
Efficacy and safety of lenvatinib for preventing tumor recurrence after liver transplantation in hepatocellular carcinoma beyond the Milan criteria
Introduction
Hepatocellular carcinoma (HCC) is a common cancer worldwide and a leading cause of cancer-related death (1,2). Despite improvements in screening programs and diagnostic tools, only 30–40% of patients with HCC are eligible for curative treatments such as surgical resection, liver transplantation (LTx), and radiofrequency ablation (3). Among them, LTx, which has the advantages of removing tumor lesions completely and simultaneously restoring normal liver function, is considered the best option for patients with tumors meeting the Milan criteria (solitary tumor ≤5 cm or up to 3 nodules ≤3 cm) (4,5). Candidate selection for LTx has increasingly been extended to include patients with HCC beyond the Milan criteria; however, a substantial proportion of these patients experience postsurgical tumor recurrence, with 5-year overall survival (OS) and disease-free survival rates of 65% and 62%, respectively (6). Therefore, an urgent need exists for an effective adjuvant therapy to improve outcomes after LTx for patients with HCC beyond the Milan criteria.
Lenvatinib is a multikinase inhibitor that targets vascular endothelial growth factor (VEGF) receptors 1–3, fibroblast growth factor (FGF) receptors 1–4, platelet-derived growth factor (PDGF) receptor alpha, rearranged during transfection (RET) proto-oncogene receptor, and the receptor tyrosine kinase (KIT) (7-10). The phase III REFLECT trial demonstrated noninferiority of lenvatinib to sorafenib for OS in patients with unresectable HCC, as well as favorable progression-free survival and time to progression (11). Based on these findings, lenvatinib was approved as one of the first-line treatments for unresectable HCC. However, there is a lack of data available in the literature regarding the use of lenvatinib in patients with HCC who have undergone LTx, particularly for those with HCC beyond the Milan criteria.
In this study, the data of 242 consecutive HCC patients with LTx at our institute during the past 2 years were retrospectively analyzed, including 102 cases within the Milan criteria and 140 beyond the Milan criteria. The safety and efficacy of lenvatinib in preventing early recurrence and improving OS after LTx for HCC patients were further evaluated, especially for those beyond the Milan criteria. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1353/rc).
Methods
Patients
Consecutive patients with HCC beyond the Milan criteria who underwent LTx at Zhongshan Hospital from August 2018 to December 2020 were retrospectively enrolled. The inclusion criteria were as follows: HCC confirmed by pathological examination, no history of other tumor types, complete patient information, and no perioperative mortality. Patients were divided into 2 groups according to the use of lenvatinib for the prevention of recurrence after LTx. Tumor differentiation was assessed using the Edmondson grading system and liver function was evaluated using the Child-Pugh scoring system. The Guidelines of Primary Liver Cancer in China (12) were used to determine tumor stage. The Milan criteria and Fudan criteria were used to evaluate the prognosis of patients with HCC after LTx (5,12). Positron emission tomography computed tomography (PET-CT) examination was performed prior to transplantation in all patients to confirm the absence of extrahepatic metastasis. The preoperative treatments and surgical parameters, including duration of surgery, blood loss, and warm ischemic time, were collected. Patient selection is summarized in Figure 1.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the committee of Zhongshan Hospital (No. B2020-402) and individual consent for this retrospective analysis was waived. All grafts used in transplantation were obtained from deceased donors, none of whom were executed prisoners.
LTx procedure and follow-up
A standard procedure was implemented for LTx (13). Triple-drug immunosuppressive therapy, including cyclosporine or tacrolimus combined with corticosteroids and/or mycophenolate mofetil (MMF), was administered after LTx, as described previously (14,15). Corticosteroids and MMF were discontinued 3 months after LTx, and most patients subsequently received tacrolimus monotherapy. Tacrolimus was substituted with rapamycin if patients showed renal insufficiency or inadequate blood concentration of tacrolimus. A 2-drug regimen of hepatitis B immunoglobulin combined with lamivudine or entecavir was used to prevent hepatitis B virus reactivation (15).
Follow-up assessments were conducted every month for the first 6 months after LTx and every 3 months thereafter and consisted of serum alpha-fetoprotein (AFP), prothrombin induced by vitamin K absence-II (PIVKA-II), abdominal ultrasonography, chest computed tomography (CT), and enhanced magnetic resonance imaging (MRI). Suspected recurrence was confirmed by imaging examination (CT, MRI, bone scan, or PET-CT).
The OS was defined as the interval from the date of surgery to either the date of death or the last follow-up visit. Time to recurrence (TTR) was defined as the interval between LTx and the first recurrence or metastasis after LTx (13). When recurrence was confirmed, treatment options were discussed by a multidisciplinary team, and patients were given appropriate treatments, such as hepatectomy, radiofrequency ablation, transcatheter arterial chemoembolization, external beam radiotherapy, systemic chemotherapy, and/or tyrosine kinase inhibitors (TKIs; sorafenib, regorafenib, or lenvatinib), according to the pattern of recurrence, liver function, and general condition of the patient. Follow-up was terminated on 1 January 2022.
Post-LTx adjuvant lenvatinib treatment
After liver transplantation, the application of adjuvant lenvatinib was recommended for patients with a high risk of recurrence [multiple lesions, microvascular invasion (mVI), poor differentiation, or postoperative AFP/PIVKA-II positive], with the intention of reducing relapse and improving survival. The recommended duration of lenvatinib treatment was 2 years. Patients were treated with lenvatinib orally at a dosage of 8 mg/day (body weight <60 kg) or 12 mg/day (body weight ≥60 kg) starting 1 month after LTx. Treatment was withdrawn if there was unacceptable toxicity or upon patient request. Dose adjustments due to adverse events (AEs) were performed according to routine clinical practice. Any AEs during lenvatinib treatment were assessed and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) (16).
Propensity score matching
To overcome the potential for confounding from selection bias between patients who did and did not receive adjuvant lenvatinib, propensity score matching (PSM) was conducted using R software v. 4.1.2 with “Matchit” package (https://www.r-project.org; R Foundation for Statistical Computing, Vienna, Austria) (17). Based on clinical knowledge and experience, factors considered to have an influence on the decision to use adjuvant lenvatinib and/or influence clinical outcomes included gender (male or female), age (≤50 or >50 years), hepatitis B surface antigen (HBsAg) status (negative or positive), AFP level (≤400 or >400 ng/mL), PIVKA-II (≤100 or >100 mAU/mL), Child-Pugh class (A or B/C), cirrhosis (no or yes), tumor size (≤5 or >5 cm), tumor number (solitary or multiple), Edmondson stage (I–II or III–IV), and mVI (no or yes). A 1:2 matching was applied using the nearest-neighbor matching algorithm to select matched pairs of patients.
Statistical analysis
Categorical variables were analyzed as frequency (%) and compared between groups via chi-square test. Continuous variables were summarized as median [interquartile range (IQR)] and compared using the Wilcoxon rank-sum test. The Kaplan-Meier method was used to calculate survival rates, and the log-rank test was applied to compare survival differences between groups. Univariate and multivariate Cox regression analyses were used to identify independent risk factors for OS and estimate the hazard ratio (HR). Variables with P<0.05 in the univariate model were selected for multivariate analysis. Since mortality ahead of relapse might have precluded recurrence in patients undergoing LTx, use of the usual Kaplan-Meier method would have misestimated the recurrence rate. Therefore, a competing risk analysis and the Fine-Gray test were applied to calculate and compare the cumulative incidence of recurrence, respectively (18). A multivariate competing model was used to identify the risk factors of recurrence after LTx and estimate the sub-hazard ratio (sHR) using the subdistribution analysis of competing risks (cmprsk) v. 2.2.11 package for R. Subgroup analyses were conducted using a competing risk model in patients stratified according to the clinical characteristics, including tumor size, tumor number, tumor differentiation, mVI, and postoperative AFP or PIVKA-II. All statistical tests were 2-tailed and a P-value <0.05 was considered statistically significant. All statistical analyses were performed using R version 4.1.2.
Results
Patient baseline characteristics
Baseline characteristics of all included patients (n=242) are summarized in Table 1. A total of 42 (17.4%) patients received adjuvant lenvatinib after LTx, while 200 (82.6%) did not (control). Lenvatinib-treated patients showed a significantly larger tumor size (P=0.002), a higher PIVKA-II level (P=0.006), and a more advanced China Liver Cancer (CNLC) stage (P<0.001) compared with the control group.
Table 1
| Variable | Items | All patients (n=242) | Patients beyond Milan criteria (n=140) | |||||
|---|---|---|---|---|---|---|---|---|
| Control (n=200) | Lenvatinib (n=42) | P value | Control (n=102) | Lenvatinib (n=38) | P value | |||
| Gender | Male | 173 (86.5) | 40 (95.2) | 0.113 | 90 (88.2) | 36 (94.7) | 0.410a | |
| Female | 27 (13.5) | 2 (4.8) | 12 (11.8) | 2 (5.3) | ||||
| Age (years) | ≤50 | 99 (49.5) | 21 (50.0) | 0.953 | 49 (48.0) | 20 (52.6) | 0.629 | |
| >50 | 101 (50.5) | 21 (50.0) | 53 (52.0) | 18 (47.4) | ||||
| HBsAg | Negative | 39 (19.5) | 11 (26.2) | 0.330 | 19 (18.6) | 10 (26.3) | 0.318 | |
| Positive | 161 (80.5) | 31 (73.8) | 83 (81.4) | 28 (73.7) | ||||
| AFP (ng/mL) | ≤400 | 161 (80.5) | 34 (81.0) | 0.946 | 76 (74.5) | 31 (81.6) | 0.381 | |
| >400 | 39 (19.5) | 8 (19.0) | 26 (25.5) | 7 (18.4) | ||||
| PIVKA-II (mAU/mL) | <100 | 104 (52.0) | 12 (28.6) | 0.006 | 39 (38.2) | 10 (26.3) | 0.189 | |
| >100 | 96 (48.0) | 30 (71.4) | 63 (61.8) | 28 (73.7) | ||||
| Child-Pugh class | A | 128 (64.0) | 33 (78.6) | 0.069 | 69 (67.6) | 30 (78.9) | 0.191 | |
| B-C | 72 (36.0) | 9 (21.4) | 33 (32.4) | 8 (21.1) | ||||
| Cirrhosis | No | 32 (16.0) | 7 (16.7) | 0.915 | 23 (22.5) | 7 (18.4) | 0.597 | |
| Yes | 168 (84.0) | 35 (83.3) | 79 (77.5) | 31 (81.6) | ||||
| Tumor size (cm) | ≤5 | 159 (79.5) | 24 (57.1) | 0.002 | 61 (59.8) | 20 (52.6) | 0.445 | |
| >5 | 41 (20.5) | 18 (42.9) | 41 (40.2) | 18 (47.4) | ||||
| Tumor number | Single | 74 (37.0) | 9 (21.4) | 0.053 | 9 (8.8) | 6 (15.8) | 0.380a | |
| Multiple | 126 (63.0) | 33 (78.6) | 93 (91.2) | 32 (84.2) | ||||
| Edmondson stage | I-II | 111 (55.5) | 20 (47.6) | 0.351 | 51 (50.0) | 18 (47.4) | 0.782 | |
| III-IV | 89 (44.5) | 22 (52.4) | 51 (50.0) | 20 (52.6) | ||||
| mVI | No | 96 (48.0) | 16 (38.1) | 0.242 | 39 (38.2) | 14 (36.8) | 0.880 | |
| Yes | 104 (52.0) | 26 (61.9) | 63 (61.8) | 24 (63.2) | ||||
| CNLC stage | I | 107 (53.5) | 10 (23.8) | <0.001 | 9 (8.8) | 6 (15.8) | 0.380a | |
| II | 93 (46.5) | 32 (76.2) | 93 (91.2) | 32 (84.2) | ||||
| Milan criteria | Within | 98 (49.0) | 4 (9.5) | <0.001 | 0 | 0 | / | |
| Beyond | 102 (51.0) | 38 (90.5) | 102 (100.0) | 38 (100.0) | ||||
| Fudan criteria | Within | 124 (62.0) | 11 (26.2) | <0.001 | 26 (25.5) | 7 (18.4) | 0.381 | |
| Beyond | 76 (38.0) | 31 (73.8) | 76 (74.5) | 31 (81.6) | ||||
| Preoperative TACE | No | 134 (67.0) | 24 (57.1) | 0.222 | 55 (53.9) | 22 (57.9) | 0.674 | |
| Yes | 66 (33.0) | 18 (42.9) | 47 (46.1) | 16 (42.1) | ||||
| Duration of surgery (minutes) | 308.0 [278.0, 332.0] |
308.0 [272.0, 329.3] |
0.563 | 308.00 [284.0, 332.0] |
305.0 [272.0, 330.0] |
0.431b | ||
| Blood loss (mL) | 800.0 [400.0, 1,500.0] |
800.0 [600.0, 1,175.0] |
0.433 | 900.0 [500.0, 1,500.0] |
800.0 [600.0, 1,200.0] |
0.82b | ||
| Warm ischemic time (minutes) | 40.0 [36.0, 44.0] |
40.0 [36.0, 45.0] |
0.822 | 40.0 [36.3, 44.0] |
39.5 [36.0, 45.0] |
0.365b | ||
Categorical variables were summarized as n (%); continuous variables were summarized as median [interquartile range]. a, continuous correction. b, Wilcoxon rank-sum test. AFP, alpha-fetoprotein; CNLC, China Liver Cancer Stage; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; mVI, microvascular invasion; PIVKA-II, protein induced by vitamin K absence or antagonist-II; TACE, transarterial chemoembolization.
Overall outcomes
The median follow-up time was 18.7 (range, 3.6 to 35.6) months. Overall, the 6-, 12-, and 24-month OS rates for all cases were 98.3%, 95.2%, and 88.0%, respectively (Figure S1A). In the competing risk analysis, the 6-, 12-, and 24-month cumulative incidence of recurrence were 5.4%, 13.4%, and 24.2%, respectively (Figure S1B). The clinical characteristics that had an impact on OS and TTR are shown in Table S1.
Among all cases, 23 (9.5%) died and 49 (20.2%) had tumor recurrence during follow-up. Tumor recurrence was observed in the liver (n=10), at extrahepatic sites (n=35), and at both intrahepatic and extrahepatic sites (n=4).
Efficacy of adjuvant lenvatinib in the whole cohort
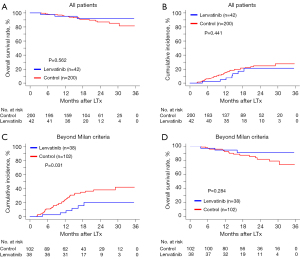
There were no significant differences between the levantinib and control groups in postoperative OS [HR, 0.73; 95% confidence interval (CI): 0.25 to 2.13; P=0.562; Figure 2A] or TTR (sHR, 0.73; 95% CI: 0.33 to 1.57; P=0.441; Figure 2B). The 6-, 12-, and 24-month OS rates in the lenvatinib group were 97.6%, 95.2%, and 91.8%, compared with 98.5%, 95.2%, and 87.1% in the control group, respectively (Figure 2A). Competing risk analysis showed that the 6-, 12-, and 24-month cumulative incidences of recurrence in the lenvatinib group were 2.4%, 4.8%, and 21.4%, compared with 6.0%, 15.2%, and 24.7% in the control group, respectively (Figure 2B).
Efficacy of adjuvant lenvatinib in HCC within Milan criteria
Considering that patients beyond the Milan criteria experienced a high risk of recurrence after LTx, lenvatinib may achieve clinical benefits in these patients instead of in those who meet the Milan criteria. Thus, we further investigated the impact of adjuvant lenvatinib on patients who underwent LTx with HCC within and beyond the Milan criteria, respectively.
For patients within the Milan criteria (n=102), only 4 cases (3.9%) received adjuvant lenvatinib. There was no difference in OS (P=0.562; Figure S1C) or TTR (P=0.208; Figure S1D) between the lenvatinib group and the control group, using the Kaplan-Meier analysis and a competing risk analysis, respectively.
Efficacy of adjuvant lenvatinib in HCC beyond Milan criteria
Of the patients with HCC beyond the Milan criteria (n=140), 38 patients (27.1%) took lenvatinib as postoperative adjuvant therapy, and these patients showed a comparable baseline with those who did not use lenvatinib after LTx (Table 1). After using death without recurrence as a competing risk, patients who received lenvatinib had a significantly longer TTR than those in the control group (sHR, 0.40; 95% CI: 0.17 to 0.93; P=0.031; Figure 2C). The cumulative incidence of recurrence was also lower in the lenvatinib group versus the control group at 6 (2.6% vs. 10.7%), 12 (5.3% vs. 24.4%), and 24 (20.0% vs. 37.9%) months (Figure 2C). In the multivariate competing risk analysis that adjusted AFP, PIVKA-II, tumor size, Edmondson stage, and mVI, adjuvant lenvatinib was identified as an independent protective factor for recurrence after LTx and was associated with a 67% reduction in the risk of recurrence (sHR, 0.33; 95% CI: 0.13 to 0.83; P=0.018; Table 2).
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| sHR (95% CI) | P value | sHR (95% CI) | P value | ||
| Gender (female) | 0.89 (0.35–2.24) | 0.810 | NA | NA | |
| Age (>50 years) | 0.88 (0.48–1.62) | 0.690 | NA | NA | |
| HBsAg (positive) | 1.90 (0.75–4.78) | 0.170 | NA | NA | |
| AFP (>400 ng/mL) | 3.19 (1.72–5.91) | <0.001 | 2.33 (1.27–4.26) | 0.006 | |
| PIVKA-II (>100 mAU/mL) | 2.56 (1.18–5.56) | 0.018 | 1.97 (0.85–4.57) | 0.120 | |
| Child-Pugh class (B-C) | 1.36 (0.69–2.65) | 0.370 | NA | NA | |
| Cirrhosis (yes) | 0.72 (0.37–1.41) | 0.330 | NA | NA | |
| Tumor size (>5 cm) | 1.72 (0.94–3.16) | 0.078 | NA | NA | |
| Tumor number (multiple) | 0.77 (0.30–2.02) | 0.600 | NA | NA | |
| Edmondson stage (III-IV) | 2.43 (1.25–4.72) | 0.009 | 2.00 (1.00–4.00) | 0.050 | |
| mVI (yes) | 2.18 (1.05–4.53) | 0.037 | 1.20 (0.52–2.79) | 0.660 | |
| Preoperative TACE | 1.27 (0.69–2.33) | 0.450 | NA | NA | |
| Duration of surgery (minutes) | 1.00 (0.99–1.00) | 0.380 | NA | NA | |
| Blood loss (mL) | 0.97 (0.93–1.01) | 0.120 | NA | NA | |
| Warm ischemic time (minutes) | 1.00 (1.00–1.00) | 0.850 | NA | NA | |
| Lenvatinib (yes) | 0.40 (0.17–0.92) | 0.032 | 0.33 (0.13–0.83) | 0.018 | |
AFP, alpha-fetoprotein; CI, confidence interval; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; NA, not applicable; mVI, microvascular invasion; PIVKA-II, protein induced by vitamin K absence or antagonist-II; sHR, sub-hazard ratio; TACE, transarterial chemoembolization.
In addition, there was similar OS in the lenvatinib group and the control group (HR, 0.57; 95% CI: 0.21 to 1.59; P=0.284; Figure 2D). The 6-, 12-, and 24-month survival rates in the lenvatinib group were 97.4%, 94.7%, and 91.1%, compared with 98.0%, 92.7%, and 81.8% in the control group, respectively (Figure 2D).
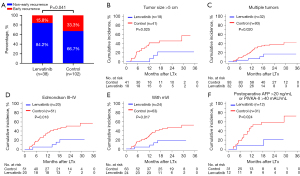
We also investigated the role of postoperative lenvatinib treatment in reducing early tumor recurrence (≤2 years) after LTx, and found that early recurrences were significantly less frequent in the lenvatinib group (6/38, 15.8%) than in the control group (34/102, 33.3%; P=0.041; Figure 3A).
Furthermore, we performed PSM and matched 99 patients in a 1:2 ratio; 33 in the lenvatinib group and 66 matched controls. The clinical features of the 2 groups after PSM were well balanced (Table S2). As expected, the competing risk analysis showed a longer TTR in the lenvatinib group than the control group in the PSM cohort (sHR, 0.31; 95% CI: 0.12 to 0.78; P=0.013; Figure S2A). The early recurrence rates were also lower in the lenvatinib group than in the control group (15.2% vs. 40.9%; P=0.010; Figure S2B). Notably, the OS did not differ between the 2 groups, but there was a trend of longer OS in the lenvatinib group after PSM (HR, 0.40; 95% CI: 0.13 to 1.19; P=0.099; Figure S2C).
Subgroup analyses in HCC beyond Milan criteria
Compared with the control group, adjuvant lenvatinib significantly decreased the cumulative incidence of recurrence rate in patients with tumor size >5 cm (P=0.025), multiple tumors (P=0.020), Edmondson III–IV grade (P=0.018), and mVI (P=0.017) (Figure 3B-3E). Furthermore, patients with AFP >20 ng/mL or PIVKA-II >40 mAU/mL at 1 month after LTx could benefit from adjuvant lenvatinib (P=0.024, Figure 3F). The sHRs and 95% CIs for TTR in the exploratory subgroups are presented in Figure S3.
Safety of adjuvant lenvatinib
Among the 42 patients using lenvatinib, 27 patients discontinued. The median duration of adjuvant lenvatinib treatment was 13.0 months, with a range of 3.1 to 26.7 months. Treatment-related AEs (TRAE) are summarized in Table 3. Any-grade TRAEs occurred in most patients (40/42, 95.2%), and grade ≥3 TRAEs occurred in approximately one third of patients (13/42, 31.0%). The most common TRAEs were hypertension (n=18, 42.9%), diarrhea (n=15, 35.7%), decreased appetite (n=10, 23.8%), and fatigue (n=10, 23.8%). Grade ≥3 TRAEs were hypertension (n=6, 14.3%), diarrhea (n=4, 9.5%), proteinuria (n=2, 4.8%), decreased appetite (n=1, 2.4%), and dysphonia (n=1, 2.4%). There were 3 patients (7.1%) who discontinued levantinib due to unbearable TRAEs and there were other patients who could tolerate the TRAEs after dose reduction (n=7, 16.6%), transient discontinuation (n=5, 11.9%), or symptomatic treatment (n=25, 59.5%). No treatment-related deaths were reported.
Table 3
| Event | Lenvatinib (n=42) | |
|---|---|---|
| Any grade | Grade ≥3 | |
| Any | 40 [95] | 13 [31] |
| Hypertension | 18 [43] | 6 [14] |
| Diarrhea | 15 [36] | 4 [10] |
| Decreased appetite | 10 [24] | 1 [2] |
| Fatigue | 10 [24] | 0 |
| Proteinuria | 7 [17] | 2 [5] |
| Palmar-plantar erythrodysesthesia | 7 [17] | 0 |
| Decreased weight | 6 [14] | 0 |
| Mucositis | 6 [14] | 0 |
| Nausea | 5 [12] | 0 |
| Dysphonia | 4 [10] | 1 [2] |
| Decreased platelet count | 4 [10] | 0 |
| Rash | 3 [7] | 0 |
| Bleed | 3 [7] | 0 |
Data are reported as n [%].
We also investigated whether adjuvant lenvatinib increased the postoperative complications of LTx. The incidence rates of infection, rejection, and bile duct stenosis in the lenvatinib group were comparable with those in the control group (4.8% vs. 4.0%, 4.8% vs. 2.0%, and 2.4% vs. 10.0%, respectively; all P>0.05).
Discussion
To our knowledge, this is the first large sample study of the use of lenvatinib as adjuvant treatment following LTx in patients with HCC. For all patients who underwent LTx with HCC, there were no significant differences in postoperative OS and TTR between patients who received adjuvant lenvatinib and those who did not. However, this observation was confounded because most patients in the lenvatinib group had tumors beyond the Milan criteria. Thus, further analysis focused on the patients with HCC beyond the Milan criteria, in which baseline characteristics were similar between the lenvatinib group and the control group. Interestingly, adjuvant lenvatinib significantly prolonged TTR and reduced the risk of tumor recurrence compared with the control group. The subsequent PSM analysis also validated the decreased recurrence rate in the lenvatinib group. The greatest benefits in reduction of tumor recurrence with adjuvant lenvatinib were seen in patients with tumor size >5 cm, multiple tumors, tumor differentiation grades III–IV, mVI, and postoperative AFP >20 ng/mL or PIVKA-II >40 mAU/mL. However, no significant difference in OS was observed between the groups, which might be due to the short follow-up time and relatively small sample size. Furthermore, the reserved liver function after LTx increased the probabilities of undergoing secondary resection and other locoregional therapy after recurrence. Notably, after further balancing the baselines via PSM, there emerged a trend toward better OS for the lenvatinib group.
It is well accepted that minimal residual disease is a major cause of metastasis and recurrence after LTx for HCC patients (19-22). We found that postoperative lenvatinib treatment significantly decreased the incidence of recurrence after LTx (sHR, 0.40; 95% CI: 0.17 to 0.93; P=0.031), which typically results from pre-operative occult micrometastases (23,24). The antitumor activity of lenvatinib has been associated with anti-angiogenesis and inhibition of tumor cell proliferation (25,26). Therefore, postoperative lenvatinib administration in patients with HCC may delay the appearance of new recurrent lesions via inhibition of tumor neovascularization and the proliferation of minimal residual disease.
Our data showed that lenvatinib was well tolerated in patients who underwent LTx, with a safety profile consistent with that previously reported in patients with advanced HCC in the REFLECT study (11). In the present study, patients had a similar rate of any grade TRAEs compared with those in the REFLECT study (95.2% vs. 93.9%), but a lower rate of grade ≥3 TRAEs (31.0% vs. 56.7%), which might be related to the improvement of liver function after LTx. In addition, it was shown that adjuvant lenvatinib did not increase the risk of complications after LTx.
The Milan criteria are the gold standard candidate selection criteria to minimize the risk of disease recurrence after LTx in patients with HCC (5,27). However, there are growing concerns that the Milan criteria may be too restrictive in view of the increasing candidate list, particularly in China (28,29). The eligibility criteria for LTx have been gradually expanded for HCC, and in China, around 50% of patients with HCC who receive LTx are beyond the Milan criteria (30,31). As a result, this will lead to a higher proportion of tumor recurrence after LTx (28.6% in our cohort within 2 years) (31,32).
The application of TKIs as adjuvant therapy in HCC after radical surgery remains controversial. Several pieces of evidence have demonstrated that TKIs, like sorafenib, improve the outcomes of HCC with a high risk of recurrence after radical resection or LTx (33-36). However, the phase III STORM trial failed to identify the clinical benefit of adjuvant sorafenib (37). In the present study, we initially found that postoperative lenvatinib administration could significantly reduce tumor recurrence (15.8% vs. 33.3%) and prolong median TTR (not reached vs. 14.6 months, P=0.019) after LTx for those HCC patients beyond the Milan criteria, compared with untreated controls. However, no significant difference in OS was observed, which might be related to the short follow-up time and small sample size, since lenvatinib only became available in China as recently as 2 years ago. Thus, our study indicated that postoperative lenvatinib administration could significantly decrease tumor recurrence for HCC patients beyond the Milan criteria who underwent LTx, especially early recurrence (within 2 years) after LTx.
The results also suggest some indications for patients within the Milan criteria. Firstly, the application of lenvatinib after LTx was safe and did not increase the incidence of complications, indicating that lenvatinib could be used in LTx patients. Besides, our data showed that adjuvant lenvatinib therapy might reduce the recurrence rate of HCCs in patients with multiple lesions, mVI, poor differentiation, or postoperative positive AFP/PIVKA-II, offering the potential for lenvatinib to confer clinical benefit even to those patients within the Milan criteria. Further evidence from large-scale, prospective clinical trials is needed.
It is challenging to conduct a prospective study to explore treatment outcomes in patients with HCC who receive LTx, particularly for those beyond the Milan criteria. Therefore, we performed a retrospective study. However, the present study had several limitations inherent to the retrospective, single-center design, and small sample size. Consequently, the results should be interpreted with caution. A prospective, multicenter, randomized study with a large sample size is required to confirm the efficacy and safety of lenvatinib in this setting. Furthermore, the efficacy of other TKIs, such as sorafenib, in preventing recurrence after LTx was still unclear in the present study, and further studies are needed.
In conclusion, our study demonstrated that postoperative lenvatinib administration might be a promising modality to reduce tumor recurrence and improve the prognosis of HCC patients beyond the Milan criteria who undergo LTx. Meanwhile, lenvatinib is well tolerated in patients with HCC who receive LTx.
Acknowledgments
We gratefully acknowledge the assistance of Fei Liang for his advice on statistical analysis. And we would like to thank Sharon Gladwin and Jake Burrell for their help in polishing our paper.
Funding: This study was jointly supported by the National Key R&D Program of China (Nos. 2019YFC1315800, 2019YFC1315802), the State Key Program of National Natural Science of China (No. 81830102), the National Natural Science Foundation of China (Nos. 81772578, 81772551, 81872355, and 82072715), the Shanghai Municipal Health Commission Collaborative Innovation Cluster Project (No. 2019CXJQ02), the Shanghai “Rising Stars of Medical Talent” Youth Development Program (Outstanding Youth Medical Talents), the Projects from the Shanghai Science and Technology Commission (Nos. 19441905000 and 21140900300), the Projects from Science Foundation of Zhong Shan Hospital, Fudan University (Nos. 2021ZSCX28, 2020ZSLC31), and the Shanghai Municipal Key Clinical Specialty.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1353/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1353/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1353/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1353/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the committee of Zhongshan Hospital (No. B2020-402) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Zaydfudim VM, Vachharajani N, Klintmalm GB, et al. Liver Resection and Transplantation for Patients With Hepatocellular Carcinoma Beyond Milan Criteria. Ann Surg 2016;264:650-8. [Crossref] [PubMed]
- Boss DS, Glen H, Beijnen JH, et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer 2012;106:1598-604. [Crossref] [PubMed]
- Matsui J, Funahashi Y, Uenaka T, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 2008;14:5459-65. [Crossref] [PubMed]
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 2008;122:664-71. [Crossref] [PubMed]
- Yamada K, Yamamoto N, Yamada Y, et al. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin Cancer Res 2011;17:2528-37. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020;9:682-720. [Crossref] [PubMed]
- Fan J, Yang GS, Fu ZR, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol 2009;135:1403-12. [Crossref] [PubMed]
- Zhou J, Fan J, Wang Z, et al. Conversion to sirolimus immunosuppression in liver transplantation recipients with hepatocellular carcinoma: Report of an initial experience. World J Gastroenterol 2006;12:3114-8. [Crossref] [PubMed]
- Wei Q, Gao F, Zhuang R, et al. A national report from China Liver Transplant Registry: steroid avoidance after liver transplantation for hepatocellular carcinoma. Chin J Cancer Res 2017;29:426-37. [Crossref] [PubMed]
- Bennett AV, Dueck AC, Mitchell SA, et al. Mode equivalence and acceptability of tablet computer-, interactive voice response system-, and paper-based administration of the U.S. National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Health Qual Life Outcomes 2016;14:24. [Crossref] [PubMed]
- Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics 1996;52:249-64. [Crossref] [PubMed]
- Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496-509. [Crossref]
- Ye Q, Ling S, Zheng S, et al. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer 2019;18:114. [Crossref] [PubMed]
- Guo W, Sun YF, Shen MN, et al. Circulating Tumor Cells with Stem-Like Phenotypes for Diagnosis, Prognosis, and Therapeutic Response Evaluation in Hepatocellular Carcinoma. Clin Cancer Res 2018;24:2203-13. [Crossref] [PubMed]
- Scortegagna E Jr, Karam AR, Sioshansi S, et al. Hepatocellular carcinoma recurrence pattern following liver transplantation and a suggested surveillance algorithm. Clin Imaging 2016;40:1131-4. [Crossref] [PubMed]
- Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017;14:203-17. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Tumour microenvironment: informing on minimal residual disease in solid tumours. Nat Rev Clin Oncol 2017;14:325-6. [Crossref] [PubMed]
- Hiller JG, Perry NJ, Poulogiannis G, et al. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 2018;15:205-18. [Crossref] [PubMed]
- Adachi Y, Matsuki M, Watanabe H, et al. Antitumor and Antiangiogenic Activities of Lenvatinib in Mouse Xenograft Models of Vascular Endothelial Growth Factor-Induced Hypervascular Human Hepatocellular Carcinoma. Cancer Invest 2019;37:185-98. [Crossref] [PubMed]
- Matsuki M, Hoshi T, Yamamoto Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med 2018;7:2641-53. [Crossref] [PubMed]
- Yokoyama I, Todo S, Iwatsuki S, et al. Liver transplantation in the treatment of primary liver cancer. Hepatogastroenterology 1990;37:188-93. [PubMed]
- Rudnick SR, Russo MW. Liver transplantation beyond or downstaging within the Milan criteria for hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2018;12:265-75. [Crossref] [PubMed]
- Kaido T. Selection Criteria and Current Issues in Liver Transplantation for Hepatocellular Carcinoma. Liver Cancer 2016;5:121-7. [Crossref] [PubMed]
- Xu X, Lu D, Ling Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut 2016;65:1035-41. [Crossref] [PubMed]
- Lu TF, Hua XW, Cui XL, et al. Liver transplantation for hepatocellular carcinoma: recent advances in China. J Dig Dis 2014;15:51-3. [Crossref] [PubMed]
- Xu DW, Wan P, Xia Q. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria: A review. World J Gastroenterol 2016;22:3325-34. [Crossref] [PubMed]
- Yan J, Tan C, Gu F, et al. Sorafenib delays recurrence and metastasis after liver transplantation in a rat model of hepatocellular carcinoma with high expression of phosphorylated extracellular signal-regulated kinase. Liver Transpl 2013;19:507-20. [Crossref] [PubMed]
- Li J, Hou Y, Cai XB, et al. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol 2016;22:4034-40. [Crossref] [PubMed]
- Li Q, Song T. Association Between Adjuvant Sorafenib and the Prognosis of Patients With Hepatocellular Carcinoma at a High Risk of Recurrence After Radical Resection. Front Oncol 2021;11:633033. [Crossref] [PubMed]
- Xia F, Wu LL, Lau WY, et al. Adjuvant sorafenib after heptectomy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma patients. World J Gastroenterol 2016;22:5384-92. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
(English Language Editors: K. Gilbert and J. Jones)



