultaneously, the protein expression level of GFAP decreased to 0.5103 (Figure 11).
Discussion
There are 17,000 new SCI cases in the United States per year, and the annual incidence is 54 cases per million. Only 1% of patients will experience a full neurological recovery when discharged from hospital, 30% of patients will be re-hospitalized several times, and only 1/3 of patients will return to work after injury (1). The high disability rate of SCI places a heavy burden on patients and society. SCI can be categorized into primary and secondary phases The direct mechanical injury comes from the fracture fragment and the intervertebral disc, which cause spinal cord bleeding and white-matter edema. This is called the primary injury. The biochemical cascade at the cellular and molecular levels caused by the primary injury induces ischemia, programmed cell death, axonal demyelination, astrocyte proliferation and glial scar formation, which are called the secondary injury (2-4,6).
Spinal cells contain a large amount of polyunsaturated fatty acid, which has no antioxidant capacity. Ischemia and hypoxia induce cell membrane and organelle damage, which cause ion channel function abnormal and calcium influx (20). And calcium influx will activate a series of cytokines that mediate the oxygen-free radical reaction and lipid peroxidation, which cause further damage to the cell membrane and organelle, leading to the release of MDA and ROS. Cell lysis induces the aggregation of neutrophils, macrophages, and monocytes, which release a large amount of inflammatory factors, such as IL-6, IL-1β, and TNF-α. These inflammatory factors further aggravate the inflammatory response (3,4). Conversely, the damage induces spinal cell Glu metabolism dysfunction, which causes local excitatory neurotransmitter Glu excess. An overdose of Glu is the most important neurotoxicity substance after spinal injury, and causes further damage to spinal cells, especially neuronal cells (21,22). Neuroglia activates and begins proliferation. The proliferation of glia cells can fill the tissue defect and restore the basic structure and barrier of the spine. However, this proliferation of glia cells also leads to the formation of a glia scar and blocks how neuron cells grow and physically stops spinal function regeneration (9,23).
From the above, we can see the key point for the treatment of SCI is to relieve spinal cord compression as soon as possible and stop the secondary injury process. The decompression relies on a surgical operation. Thus, the prevention of secondary injuries still relies on medical treatments. Ideally, drugs used to treat SCI should have 4 functions: (I) an ability to prevent further damage to spinal cells and palliate progressive cell death and dysfunction; (II) an anti-inflammatory ability to reduce the release of inflammatory factors and break off the cascade of the inflammatory response; (III) an ability to reduce toxic neurotransmitters, especially Glu; and (IV) an ability to stop glia cell proliferation and glia scar formation.
Methylprednisolone (MP) has an immediate anti-inflammatory ability and has been long used in the clinical treatment of SCI. However, there is evidence that MP does not improve the long-term prognosis of SCI patients. Additionally, like many other glucocorticoids, MP has many side effects (24-26). Gangliosides are another frequently used medicine in clinical settings. However, there is still no evidence that gangliosides improve the neuro function of SCI patients (27,28) Thus, a medicine to cure SCI is urgently needed (29).
Muscone is the active ingredient of musk. The ancient Chinese believed that it has the ability to relieve uneasiness of the mind and tranquilize the body. Modern medical research has shown that muscone has an anti-inflammatory ability and can pass through the blood-brain barrier (4). There is evidence that muscone can reduce the neuron death of neonatal mice after ketamine anesthesia and ease brain edema and cell damage after cerebral ischemia (17-19,30,31). Muscone has a similar anti-inflammatory effect to that of glucocorticoids, but does not have the side effects of glucocorticoids (32). It has been frequently used in clinical settings to treat brain and cardiovascular injuries.
We sought to prove the therapeutic effect of muscone on SCI. In this study, we isolated and purified astrocytes from fetal mice. An astrocyte is a major cell that makes up the spinal cord; the ratio of astrocytes to neurons is about 10:1 (13,14). Astrocytes are the most important cells for Glu recycling and play a key role in maintaining Glu concentration in the spinal cord (33,34). Unlike neurons, astrocytes have the ability to proliferate and are the most important cells in spinal regeneration. Over proliferation also blocks the way in which the neuron axon grows and the formation of the glia scar. Additionally, astrocytes can be cultured in vitro (13).
A MCD model of astrocytes was established to examine the protective effects of muscone. Muscone was shown to have a definite protective effect on the damaged astrocytes. In the MTT assay, the live cells significantly increased when treated with muscone. Additionally, the leaking of LDH was significantly reduced by muscone, and the release of MDA produced trends similar to those of LDH. The calcium influx was measured by flow cytometry, and muscone also significantly reduced the calcium levels of astrocytes after damage. All these protective effects reached their maximum performance at the muscone concentration of 6 µg/mL, and higher concentrations produced no further improvements. The above-mentioned results demonstrate that muscone has the ability to protect astrocytes from further damage.
We also sought to determine whether muscone has an anti-inflammatory ability in a SCI model. SOD catalyzes the conversion of superoxide to oxygen and hydrogen peroxide by disproportionation (35,36). SOD is the most important antioxidant in body that can protect cells from oxidation damage. The SOD concentration of the MCD astrocytes decreased progressively with time. When treated with muscone, the SOD concentration significantly increased, and it was even higher than that of undamaged astrocytes when treated with 6 µg/mL of muscone. We found that muscone enhanced the anti-oxidation ability of astrocytes. However, this enhancing effect did not last long and ceased over time. Next, we used ELISA tests to measure the abundance of TNF-α in the experimental groups. The concentration of TNF-α increased significantly after damage. Muscone also significantly reduced the TNF-α concentration of the MCD group. The administration of 6 µg/mL of muscone produced the maximum effect once again.
These experiments confirmed that muscone has the ability to protect astrocytes and has an anti-inflammatory effect. To determine the possible mechanism and compare the cure ability of muscone and MK801, we redesigned the experimental group (Table 2). We then measured the Glu concentration of each group, and found that Glu concentration increased significantly after mechanical damage. The muscone and MK801 both reduced the Glu concentration of the damaged group, but muscone has a superior effect to that of MK801. The muscone and MK801 has a cooperative effect when combined.
Glu is the most important toxic neurotransmitter after spinal injury, and an overdose of Glu will damage neurons and enhance neuron damage (6,37). Astrocytes are the main cells that recycle extracellular Glu and convert it into Gln via glutamine synthetase. We found that muscone enhanced the cycle of Glu-glutamine in astrocytes after injury (38,39). We have 3 hypotheses as to the possible mechanisms by which this occurs. First, it may be that the protective effect of muscone reduces the cell death of astrocytes and enables astrocytes to maintain their normal recycling function. Second, muscone may stimulate the proliferation of astrocytes and the total recycle capacity may increase with the multiplication of astrocytes. Third, muscone may enhance the Glu recycle ability of a single astrocyte. From the result of current study proved that muscone protects astrocytes from further damage and cell death. However, we also remeasured the intracellular calcium of the newly designed experimental group. As predicted muscone once more reduced the intracellular calcium levels of the damaged astrocytes. This protection ability is stronger than MK801 and can be combined with MK801.
EAAT is the main transporter of astrocytes for Glu transportation. It plays a key role in maintaining the Glu concentration of the intercellular space and in preventing the neuron toxicity of Glu. However, the activation of EAAT also increases the calcium influx of cells and induces organelle dissolution and programmed cell death (40-42). To determine whether muscone reduces intercellular calcium by mediating the upregulation of the expression of EAAT, we measured the mRNA and protein expression of the EAATs of the astrocytes. The mRNA and protein expression levels were highly consistent. The MCD upregulated the expression of EAAT. This may be a result of the increase in intercellular calcium. Muscone significantly suppressed the expression of EAAT. This suppression effect may be the result of the decreasing of intercellular calcium or some unknown mechanism. It is clear that muscone does not enhance Glu recycling via the activation of EAAT expression. Additionally, MK801 also had a suppressive effect, but the effect was lower than that of muscone, and MK801 can be combined with muscone to enhance the suppressive effect.
The question arises as to whether muscone restrains the proliferation of astrocytes to enhance the Glu recycling ability. GFAP is a specific protein of astrocytes, and its abundance reflects the amount of astrocytes (43). By measuring the GFAP mRNA and protein abundance, we found out that GFAP mRNA and protein abundance is significantly increased after damage. It may be that the cell damage and increased concentration of intercellular Glu stimulated the proliferation of astrocytes in a manner similar to that of the glia scar formation process in spinal injury. Muscone suppressed the over proliferation and significantly reduced the GFAP abundance. However, this time. MK801 showed a greater suppression ability than muscone. When combined together, they also further reduced the GFAP abundance.
Conclusions
Muscone is a significant biomarker for SCI treatment and does not have the side-effects of glucocorticoids. It has an anti-inflammatory ability, reduces calcium influx, protects astrocytes from further damage and increases cell viability after damage. It also reduces intercellular Glu, which may protect neurons from damage by toxic neurotransmitters. This effect is not achieved by upregulating the expression of EAAT or stimulating astrocyte proliferation; rather, muscone suppresses the over expression of EAAT and reduces the proliferation of astrocytes, which may stop the formation of glia scars. The mechanism by which muscone reduces the concentration of intercellular Glu and suppresses the activation of EAAT and the proliferation of astrocytes at the same time is still unknown. It may be that muscone protects astrocytes from further damage and reduces the cell death rate by exerting an anti-inflammatory effect and reducing the calcium influx. This protective effect increases the number of viable cells and maintains the normal function of astrocytes. Thus, the intercellular Glu is efficiently recycled and does not stimulate the expression of EAAT and the proliferation of astrocytes.
Acknowledgments
Funding: This work was supported by the Chongqing Science and Technology Project (contract No. cstc2016jcyjA0299).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3848/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3848/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3848/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Review Board of the University-Town Hospital of Chongqing Medical University (No. LL-202133). The experimental procedures followed the national guidelines for the Care and Use of Laboratory Animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shank CD, Walters BC, Hadley MN. Current Topics in the Management of Acute Traumatic Spinal Cord Injury. Neurocrit Care 2019;30:261-71. [Crossref] [PubMed]
- Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers 2017;3:17018. [Crossref] [PubMed]
- Quadri SA, Farooqui M, Ikram A, et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev 2020;43:425-41. [Crossref] [PubMed]
- Du Y, Gu X, Meng H, et al. Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome. Am J Transl Res 2018;10:4235-46. [PubMed]
- Pei JP, Fan LH, Nan K, et al. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J Neuroinflammation 2017;14:97. [Crossref] [PubMed]
- Shahsavani N, Alizadeh A, Kataria H, et al. Availability of neuregulin-1beta1 protects neurons in spinal cord injury and against glutamate toxicity through caspase dependent and independent mechanisms. Exp Neurol 2021;345:113817. [Crossref] [PubMed]
- Ahuja CS, Fehlings M. Concise Review: Bridging the Gap: Novel Neuroregenerative and Neuroprotective Strategies in Spinal Cord Injury. Stem Cells Transl Med 2016;5:914-24. [Crossref] [PubMed]
- Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience 2016;322:377-97. [Crossref] [PubMed]
- Pannese E. Neurocytology: fine structure of neurons, nerve processes, and neuroglial cells. 2nd edition. Berlin: Springer, 2015.
- Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 2021;24:312-25. [Crossref] [PubMed]
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci 2016;19:182-9. [Crossref] [PubMed]
- Danbolt NC, Furness DN, Zhou Y. Neuronal vs glial glutamate uptake: Resolving the conundrum. Neurochem Int 2016;98:29-45. [Crossref] [PubMed]
- Zhang M, Yang H, Chen Z, et al. Long Noncoding RNA X-Inactive-Specific Transcript Promotes the Secretion of Inflammatory Cytokines in LPS Stimulated Astrocyte Cell Via Sponging miR-29c-3p and Regulating Nuclear Factor of Activated T cell 5 Expression. Front Endocrinol (Lausanne) 2021;12:573143. [Crossref] [PubMed]
- Kim JY, Park J, Chang JY, et al. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp Neurobiol 2016;25:241-51. [Crossref] [PubMed]
- O'Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest 2017;127:3259-70. [Crossref] [PubMed]
- Yu L, Wang N, Zhang Y, et al. Neuroprotective effect of muscone on glutamate-induced apoptosis in PC12 cells via antioxidant and Ca(2+) antagonism. Neurochem Int 2014;70:10-21. [Crossref] [PubMed]
- Zhang X, Zhang Y, Tang S, et al. Pien-Tze-Huang protects cerebral ischemic injury by inhibiting neuronal apoptosis in acute ischemic stroke rats. J Ethnopharmacol 2018;219:117-25. [Crossref] [PubMed]
- Jin Y, Wei F, Dai X, et al. Anti-inflammatory effect of 4-methylcyclopentadecanone in rats submitted to ischemic stroke. Fundam Clin Pharmacol 2018;32:270-8. [Crossref] [PubMed]
- Yu S, Zhao G, Han F, et al. Muscone relieves inflammatory pain by inhibiting microglial activation-mediated inflammatory response via abrogation of the NOX4/JAK2-STAT3 pathway and NLRP3 inflammasome. Int Immunopharmacol 2020; Epub ahead of print. [Crossref] [PubMed]
- Ribas VT, Schnepf B, Challagundla M, et al. Early and sustained activation of autophagy in degenerating axons after spinal cord injury. Brain Pathol 2015;25:157-70. [Crossref] [PubMed]
- Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci 1997;17:1055-63. [Crossref] [PubMed]
- Anneken JH, Collins SA, Yamamoto BK, et al. Chapter 38 - MDMA and Glutamate: Implications for Hippocampal Neurotoxicity. In: Preedy VR, editor. Neuropathology of Drug Addictions and Substance Misuse. San Diego: Academic Press, 2016:406-14.
- Li X, Li M, Tian L, et al. Reactive Astrogliosis: Implications in Spinal Cord Injury Progression and Therapy. Oxid Med Cell Longev 2020;2020:9494352. [Crossref] [PubMed]
- Evaniew N, Belley-Côté EP, Fallah N, et al. Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Systematic Review and Meta-Analysis. J Neurotrauma 2016;33:468-81. [Crossref] [PubMed]
- Andersen JV, Markussen KH, Jakobsen E, et al. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021;196:108719. [Crossref] [PubMed]
- Michaluk P, Heller JP, Rusakov DA. Rapid recycling of glutamate transporters on the astroglial surface. Elife 2021;10:64714. [Crossref] [PubMed]
- Donovan J, Kirshblum S. Clinical Trials in Traumatic Spinal Cord Injury. Neurotherapeutics 2018;15:654-68. [Crossref] [PubMed]
- Hernon M, Kasotakis G. Spinal Cord Injury. In: Salim A, Brown C, Inaba K, editors. Surgical Critical Care Therapy: A Clinically Oriented Practical Approach. Cham: Springer International Publishing, 2018:29-36.
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2015;76:S71-83. [Crossref] [PubMed]
- Zhang W, Hong J, Zhang H, et al. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY) 2021;13:21642-58. [Crossref] [PubMed]
- Guo YJ, Luo SH, Tang MJ, et al. Muscone exerts protective roles on alcohol-induced osteonecrosis of the femoral head. Biomed Pharmacother 2018;97:825-32. [Crossref] [PubMed]
- Yu C, Gui F, Huang Q, et al. Protective effects of muscone on traumatic spinal cord injury in rats. Ann Transl Med 2022;10:685. [Crossref] [PubMed]
- Gwak YS, Luo ZD. Spinal GABA mechanism in neuropathic pain after spinal cord injury, in Spinal Cord Injury Pain. Elsevier 2022;275-96.
- Sonnewald U, Schousboe A. Introduction to the Glutamate-Glutamine Cycle. Adv Neurobiol 2016;13:1-7. [Crossref] [PubMed]
- Azadmanesh J, Borgstahl GEO. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants (Basel) 2018;7:25. [Crossref] [PubMed]
- Wang J, Zhang H, Zhang T, et al. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int J Biol Macromol 2015;77:59-67. [Crossref] [PubMed]
- Goldshmit Y, Jona G, Schmukler E, et al. Blood Glutamate Scavenger as a Novel Neuroprotective Treatment in Spinal Cord Injury. J Neurotrauma 2018;35:2581-90. [Crossref] [PubMed]
- Vidor MV, Panzenhagen AC, Martins AR, et al. Emerging findings of glutamate-glutamine imbalance in the medial prefrontal cortex in attention deficit/hyperactivity disorder: systematic review and meta-analysis of spectroscopy studies. Eur Arch Psychiatry Clin Neurosci 2022; Epub ahead of print. [Crossref] [PubMed]
- Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci 2000;20:1190-8. [Crossref] [PubMed]
- Fahlke C, Kortzak D, Machtens JP. Molecular physiology of EAAT anion channels. Pflugers Arch 2016;468:491-502. [Crossref] [PubMed]
- Kovermann P, Engels M, Müller F, et al. Cellular Physiology and Pathophysiology of EAAT Anion Channels. Front Cell Neurosci 2021;15:815279. [Crossref] [PubMed]
- Machtens JP, Kortzak D, Lansche C, et al. Mechanisms of anion conduction by coupled glutamate transporters. Cell 2015;160:542-53. [Crossref] [PubMed]
- Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol 1985;8:203-14. [Crossref] [PubMed]


