
Long-term effects of vitamin D on exacerbation rate, health care utilization and lung function in children with asthma
Introduction
Asthma is one of the most common chronic diseases in children, currently affecting an estimated 5.1 million children under 18 years (1). Much of the disease burden is caused by asthma exacerbations (2). Unscheduled emergency department (ED) visits and hospitalizations due to asthma exacerbations increase healthcare expenditure and thus cause a substantial financial burden on families (3). In addition, frequent exacerbations may lead to reduced lung function, resulting in more severe airway obstruction and airway remodeling (4). Early identification of protective factors against asthma exacerbations would allow a proactive management approach.
Optimal vitamin D level plays a key role in keeping the lungs healthy in asthmatic children (5). Airway epithelia contain high levels of the enzyme that converts inactive vitamin D to its active form, 25-dihydroxy-vitamin D3 [25(OH)D3] (6). This active form of vitamin D contributes to the integrity of the mucosal barrier, promotes the killing of pathogens, and modulates both innate and adaptive immune responses (5). Through these mechanisms, vitamin D may be protective against exacerbations in asthmatic children.
Many studies have reported a possible relationship between asthma outcomes and vitamin D level; however, the evidence remains inconclusive. Some epidemiologic studies suggested that low serum vitamin D in children with asthma was associated with asthma exacerbations, reduced lung function, and increased asthma severity (7-9), whereas other studies did not observe such a relationship (10,11) or even found the inverse to be true (12,13). Therefore, these inconsistent findings require further clarification. In addition, most studies focused on the short-term effects of vitamin D on asthma outcomes (7-13). Whether the observed effect of vitamin D on asthma is persistent and clinically important in the long term remains unclear. As far as we know, the temporal trends of these effects have not been studied. Asian children have differences in the genetic polymorphisms of vitamin D pathway genes and dietary intake compared with Caucasian children (14). It has also been reported that serum vitamin D levels are much lower in Asian children than in European and American children (15). However, most of the existing studies were conducted in Europe or North America (7-9), and it thus remains unclear whether the association between lower vitamin D serum levels and asthma exacerbations also applies for Asian children. Therefore, this study aims to examine the long-term associations between vitamin D levels and asthma exacerbations, ED visits or hospitalizations, and lung function among Chinese children with asthma, including the temporal trends of the potential protective effect. We present the following article in accordance with the STROBE reporting checklist (16) (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2750/rc).
Methods
Study design and participants
This retrospective cohort study was conducted in the Children’s Hospital of Chongqing Medical University. Patients that met all the following criteria were included: (I) being newly diagnosed with asthma between 2017 and 2021 by a pediatrician using the criteria of the Chinese Guidelines for the Diagnosis and Prevention of Childhood Asthma and the Global Initiative for Asthma (GINA) (17,18); (II) age between 3 and 18 years old; and (III) with results of serum 25(OH)D3 measurement available. Patients that met any of the following criteria were excluded: (I) having a chronic systemic disease such as a cardiac disease, renal disease, liver disease, endocrinal disorder, orthopedic problem or neuromuscular limitation; or (II) having a history of consumption of any supplements of vitamin D or drugs that modulate serum vitamin D levels, such as systemic corticosteroids or anticonvulsants. The follow-up began at the time of asthma diagnosis and ended in December 2021 or when the patient was lost to follow-up. Outcomes were assessed during the first, second and third years after asthma diagnosis separately. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of the Children’s Hospital of Chongqing Medical University (No. 2021-470). As this was a retrospective analysis, no informed consent was required.
Data collection
Patient data were extracted from the medical records by two trained pediatricians using a standard collection form. Demographic characteristics (age, sex, height, and weight), comorbidities (pneumonia, allergic rhinitis and atopic dermatitis/eczema), family history (maternal and paternal) of asthma, severity of asthma exacerbation, peripheral and bronchoalveolar lavage (BAL) eosinophil count and proportion, skin prick test results, and serum 25(OH)D3 level at baseline were collected. The number of asthma exacerbations, the number of ED visits or hospitalizations, and every lung function measurement during the 3-year follow-up were also collected.
Clinical outcomes
The primary outcome was the number of asthma exacerbations during the first year of the follow-up. The secondary outcomes were the number of asthma exacerbations during the second and third years of follow-up; the number of ED visits or hospitalizations; and the prevalence of large airway dysfunction (LAD) and small airway dysfunction (SAD) during the first, second and third years of follow-up.
Definition of covariates
Serum levels of 25(OH)D3 were measured using the chemiluminescence immunoassay method with ADVIA Centaur XP and Atellica Solution (SIEMENS, USA). Vitamin D levels were defined as deficient (<20 ng/mL), insufficient (20–30 ng/mL), and sufficient (>30 ng/mL) (19). Obesity and overweight were defined as having a body mass index (BMI) more than two or one standard deviations (SDs) above the age-specific World Health Organization (WHO) Growth Reference median, respectively. BMI between 2 SD below and 1 SD above the WHO median was considered normal weight, and BMI more than 2 SD below the median as underweight (20). Peripheral eosinophilia was defined as having a blood eosinophil count above 0.5×109/L (21). BAL eosinophilia was defined as a BAL eosinophil percentage above 1.2% of the total BAL cell count (22). Skin prick test was performed to detect food and aeroallergen sensitization, a test was considered positive if the maximal diameter of the wheal was 3 mm greater than the negative control (23). Food sensitization was defined as a positive skin test to any food allergen including milk, apple, sea crab, egg, beef, peanut, and soybean. Aeroallergen sensitization was defined as a positive skin test to any aeroallergen including dust mite, cat hair, dog hair, duck feathers, cockroach, corn pollen, artemisia pollen, penicillium, yeast, and cigarette smoke. The severity of asthma exacerbation was assessed according to GINA (15) and categorized as mild and severe for children aged 3 to 5 years, and mild, moderate and severe for children aged 6 to 18 years. Asthma exacerbation was defined as having at least one of the following: an asthma-related ED visit or hospitalization; taking systemic corticosteroids; or taking short-acting β-agonists as quick-relief medications (24). Repeated exacerbations occurring within 14 days were calculated as one single event (24). Forced expiratory volume in one second (FEV1) % less than 80% and forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) % less than 65% were considered as LAD and SAD, respectively (25).
Statistical analysis
Mann-Whitney U test or Kruskal-Wallis test was used to analyze the relationships between vitamin D levels and categorical variables. Multivariable negative binomial regression or Poisson regression was performed to investigate the effects of vitamin D on the number (count) of asthma exacerbations, emergency room visits and hospitalizations during the 3-year follow-up. Multivariable logistic regression was used to investigate the association between vitamin D and binary outcomes (LAD and SAD during the 3-year follow-up). All multivariable models were adjusted for age, sex, BMI z-score, severity of asthma exacerbation, combination with pneumonia and/or allergic rhinitis. Multivariate imputation was used to fill the missing data. BMI z scores were calculated based on WHO reference standards (20). Associations were considered statistically significant at P values less than 0.05 using two-sided tests. Statistical analysis was performed using R software (version: 4.1.1, https://www.r-project.org/).
Results
This study included 370 patients at baseline. Among them, 307, 285, and 212 patients visited at the outpatient clinic during the first, second and third years of follow-up, respectively. Further information about patient selection and follow-up is presented in Figure S1.
Baseline characteristics
The baseline characteristics of the study population are shown in Table 1. The median [interquartile range (IQR)] age of children with asthma was 4.3 (3.5–5.8) years. Among the 370 participants, 57.6% [95% confidence interval (CI): 52.6–62.6%] were male and 5.6% (95% CI: 3.0–8.2%) had obesity. Five percent (95% CI: 0.2–9.8%) of the participants aged 6 to 18 years and 12.1% (95% CI: 8.3–15.9%) of the participants aged 3 to 5 years had severe exacerbation at baseline.
Table 1
| Characteristics | Number, percentage (95% confidence interval) |
Vitamin D level, median (interquartile range) (ng/mL) |
P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (n=370) | 0.001 | ||
| Children 3 to 5 years | 290, 78.4 (74.2–82.6) | 20.6 (16.1–25.7) | |
| Children 6 to 18 years | 80, 21.6 (17.4–25.8) | 17.2 (13.2–21.8) | |
| Sex (n=370) | 0.768 | ||
| Male | 213, 57.6 (52.6–62.6) | 19.8 (15.5–25.1) | |
| Female | 157, 42.4 (37.4–47.4) | 19.8 (15.3–24.5) | |
| BMI class (n=301) | <0.001 | ||
| Normal | 214, 71.1 (66.0–76.2) | 20.9 (15.6–26.2) | |
| Overweight | 62, 20.6 (16.0–25.2) | 19.4 (15.5–23.8) | |
| Obesity | 17, 5.6 (3.0–8.2) | 15.4 (9.1–19.5) | |
| Underweight | 8, 2.7 (3.0–8.2) | 14.0 (12.2–17.6) | |
| Comorbidities | |||
| Pneumonia (n=370) | 0.069 | ||
| Yes | 237, 64.1 (59.2–69.0) | 20.5 (16.1–25.1) | |
| No | 133, 35.9 (31.0–40.8) | 19.1 (13.4–24.0) | |
| Allergic rhinitis (n=364) | 0.976 | ||
| Yes | 77, 21.2 (17.0–25.4) | 19.4 (15.0–27.2) | |
| No | 287, 78.8 (74.6–83.0) | 19.8 (15.4–24.2) | |
| Atopic dermatitis/eczema (n=364) | 0.978 | ||
| Yes | 95, 26.1 (21.6–30.6) | 19.4 (15.1–26.5) | |
| No | 269, 73.9 (69.4–78.4) | 19.9 (15.4–24.3) | |
| Family history of asthma | |||
| Maternal history of asthma (n=359) | 0.806 | ||
| Yes | 4, 1.1 (0.0–2.2) | 19.7 (17.2–20.8) | |
| No | 355, 98.9 (97.8–100.0) | 19.8 (15.3–24.9) | |
| Paternal history of asthma (n=359) | 0.836 | ||
| Yes | 5, 1.4 (0.2–2.6) | 18.6 (17.1–25.7) | |
| No | 354, 98.6 (97.4–99.8) | 19.8 (15.3–24.9) | |
| Severity of asthma exacerbation | |||
| Children aged 6 to 18 years (n=80) | <0.001 | ||
| No exacerbation | 10, 12.5 (5.3–19.7) | 23.7 (19.3–37.1) | |
| Mild | 37, 46.3 (35.4–57.2) | 19.5 (16.4–24.1) | |
| Moderate | 29, 36.2 (25.7–46.7) | 13.3 (10.6–16.5) | |
| Severe | 4, 5.0 (0.2–9.8) | 12.0 (5.9–15.4) | |
| Children aged 3 to 5 years (n=290) | <0.001 | ||
| No exacerbation | 13, 4.5 (2.1–6.9) | 25.1 (21.0–31.0) | |
| Mild | 242, 83.4 (79.1–87.7) | 21.1 (17.5–26.6) | |
| Severe | 35, 12.1 (8.3–15.9) | 11.1 (8.6–16.1) | |
| Allergies | |||
| Peripheral eosinophilia (n=334) | 0.492 | ||
| Yes | 42, 12.6 (9.0–16.2) | 19.2 (15.9–22.9) | |
| No | 292, 87.4 (83.8–91.0) | 19.9 (15.2–25.1) | |
| BAL eosinophilia (n=95) | 0.566 | ||
| Yes | 13, 13.7 (6.8–20.6) | 19.1 (14.7–26.1) | |
| No | 82, 86.3 (79.4–93.2) | 18.6 (14.8–22.7) | |
| Food sensitization (n=126) | 0.435 | ||
| Yes | 20, 15.9 (9.5–22.3) | 19.9 (15.3–28.0) | |
| No | 106, 84.1 (77.7–90.5) | 19.8 (13.8–25.7) | |
| Aeroallergen sensitization (n=126) | 0.112 | ||
| Yes | 64, 50.8 (42.1–59.5) | 19.1 (13.4–24.2) | |
| No | 62, 49.2 (40.5–57.9) | 20.9 (15.6–27.4) | |
Overweight is defined as a BMI above +1 SD, obesity as above +2 SD, and underweight as below −2 SD; peripheral eosinophilia: peripheral eosinophil count more than 0.5×109/L; BAL eosinophilia: BAL eosinophil percentage more than 1.2%; food sensitization: a positive skin test to any food allergen including milk, apple, sea crab, egg, beef, peanut, and soybean; aeroallergen sensitization: a positive skin test to any aeroallergen including dust mite, cat hair, dog hair, duck feathers, cockroach, corn pollen, artemisia pollen, penicillium, yeast, and cigarette smoke. BAL, bronchoalveolar lavage; BMI, body mass index; SD, standard deviation.
Distribution and determinants of serum vitamin D levels
Of the 370 participants, 51.6% (95% CI: 46.5–56.7%) had vitamin D deficiency, 36.2% (95% CI: 31.3–41.4%) had vitamin D insufficiency, and only 12.2% (95% CI: 8.9–15.5%) had normal vitamin D levels at baseline. The median (IQR) baseline vitamin D level of all participants was 19.8 (15.4–24.9) ng/mL. The distribution of vitamin D levels is shown in Figure 1A.
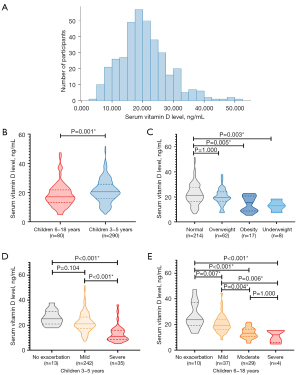
Vitamin D level was associated with age, BMI, and asthma exacerbation severity (Table 1). Vitamin D levels were significantly lower in asthmatic children aged 6 to 18 years than in those aged 3 to 5 years (P=0.001) (Figure 1B). Compared with children with normal BMI, obese children (P=0.005) and underweight children (P=0.003) had significantly lower vitamin D levels (Figure 1C). More severe asthma exacerbation was significantly associated with lower vitamin D level in children aged 3 to 18 years (Figure 1D,1E). No statistically significant association was found between vitamin D levels and sex, allergic rhinitis, atopic dermatitis/eczema, parental history of asthma, peripheral and BAL eosinophilia, food sensitization, and aeroallergen sensitization (Table 1).
Asthma exacerbation and health care utilization
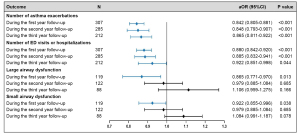
The prevalence of exacerbation and health care utilization in asthmatic children during the 3-year period is presented in Table S1. After adjustment for age, sex, BMI z-score, severity of asthma exacerbation, combination with pneumonia and/or allergic rhinitis, higher vitamin D levels in asthmatic children were significantly associated with a reduced number of asthma exacerbations during the first [adjusted odds ratio (aOR) 0.842, 95% CI: 0.805–0.881; P<0.001], second (aOR 0.848, 95% CI: 0.793–0.907; P<0.001) and third years (aOR 0.865, 95% CI: 0.811–0.922; P<0.001) of follow-up (Figure 2). Similarly, higher vitamin D levels in asthmatic children were significantly associated with a reduced number of ED visits or hospitalizations during the first (aOR 0.880, 95% CI: 0.842–0.920; P<0.001), second (aOR 0.885, 95% CI: 0.832–0.941; P<0.001) and third years (aOR 0.922, 95% CI: 0.851–0.998; P=0.044) of follow-up (Figure 2). In addition, the protective effect of vitamin D levels on asthma exacerbation and health care utilization decreased over time (Figure 2). In other words, the protective effect was highest in the first year and lowest in the third year. To further demonstrate the robustness of these results, we repeated the multivariable analysis based on a data set with no missing values. The obtained results were similar to those of the main analysis (Table S2).
Lung function
The prevalence of LAD and SAD in asthmatic children during the 3-year follow-up is presented in supplementary Table S1. After adjustment for age, sex, BMI z-score, severity of asthma exacerbation, combination with pneumonia and/or allergic rhinitis, higher vitamin D levels in asthmatic children were significantly associated with reduced probability of LAD (aOR 0.865, 95% CI: 0.771–0.970; P=0.013) and SAD (aOR 0.922, 95% CI: 0.855–0.996; P=0.038) during the first year of follow-up (Figure 2). The associations between vitamin D, LAD and SAD during the second and third years of follow-up attenuated and became statistically insignificant (Figure 2). Further information about the associations between vitamin D level and lung function can be found in Table S2.
Discussion
In this study, we demonstrated that higher baseline vitamin D levels in asthmatic children were associated with decreased number of asthma exacerbations, ED visits and hospitalizations over the 3-year follow-up period of the study. In addition, asthmatic children with higher baseline vitamin D levels had lower probability of LAD and SAD during the first year of follow-up than children with lower vitamin D levels. Furthermore, the protective effect of vitamin D against asthma exacerbations and reduction in lung function attenuated over time.
Consistent with our findings, high vitamin D level has shown to be protective against exacerbation and the need of health care utilization in asthmatic children in Costa Rica, United States, and United Kingdom (7-9). These results can be explained in several ways. Firstly, vitamin D protects against infections by enhancing epithelial barrier function (26) and production of antimicrobial proteins which have a broad-spectrum antimicrobial activity (27). Secondly, vitamin D induces tolerance and dampens proinflammatory responses in various cell types of the airway mucosa (28). Thirdly, vitamin D enhances the uptake and effectiveness of steroids in asthmatic patients (29).
We found a significant association between vitamin D level and large airway function. Our results were consistent with those from previous studies (8,9). Mechanisms contributing to this association may include effects on airway smooth muscle (ASM) and epithelial-to-mesenchymal transition (EMT). Gupta et al. found an association between low vitamin D levels, poor lung function and increased ASM mass (8). The association was further supported by an in vitro study that showed that vitamin D inhibited smooth muscle cells proliferation (30). Another in vitro study found that vitamin D supplementation inhibited EMT and fibrosis, in particular when this process is induced by tumor growth factor β1 (TGF-β1) (31). In contrast to our results, vitamin D was not associated with lung function in Costa Rican children (7). One possible reason for the inconsistent findings could be the differences in the magnitude of vitamin D deficiency. People in Chongqing, the city where our study was conducted (Latitude 30°N), may be exposed to less sunshine than people in Costa Rica (Latitude 10°N). Moreover, in Chongqing, food products are usually not fortified with vitamin D, and it is very common to use sunscreen and sunshades on sunny days. Therefore, it is plausible that the prevalence of vitamin D deficiency in the patients of our study is markedly higher than that reported in patients living in Costa Rica (3.4%) (7). The effect of vitamin D on lung function is dose-dependent, and lung function impairment is more noticeable with lower vitamin D levels (8). Therefore, the significant association between vitamin D and lung function found in our study may be attributable to the higher prevalence of vitamin D deficiency. Further investigation will be required to resolve the reasons behind these inconsistencies. Our study not only explored the effect of vitamin D on large airway function, but also on small airway function. Interestingly, we observed a positive association between vitamin D levels and small airway function, which to our knowledge has not been found in other studies so far. This could be an incidental finding due to small sample size but could also represent an increased awareness of the disease in this subgroup of children. More studies are needed to confirm this finding.
More importantly, we found a temporal relationship between vitamin D and asthma exacerbations, which remained over the entire 3-year follow-up period but attenuated over time. To our knowledge, this finding has not been reported before. Therefore, the underlying mechanisms are unclear. We speculated that the findings could partly be explained by the reduced protective effect of vitamin D. As we discussed before, the main protective mechanisms of vitamin D on asthma are through enhancement of anti-infection effect and reduction in airway inflammatory response, but the two mechanisms occur predominantly in the acute stage of exacerbation. In later stages of the disease, the protective effect of vitamin D may be more due to the enhanced response to glucocorticoids. However, the protective effect may diminish as the patient’s condition improves and the use of glucocorticoids decreases. Vitamin D levels could be affected by many factors, such as sunlight exposure and vitamin D supplementation intake (5). It is possible that low baseline vitamin D levels gradually return to normal levels over time, so the effect of low baseline vitamin D levels on asthma is weakened. We were unable to obtain multiple measurements of vitamin D over time due to the retrospective design, so this hypothesis needs to be confirmed by future studies. Considering the short protective effect of vitamin D, long duration of vitamin D supplementation intake may be necessary to better prevent asthma exacerbation in the long term. In addition, our finding may also explain why the efficacy of vitamin D in the treatment of asthma varies greatly across different randomized controlled trials (RCTs), as it may be related to the course of the medication (32).
We examined the long-term effects of vitamin D levels on asthma exacerbations and lung functions and found a temporal trend of the effects for the first time. However, we acknowledge several limitations of our study. Firstly, we cannot exclude confounding effects by unmeasured variables, such as the use of asthma medication, medication adherence, outdoor activities, environment change, variation in vitamin D measurements, and intake of other nutrients. Secondly, many patients without vitamin D measurements were excluded from this study. The selective population may limit the generalizability of these findings. A possible explanation for this issue might be the majority of pediatricians were unaware that children with asthma were at high risk for vitamin D deficiency, hence they did not examine the vitamin D levels in asthmatic patients. In the future, pediatricians need to pay more attention to the vitamin D level of children with asthma, particularly those with obesity and having severe asthma, and timely supplement vitamin D in children with vitamin D deficiency.
In conclusion, we demonstrate that sufficient level of vitamin D in children with asthma leads to decreased rate of asthma exacerbations and health care utilization over a 3-year period, and decreased the probability of LAD and SAD during the first year after asthma diagnosis. The protective effect of vitamin D on asthma decreased over time. These results confirm and extend the previous findings and may provide a basis for asthma management and future research.
Acknowledgments
The authors thank Dr. Janne Estill for editing our paper. We thank patients who participated in this study.
Funding: This study was supported by National Clinical Research Center for Child Health and Disorders (No. NCRCCHD-2020-GP-05, to Zhengxiu Luo; No. NCRCCHD-2021-YP-01, to Qinyuan Li) and Ministry of Education Key Laboratory of Child Development and Disorders (No. GBRP-202112, to Qinyuan Li).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2750/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2750/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2750/coif). QL reports that she has received the funds from National Clinical Research Center for Child Health and Disorders (No. NCRCCHD-2021-YP-01) and Ministry of Education Key Laboratory of Child Development and Disorders (No. GBRP-202112). ZL reports that she has received the fund from National Clinical Research Center for Child Health and Disorders (No. NCRCCHD-2020-GP-05). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of the Children’s Hospital of Chongqing Medical University (No. 2021-470) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Centers for Disease Control and Prevention. 2019 National Health Interview Survey data. U.S. Department of Health & Human Services 2020. Available online: https://www.cdc.gov/asthma/nhis/2019/data.htm
- Gokhale M, Hattori T, Evitt L, et al. Burden of asthma exacerbations and health care utilization in pediatric patients with asthma in the US and England. Immun Inflamm Dis 2020;8:236-45. [Crossref] [PubMed]
- Ferreira de Magalhães M, Amaral R, Pereira AM, et al. Cost of asthma in children: A nationwide, population-based, cost-of-illness study. Pediatr Allergy Immunol 2017;28:683-91. [Crossref] [PubMed]
- Tang MF, Leung ASY, Ngai NA, et al. Prospective study of disease persistence and lung function trajectories of childhood asthma. Pediatr Allergy Immunol 2022;33:e13726. [Crossref] [PubMed]
- Schrumpf JA, van der Does AM, Hiemstra PS. Impact of the Local Inflammatory Environment on Mucosal Vitamin D Metabolism and Signaling in Chronic Inflammatory Lung Diseases. Front Immunol 2020;11:1433. [Crossref] [PubMed]
- Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 2008;181:7090-9. [Crossref] [PubMed]
- Brehm JM, Celedón JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009;179:765-71. [Crossref] [PubMed]
- Gupta A, Sjoukes A, Richards D, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med 2011;184:1342-9. [Crossref] [PubMed]
- Han YY, Forno E, Celedón JC, Vitamin D. Insufficiency and Asthma in a US Nationwide Study. J Allergy Clin Immunol Pract 2017;5:790-796.e1. [Crossref] [PubMed]
- Dogru M, Kirmizibekmez H, Yesiltepe Mutlu RG, et al. Clinical effects of vitamin D in children with asthma. Int Arch Allergy Immunol 2014;164:319-25. [Crossref] [PubMed]
- Dabbah H, Bar Yoseph R, Livnat G, et al. Bronchial Reactivity, Inflammatory and Allergic Parameters, and Vitamin D Levels in Children With Asthma. Respir Care 2015;60:1157-63. [Crossref] [PubMed]
- Tolppanen AM, Sayers A, Granell R, et al. Prospective association of 25-hydroxyvitamin d3 and d2 with childhood lung function, asthma, wheezing, and flexural dermatitis. Epidemiology 2013;24:310-9. [Crossref] [PubMed]
- van Oeffelen AA, Bekkers MB, Smit HA, et al. Serum micronutrient concentrations and childhood asthma: the PIAMA birth cohort study. Pediatr Allergy Immunol 2011;22:784-93. [Crossref] [PubMed]
- Zhao DD, Yu DD, Ren QQ, et al. Association of vitamin D receptor gene polymorphisms with susceptibility to childhood asthma: A meta-analysis. Pediatr Pulmonol 2017;52:423-9. [Crossref] [PubMed]
- Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr 2014;111:23-45. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Global Initiative for asthma. Global Strategy for Asthma Management and Prevention 2016. Available online: http://www.ginasthma.org
- Bao YX, Chen AH, Fu Z, et al. Guidelines for diagnosis and prevention of bronchial asthma in children (2016). Chin J Pediatr 2016;54:167-81.
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr 2020;74:1498-513. [Crossref] [PubMed]
- World Health Organization. WHO child growth standards: length/height-for- age, weight-for-age, weight-for-length, weight-for-height and body mass index-forage: methods and development 2006. Available online: https://www.who.int/publications-detail-redirect/924154693X
- Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol 2022;97:129-48. [Crossref] [PubMed]
- Payne DN, Qiu Y, Zhu J, et al. Airway inflammation in children with difficult asthma: relationships with airflow limitation and persistent symptoms. Thorax 2004;59:862-9. [Crossref] [PubMed]
- Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J 2020;13:100080. [Crossref] [PubMed]
- Niu C, Xu Y, Schuler CL, et al. Evaluation of Risk Scores to Predict Pediatric Severe Asthma Exacerbations. J Allergy Clin Immunol Pract 2021;9:4393-4401.e8. [Crossref] [PubMed]
- Jian W, Gao Y, Hao C, et al. Reference values for spirometry in Chinese aged 4-80 years. J Thorac Dis 2017;9:4538-49. [Crossref] [PubMed]
- DiFranco KM, Mulligan JK, Sumal AS, et al. Induction of CFTR gene expression by 1,25(OH)2 vitamin D3, 25OH vitamin D3, and vitamin D3 in cultured human airway epithelial cells and in mouse airways. J Steroid Biochem Mol Biol 2017;173:323-32. [Crossref] [PubMed]
- Subramanian K, Bergman P, Henriques-Normark B, Vitamin D. Promotes Pneumococcal Killing and Modulates Inflammatory Responses in Primary Human Neutrophils. J Innate Immun 2017;9:375-86. [Crossref] [PubMed]
- Pfeffer PE, Chen YH, Woszczek G, et al. Vitamin D enhances production of soluble ST2, inhibiting the action of IL-33. J Allergy Clin Immunol 2015;135:824-7.e3. [Crossref] [PubMed]
- Mehta AA, Agrawal AD, Appanna V, et al. Vitamin D improves corticosteroid efficacy and attenuates its side-effects in an animal model of asthma. Can J Physiol Pharmacol 2015;93:53-61. [Crossref] [PubMed]
- Britt RD Jr, Faksh A, Vogel ER, et al. Vitamin D attenuates cytokine-induced remodeling in human fetal airway smooth muscle cells. J Cell Physiol 2015;230:1189-98. [Crossref] [PubMed]
- Tzilas V, Bouros E, Barbayianni I, et al. Vitamin D prevents experimental lung fibrosis and predicts survival in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 2019;55:17-24. [Crossref] [PubMed]
- Kumar J, Kumar P, Goyal JP, et al. Vitamin D supplementation in childhood asthma: a systematic review and meta-analysis of randomised controlled trials. ERJ Open Res 2022;8:e00662-2021. [Crossref] [PubMed]


