
A virus preservation solution that inactivates the virus while maintaining the virus particle intact
Introduction
Severe acute respiratory syndrome coronavirus 2 is the causative agent of the respiratory disease named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO). It first widely appeared in Wuhan, the capital city of Hubei province in China, during the end of 2019 (1-3), and was declared a pandemic on March 11, 2020. Epidemiological data suggest that approximately 132,901 confirmed cases of COVID-19 and 5,699 deaths occurred in China between January 3, 2020 and January 5, 2022. To date, only 4 countries have not been infected with COVID-19 (https://covid19.who.int/). The ongoing COVID-19 pandemic has become the most serious threat to public health (4).
To manage this disease, convenient and reliable detection technologies are urgently needed (5,6). To date, fluorescent quantitative reverse transcription-polymerase chain reaction (qRT-PCR) has been the most commonly used detection technology for the diagnosis of COVID-19 infection (7,8), as described in “The Collection and Testing Technical Guide of COVID-19” issued by National Health Commission of the People’s Republic of China. However, the preservation of samples is a critical but easily overlooked factor affecting the efficacy of detection (6,9,10).
In clinical detection, various problems arise due to improper preservation of virus samples (11). For example, cold chain logistics and cryopreservation are required, because viral activity is greatly affected by temperature. In addition, the outer shell of a single-stranded RNA virus is easily broken and may release nucleic acid; thus, the integrity of the virus particles cannot be guaranteed. Moreover, healthcare workers face an increased risk of infection if the virus is not inactivated in a timely manner. At present, the disposable virus sampling tubes used in clinics are throat and nasal swabs with virus preservation solution added. Two types of virus preservation solutions are frequently used: the isotonic Hank’s balanced salt solution (8,12), which cannot inactivate the virus, and guanidine salt (13). In the COVID-19 nucleic acid sampling process, it has been found that the use of virus storage tubes containing guanidine salt can inactivate the virus immediately after sampling, maintain the stability of viral nucleic acid, make it easier to detect low copy number viruses, and reduce false negatives (13). Therefore, the importance of guanidine salts to downstream molecular biology experiments cannot be ignored. In this study, we used homogeneous reaction technology to develop a new virus sample preservation solution without guanidinium that was able to protect the integrity of viral particles to avoid RNA release, degradation, and contamination. In addition, our solution effectively inactivated the virus at room temperature, which could improve the positive rate of follow-up detection. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4295/rc).
Methods
Cell culture
HEK293T cell lines was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and grown in Dulbecco’s Modified Eagle’s Medium (DMEM; Corning Inc., Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS; Ausbian, GC, USA). Cells were cultured in a standard humidified atmosphere of 5% CO2 at 37 ℃.
Virus samples
Lentivirus GV115-pGCSIL-004 with enhanced green fluorescence protein (EGFP) labeling, lentivirus CON335, and CON098 were purchased from Genechem (Shanghai, China). All the samples were diluted to 10-fold in phosphate buffered saline (PBS) and virus preservation solution with a total concentration gradient of 7.
Extraction of nucleic acid and qRT-PCR
According to the product description, total nucleic acid was extracted using a Viral DNA/RNA Mini Kit, which was purchased from Vazyme (Nanjing, China). RNA was reverse-transcribed into complementary DNA (cDNA) using the Hiscript Q RT SuperMix for qPCR (Vazyme, Nanjing, China). cDNA was quantified by qRT-PCR, and the data were acquired using a ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). The sequences of primers or probes are shown in Table 1.
Table 1
| Sample | Sequence |
|---|---|
| Lentivirus CON335 | F: GTCCTTTCCATGGCTGCTC |
| R: CCGAAGGGACGTAGCAGA | |
| Probe: FAM-ACTCATCGCCGCCTGCCTTGCC-TAMRA | |
| Adenovirus CON098 | F: GCTTCAGCCGCTAC |
| R: TCACCTTGATGCCGT |
F, forward; R, reverse; FAM, fluorescein; TAMRA, tetramethyl rhodamine.
Virus inactivation
The lentivirus GV115-pGCSIL-004 was placed in the sample preservation solution and suspended for 5, 15, 30, 60, 120, or 180 minutes. The control group was suspended in PBS. The infection of HEK293T cells was performed according to the manufacture’s protocol at a multiplicity of infection (MOI) of 10.
Virion integrity
The adenovirus CON098 and the lentivirus CON335 were placed in the virus preservation solution for 7 days at room temperature and monitored periodically on the 1st, 3rd, and 7th day. The samples of adenovirus CON098 were diluted to 10−2 and analyzed via qPCR (SYBR). The samples of lentivirus CON335 were diluted to 10−5 and analyzed via qRT-PCR (TaqMan). In addition, the above samples of lentivirus CON335 were detected by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) to determine whether the virus protein was intact.
Statistical analysis
Statistical significance was determined using the paired two-tailed t-test. P<0.05 was considered statistically significant. All values are expressed as the means ± standard deviation.
Results
Effect of the preservation solution on inactivating the virus
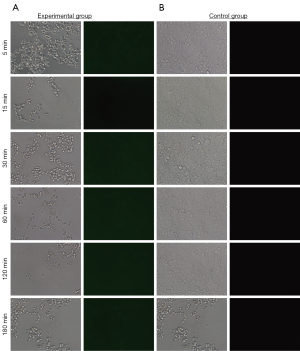
To verify whether the sample preservation solution would inactivate the virus, we divided the lentivirus GV115 samples into 6 groups and stored them at room temperature. As per the manufacturer’s instructions, the treated virus was used to infect HEK293T cells at MOI =10. It was found that the expression efficiency of green fluorescent protein (GFP) was 50–80% in the control group 48 hours after infection (Figure 1A). In comparison, no GFP signal was observed in the experimental group samples after treatment with the virus preservation solution for 6 different lengths of time, suggesting that the cells were not infected by the virus efficiently. This result indicated that regardless of the duration of suspension, the treated virus was inactivated by the virus preservation solution.
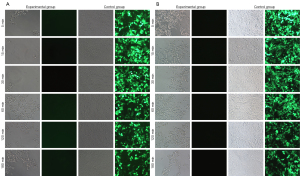
It is worth mentioning that when the infection time was extended to 72 hours, the experimental group still did not have any fluorescent signal (Figure 1B). However, the efficiency of the control groups in generating the fluorescent signal was further improved to 60–90%. These results indicated that the virus sample preservatives developed in this study can immediately inactivate the virus after sampling and greatly reduce the risk of laboratory exposure to infection, which is significant in epidemic prevention and control. Furthermore, no fluorescent signal appeared in either the control groups or the experimental groups if the MOI was 1 (Figure 2).
Stability of viral nucleic acid
To investigate whether the virus preservation solution was able to protect viral nucleic acid integrity during the inactivation progress at room temperature, we analyzed the stability of adenovirus CON098 (DNA virus) and lentivirus CON335 (RNA virus) by qPCR (SYBR) and RT-PCR, respectively. The results showed that when the DNA and RNA viruses were stored in the virus preservation solution for 7 days, the nucleic acid was hardly affected (Figures 3,4).
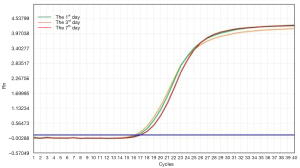
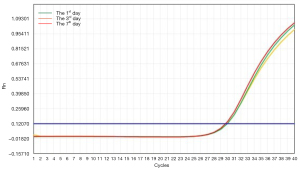
Figures 3,4 show that the virus preservation solution maintained the stability of the viral nucleic acid and made it easier to detect low copy number viruses, which could reduce false negatives. These results are similar to or better than most commercially available preservation solutions of guanidine salt. However, our preservation solution was also able to protect the stability and integrity of the nucleic acid to a great extent by destroying the ester component of the virus coat to make it noninfective. In addition, virus samples treated with our preservation solution are more suitable for subsequent downstream molecular biology experiments such as nucleic acid extraction and PCR reaction. For example, the “one-step” nucleic acid extraction kit is not a good choice for samples stored in tubes that contain guanidinium because there is no step for nucleic acid desalting and purification (13).
Integrity of viral proteins
The integrity of the viral protein is an important factor affecting the ability of the virus to infect. Therefore, we used SDS-PAGE to detect the protein of the lentivirus CON335 after being stored in the virus preservation solution for a certain length of time. It can be seen in Figure 5 that the band of viral proteins was still bright after 7 days of storage. In other words, the protein structure of the virus was intact and not damaged.
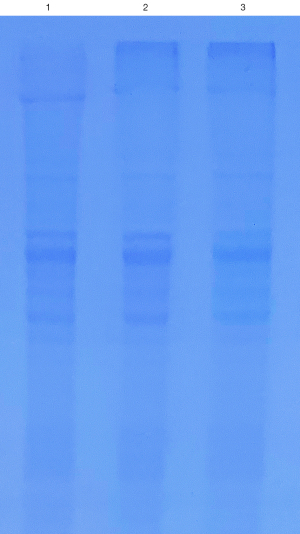
This result further showed that viruses treated with our virus preservation solution had intact virus particles, making it easier to extract the nucleic acid of low copy number viruses and reducing false negatives.
Discussion
Rapid and accurate diagnosis is crucial for successful outbreak containment. During the current COVID-19 public health emergency, the gold standard for the diagnosis of COVID-19 infection is the detection of viral RNA (14,15). Using homogeneous reaction through plasma technology, we developed a new virus preservation solution, focusing on the selection of high-efficiency anticoagulants, natural preservatives, low-toxicity cell membrane protectors, and osmotic pressure stabilizers. Our results showed that this preservation solution can not only rapidly inactivate the virus within 5 minutes, thereby blocking the route of infection, but also reduce secondary contamination caused by samples, which could help protect the safety of medical workers.
The virus preservation solution is broad-spectrum and suitable for the sampling, transportation and preservation of oropharynx of respiratory and intestinal viruses such as coronavirus, influenza, avian influenza, hand foot mouth disease (HFMD), swine influenza and other virus samples; It is also applicable to the collection of specimens of other viruses, chlamydia, mycoplasma and ureaplasma urealyticum. This preservation solution can store virus samples of different carriers, including serum, plasma, throat swab, nose swab, sputum, feces, urine, etc. The collected clinical samples do not need to be dried and are directly suspended in the preservation solution. At present, products on domestic and foreign markets can also store a variety of samples, but our preservation principles are different.
Temperature and time are two important factors that affect the preservation of virus samples (16). For example, COVID-19, as an enveloped lipid virus, is sensitive to temperature (17). The samples can be incubated at 56 ℃ for 30–45 min or higher for virus inactivation in BSL-1 laboratory. However, due to the instability of RNA nucleic acid, the high-temperature inactivation process may cause the degradation of pathogenic nucleic acid, thus affecting the accuracy of detection, resulting in false negative results (18). All experiments in this paper were performed at room temperature, except for the infection experiments. We were able to achieve room temperature preservation of the virus, which could reduce costs associated with the transportation of virus samples and aid follow-up detection work. In addition, the use of multiple types of viruses in our experiments showed that our preservation solution had good compatibility.
Most virus preservation solutions on the market will destroy the complete structure of the virus. Some preservation solutions add bovine serum albumin (BSA) as a protein stabilizer, which can form a protective film on the protein shell of the virus, making it difficult to decompose and ensuring the integrity of the virus. However, it has been pointed out that BSA is not conducive to maintaining stability (19). The virus preservation solution in this article is a non-protein chemical agent, which can maintain the integrity of virus particles, avoid nucleic acid degradation, and effectively inactivate the virus.
The most important finding of our study was that our virus preservation solution preserved the stability of the virus, thereby inhibiting the activity of RNase, which can degrade the RNA. This suggests that our solution will increase the abundance of the virus. Unlike most virus preservation solutions, our solution achieved the effect of inactivating and preserving the virus by destroying the ester component of the virus shell, thus protecting the virion integrity to a great extent. Our virus preservation solution is also applicable to the extraction of DNA and RNA of most virus nucleic acid extraction kits.
Standardize the sampling, store and transport the samples, submit them for inspection in time, and optimize the testing process, it is an effective measure to ensure and improve the accuracy of nucleic acid detection. At present, full-automatic nucleic acid detection and analysis system is widely used. It is equipped with a full-automatic platform. Each step of operation is an accurate standard action. Amplification detection and result analysis are integrated on one instrument without manual operation deviation. The virus preservation solution can be filled in the preservation tube adapted to the instrument to realize fully automatic sample processing, nucleic acid detection and result analysis.
The virus preservation solution in this study has not yet done the gradient experiment of preservation temperature and the impact of large temperature difference on the preservation of virus samples. In the process of actual large-scale application, uncertainties such as storage and transportation under normal temperature, extreme weather and harsh environment may exist. Later, we will carry out relevant experiments to verify the effect of temperature on virus preservation.
Conclusions
The virus preservation solution developed in this study can rapidly inactivate the virus and preserve its integrity at room temperature. It also reduced the possibility of secondary infection, thereby protecting the safety medical personnel. In addition, the solution is effective for at least a week at room temperature, leading to considerable economic benefits by reducing the need for cold chain transportation and cryopreservation. These results suggest that our solution has wide applicability in clinical testing, especially in the context of the current COVID-19 pandemic.
Acknowledgments
Funding: This study was supported by the Natural Science Foundation of Zhejiang Province (No. LGC21H200002).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4295/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4295/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4295/coif). JZ is from Ningbo AJcore Biosciences Inc. The company has no substantial interest relationship and conflict with this article. All authors report that this study was supported by the Natural Science Foundation of Zhejiang Province (No. LGC21H200002). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pandamooz S, Jurek B, Meinung CP, et al. Experimental Models of SARS-CoV-2 Infection: Possible Platforms to Study COVID-19 Pathogenesis and Potential Treatments. Annu Rev Pharmacol Toxicol 2022;62:25-53. [Crossref] [PubMed]
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- Fenollar F, Bouam A, Ballouche M, et al. Evaluation of the Panbio COVID-19 Rapid Antigen Detection Test Device for the Screening of Patients with COVID-19. J Clin Microbiol 2021;59:e02589-20. [Crossref] [PubMed]
- Wang C, Xiao X, Feng H, et al. Ongoing COVID-19 Pandemic: A Concise but Updated Comprehensive Review. Curr Microbiol 2021;78:1718-29. [Crossref] [PubMed]
- Rai P, Kumar BK, Deekshit VK, et al. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Appl Microbiol Biotechnol 2021;105:441-55. [Crossref] [PubMed]
- Yamayoshi S, Sakai-Tagawa Y, Koga M, et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020;12:1420. [Crossref] [PubMed]
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020;323:2249-51. [Crossref] [PubMed]
- Yang Y, Song QH, Wang X, et al. Effect of eight kinds of inactivated virus preservation solutions on the stability of virus nucleic acid. Chinese Journal of Experimental and Clinical Virology 2021;35:500-4.
- van Kasteren PB, van der Veer B, van den Brink S, et al. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol 2020;128:104412. [Crossref] [PubMed]
- Velay A, Gallais F, Benotmane I, et al. Evaluation of the performance of SARS-CoV-2 serological tools and their positioning in COVID-19 diagnostic strategies. Diagn Microbiol Infect Dis 2020;98:115181. [Crossref] [PubMed]
- Si HF. Risk Control Methods of the Adverse Events Associated with Virus Sampling Tubes. Chinese Journal of Medical Device 2021;34:75-6.
- Qin P, Chen LY, Zhao CH, et al. Corrosion behavior and mechanism of selective laser melted Ti35Nb alloy produced using pre-alloyed and mixed powder in Hank’s solution. Corrosion Science 2021;189:109609. [Crossref]
- Chen Y, Lian GW, Zheng LL, et al. Explore the role of virus inactived storage tubes with guanidine salt in improving the safety and accuracy of nucleic acid detection of COVID-19. Chinese Journal of Clinical Laboratory Management 2020;8:94-9. (Electronic Edition).
- Tang Z, Nouri R, Dong M, et al. Rapid detection of novel coronavirus SARS-CoV-2 by RT-LAMP coupled solid-state nanopores. Biosens Bioelectron 2022;197:113759. [Crossref] [PubMed]
- Dankova Z, Novakova E, Skerenova M, et al. Comparison of SARS-CoV-2 Detection by Rapid Antigen and by Three Commercial RT-qPCR Tests: A Study from Martin University Hospital in Slovakia. Int J Environ Res Public Health 2021;18:7037. [Crossref] [PubMed]
- Kim N, Kwon A, Roh EY, et al. Effects of Storage Temperature and Media/Buffer for SARS-CoV-2 Nucleic Acid Detection. Am J Clin Pathol 2021;155:280-5. [Crossref] [PubMed]
- Xiong D, Kan LJ, Wang MM, et al. Evaluation of the consistency and detection capability of seven domestic 2019-nCoV nucleic acid detection kits. Chinese Journal of Laboratory Medicine 2020;43:787-93.
- Chen PS, He YT, Hang YL, et al. Inactivation of 2019 novel coronavirus before quantitative real-time PCR testing. Chinese Journal of Laboratory Medicine 2020;43:364-7.
- Cai GJ, Lin LJ, Fang YP, et al. Repeatability of self-made indoor quality control material of human cytomegalovirus nucleic acid. Chinese Journal of Clinical Laboratory Science 2013;31:713-4.
(English Language Editor: C. Gourlay)


