
Efficacy and optimal combination timing of chemotherapy combined with PD-1 inhibitor in advanced cervical cancer: a multicenter retrospective cohort study
Introduction
Cervical cancer is a malignant reproductive system tumor that seriously affects the survival of women, and the new cases of cervical cancer are among the top 10 most common malignant tumors in females (1). Cervical cancer can be cured by radical surgical resection or radiotherapy if detected early (2). Radiotherapy, chemotherapy, and systemic therapy are the main treatments for advanced cervical cancer, but the 5-year survival rate is still very low (<20%) (3). In developing countries, >70% of cervical cancer patients are at an advanced stage at the time of diagnosis due to the poor medical conditions. Disease progression is difficult to control using surgical resection or local chemoradiotherapy. Thus, the treatment has become an important research direction for gynecological oncology researchers.
Chemotherapy and targeted therapy treatments for advanced cervical cancer have progressed continuously in recent years. With the accumulation of clinical data, the recommendation that immune checkpoint inhibitors be used in the treatment of cervical cancer has continued to increase, as checkpoint inhibitors provide a better prognosis than traditional treatments for patients. Currently, an increasing number of clinical studies are being conducted on the use of programmed cell death protein 1 (PD-1) inhibitors in the treatment of recurrent or metastatic cervical cancer. Although a previous study has observed the efficacy and safety of PD-1 inhibitors alone after failure of ≥1 line therapy in the recurrent metastatic cervical cancer (4), patients with advanced cervical cancer who are initially diagnosed also require systemic therapy in the real world. There are few reports on the efficacy and safety of combination therapy in such patients.
This study focused on advanced cervical cancer, including stage IVB and recurrent/metastatic cervical cancers. We explored the anti-tumor activity and safety of chemotherapy combined with PD-1 inhibitors and the factors affecting survival. This study also compared the efficacy of early and late combination therapies and further explored the timing of the combination of PD-1 inhibitors and chemotherapy. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4298/rc).
Methods
Study design and patient information
This was a retrospective, single-arm, multicenter study. We analyzed the antitumor activity and safety of PD-1 inhibitor combined with chemotherapy in all included patients. The age, Eastern Cooperative Oncology Group (ECOG) score, histological type, timing of combined treatment, lung metastasis, lymph node metastasis, programmed death-ligand 1 (PD-L1), and history of previous anti-tumor therapy of all included patients were recorded in detail. And we analyzed whether these clinical features are prognostic factors. From March 2020 to December 2021, patients with advanced cervical cancer were recruited from the following 4 centers: The Affiliated Cancer Hospital of Zhengzhou University, The First Affiliated Hospital of Henan University of Science and Technology, Huaihe Hospital of Henan University, and The First Affiliated Hospital of Henan University. This multicenter, retrospective, real-world study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of The Affiliated Cancer Hospital of Zhengzhou University (approval No. 2021-KY-0053-003). The Ethics Committee of other hospitals (The First Affiliated Hospital of Henan University of Science and Technology, Huaihe Hospital of Henan University, and The First Affiliated Hospital of Henan University) were informed and agreed the study. All the patients who met the inclusion criteria signed an informed consent form before enrolment.
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) be aged 18–75 years; (II) have a pathologically or histologically confirmed diagnosis of cervical cancer; (III) have Federation International of Gynecology and Obstetrics (FIGO) stage IVB or recurrent metastatic advanced cervical cancer; (IV) have an ECOG performance status score <2; and (V) have at least 1 measurable target lesion according to the solid tumor response evaluation criteria (RECIST version 1.1). Patients were excluded from the study if they met any of the following exclusion criteria: (I) had other malignant tumors; (II) had previously undergone immunotherapy; (III) had participated in other clinical trials; (IV) had incomplete data; and/or (V) had severe comorbidities, including severe heart, lung, renal, or coagulation disorders.
Treatment regimens
The chemotherapy regimen consisted of paclitaxel (135 mg/m2; Yangtze River Pharmaceutical Group, Taizhou, China) and cisplatin (50 mg/m2; Qilu Pharmaceutical, Jinan, China) administered intravenously every 3 weeks for 3–4 cycles. The immunotherapy regimen consisted of 200 mg of a PD-1 inhibitor administered intravenously every 3 weeks, and the PD-1 inhibitors included sintilimab (Innovent Pharmaceutical, Suzhou, China), tislelizumab (BeiGene Pharmaceutical, Shanghai, China), and camrelizumab (Hengrui Pharmaceutical, Lianyungang, China). Specifically, 57 patients received sintilimab, 18 received tislelizumab, and 10 received camrelizumab. When patient adverse events were grade ≥3 and determined to be related to chemotherapy, the chemotherapy dose was reduced by 20%. If adverse events continue to occur after dose adjustment, the drug was suspended, and the initial dose was resumed after the adverse events were resolved or eliminated. If the adverse events were determined to be related to the PD-1 inhibitors, the medication was suspended and did not resume until the adverse events had resolved.
Early combination therapy was defined as a combination of PD-1 inhibitors administered in the 1st cycle of chemotherapy. Late combination therapy was defined as the combination of PD-1 inhibitors administered after at least 1 cycle of chemotherapy. All the patients received chemotherapy, and the time at which the PD-1 inhibitors were administered (i.e., early or late) was decided by the doctor after considering the patients’ wishes.
Follow-up and study endpoints
Progression-free survival (PFS), the objective response rate (ORR), and treatment safety were the primary endpoints of this study. We analyzed prognostic factors affecting survival and further explored whether the timing of combining chemotherapy with PD-1 inhibitors was a prognostic factor affecting survival. Overall survival (OS) and the disease-control rate (DCR) were the secondary endpoints of this study.
The enrolled patients underwent imaging evaluations every 2 cycles of treatment. Pelvic lesions were examined using multiphase-enhanced magnetic resonance imaging and other lesions were examined using enhanced computed tomography. The tumor evaluations were performed by 2 experienced radiologists according to the RECIST 1.1 standard. When the 2 evaluation results were inconsistent, a 3rd senior doctor made the final decision. The final assessments included complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD). The proportion of patients with CR + PR was defined as the ORR, and the proportion of patients with CR + PR + SD was defined as the DCR. PFS was defined as the interval from the date of treatment initiation to the date of disease progression or death from any cause. OS was defined as the interval between the date of treatment initiation and the date of death from any cause. Adverse events were recorded through patients’ laboratory tests, telephone follow-up calls, or medical history. In this study, all the treatment-related adverse events were recorded and assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, published by the National Cancer Institute.
Statistical analysis
All the statistical analyses were performed using SPSS Statistics for Windows (version 22.0; IBM, Armonk, NY, USA). Normally, distributed continuous variables were expressed using the mean ± standard deviation (SD), and non-normally distributed continuous variables were presented using median (interquartile range). Number (percentage) were used for categorical variables. 95% confidence intervals (95% CI) of ORR and DCR were estimate by Clopper-Pearson method. The median OS and PFS were estimate using the Kaplan-Meier method. The statistical significance of the clinical characteristics was assessed by a univariate analysis, and statistically significant variables were included in the multivariate Cox regression models to identify the predictors associated with PFS and OS. A two-sided P value <0.05 was considered statistically significant.
Results
Patient characteristics
During the study period (March 2020 to December 2021), 116 patients with advanced cervical cancer were screened at 4 centers. Among these patients, 31 met the exclusion criteria and were excluded, and the remaining 85 were ultimately included in the study (see Figure 1). Follow-up was performed until June 2022, with a median follow-up time of 23.4 months (95% CI: 22.19–24.62 months). The median age of patients was 52 [46–62] years, and among the patients, 50 (58.8%) were ≥50 years old. Among the 85 patients, 46 (54.1%) had an ECOG score of 1, 68 (80.0%) had a histology of squamous cell carcinoma, 41 (48.2%) received early combination therapy, 22 (25.9%) had lung metastases, 53 (62.4%) had distant lymph node metastases, 30 (35.3%) had a PD-L1-positive cell count (combined positive score <1), and 43 (50.6 %) had been previously treated (see Table 1).
Table 1
| Variable | Chemotherapy combined with PD-1 inhibitors (n=85) | Early combination (n=41) | Late combination (n=44) |
|---|---|---|---|
| Age (years), median [IQR] | 52 [46–62] | 53 [48–60] | 50 [43–63] |
| Age (years), n (%) | |||
| <50 | 35 (41.2) | 19 (46.3) | 16 (36.4) |
| ≥50 | 50 (58.8) | 22 (53.7) | 28 (63.6) |
| ECOG, n (%) | |||
| 0 | 39 (45.9) | 22 (53.7) | 17 (38.6) |
| 1 | 46 (54.1) | 19 (46.3) | 27 (61.4) |
| Histology, n (%) | |||
| Squamous cell carcinoma | 68 (80.0) | 33 (80.5) | 35 (79.5) |
| Adenocarcinoma | 17 (20.0) | 8 (19.5) | 9 (20.5) |
| Co-treatment time, n (%) | |||
| Early combination | 41 (48.2) | 41 (100.0) | 0 (0.0) |
| Late combination | 44 (51.8) | 0 (0.0) | 44 (100.0) |
| Lung metastases | 22 (25.9) | 6 (14.6) | 16 (36.4) |
| Lymph node metastases | 53 (62.4) | 21 (51.2) | 32 (72.7) |
| PD-L1, n (%) | |||
| PD-L1 <1 | 30 (35.3) | 14 (34.1) | 16 (36.4) |
| 1≤ PD-L1 <10 | 33 (38.8) | 17 (41.5) | 16 (36.4) |
| PD-L1 ≥10 | 22 (25.9) | 10 (24.4) | 12 (27.3) |
| Previous treatment, n (%) | |||
| No | 42 (49.4) | 29 (70.7) | 13 (29.5) |
| Yes | 43 (50.6) | 12 (29.3) | 31 (70.5) |
PD-1, programmed cell death protein 1; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1.
Anti-tumor activity
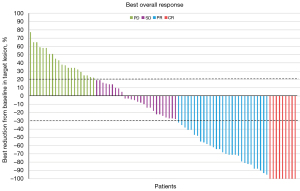
As of the final follow-up date (June 2022), the median PFS of patients enrolled in this study was 10.3 months (95% CI: 9.47–11.13 months), and the 12-month PFS rate was 64.7% (see Figure 2). The 24-month OS rate was 73.1%, and the median OS was not reached (see Figure 3). According to the RECIST 1.1 evaluation criteria, the numbers of patients evaluated as CR, PR, SD, and PD were 9, 29, 26, and 21, respectively. The ORR was 44.7% and the DCR was 75.3%. Compared to the baseline, 51 (64.7%) patients had reduced target lesions and 34 (40%) had enlarged target lesions. A total of 26 patients were assessed as SD, of whom 17 had tumor shrinkage and 9 had tumor enlargement. The tumor response and optimal changes in tumor size for all patients are shown in Table 2 and Figure 4.
Table 2
| Best tumor response | Chemotherapy combined with PD-1 inhibitors (n=85), N (%) |
|---|---|
| CR | 9 (10.6) |
| PR | 29 (34.1) |
| SD | 26 (30.6) |
| PD | 21 (24.7) |
| ORR (CR + PR) | 38 (44.7) |
| DCR (CR + PR + SD) | 64 (75.3) |
PD-1, programmed cell death protein 1; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease-control rate.
Analysis of prognostic factors
The univariate analysis showed that the main factors affecting the PFS of patients were lung metastasis [hazard ratio (HR) 1.82, 95% CI: 1.07–3.1, P=0.026], lymph node metastasis (HR 2.16, 95% CI: 1.31–3.56, P=0.003), previous treatment (HR 2.23, 95% CI: 1.39–3.61, P<0.001), and the timing of the combination therapy (HR 0.34, 95% CI: 0.20–0.55, P<0.001). Patient age, ECOG score, pathology, and PD-L1 expression were not factors affecting the PFS of patients (P>0.05). All the significant variables in the univariate analysis were included in multivariate analysis, and the results showed that the early combination of PD-1 inhibitors and chemotherapy provided better PFS than the late combination (HR 0.40, 95% CI: 0.24–0.67, P=0.001). Lymph node metastasis (HR 2.04, 95% CI: 1.24–3.38, P=0.005) and previous treatment (HR 1.79, 95% CI: 1.09–3.00, P=0.023) were also independent risk factors for PFS (see Table 3).
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≥50 vs. <50 years) | 1.08 (0.67–1.74) | 0.745 | |||
| ECOG (0 vs. 1) | 1.09 (0.69–1.75) | 0.705 | |||
| Histology (squamous cell carcinoma vs. adenocarcinoma) | 0.67 (0.36–1.22) | 0.189 | |||
| Lung metastases (yes vs. no) | 1.82 (1.07–3.1) | 0.026 | |||
| Lymph node metastases (yes vs. no) | 2.16 (1.31–3.56) | 0.003 | 2.04 (1.24–3.38) | 0.005 | |
| PD-L1 <1 vs. 1≤ PD-L1 <10 | 0.63 (0.36–1.1) | 0.105 | |||
| 1≤ PD-L1 <10 vs. PD-L1 ≥10 | 0.59 (0.32–1.1) | 0.095 | |||
| Previous treatment (yes vs. no) | 2.23 (1.39–3.61) | <0.001 | 1.79 (1.09–3.00) | 0.023 | |
| Combined treatment time (early vs. late) | 0.34 (0.20–0.55) | <0.001 | 0.40 (0.24–0.67) | 0.001 | |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1.
Safety
During the treatment and follow-up periods, the overall incidence of adverse events in this study was 56.5%, and that of grade ≥3 adverse events was 12.9%. The most common treatment-related adverse events were thrombocytopenia (9.4%), neutropenia (11.8%), and anemia (8.2%), all of which improved after symptomatic treatment. The main immune-related adverse events were hypothyroidism (5.9%) and immune-related pneumonitis (2.4%). The hypothyroidism of 5 patients was relieved after 1 month of oral euthyrox. Among the patients who discontinued immunotherapy due to adverse events of grade ≥3 (3.6%), 2 patients (2.4%) had immune-related pneumonia and 1 (1.2%) had immune-related hepatic insufficiency (see Table 4).
Table 4
| Variable | All grade, N (%) | Grade ≥3, N (%) |
|---|---|---|
| Total | 48 (56.5) | 11 (12.9) |
| Thrombocytopenia | 8 (9.4) | 2 (2.4) |
| Neutropenia | 10 (11.8) | 4 (4.7) |
| Fatigue | 4 (4.7) | 0 (0) |
| Anemia | 7 (8.2) | 1 (1.2) |
| Hypothyroidism | 5 (5.9) | 0 (0) |
| Immune-related pneumonitis | 2 (2.4) | 2 (2.4) |
| Immune-related hepatic insufficiency | 1 (1.2) | 1 (1.2) |
| Renal dysfunction | 1 (1.2) | 0 (0) |
| Decreased appetite | 3 (3.5) | 0 (0) |
| Diarrhea | 2 (2.4) | 1 (1.2) |
| Fever | 1 (1.2) | 0 (0) |
| Vomiting | 2 (2.4) | 0 (0) |
| RCCEP | 1 (1.2) | 0 (0) |
| Constipate | 1 (1.2) | 0 (0) |
RCCEP, reactive cutaneous capillary endothelial proliferation.
Discussion
The results of this study show that chemotherapy combined with PD-1 inhibitors is effective, safe and reliable in the treatment of advanced cervical cancer, and the early combination of PD-1 inhibitors and chemotherapy provides better efficacy than the late combination.
Immune checkpoint inhibitors were initially recommended for patients with high microsatellite instability, mismatch repair mutations, or PD-L1-positive tumors as 2nd- or last-line treatments. The 1st-line treatment for advanced cervical cancer is a critical factor affecting the survival of patients, and once the disease recurs, the 2nd-line treatment effect is usually unsatisfactory. A study has shown that the median OS of 1st-line treatment for advanced cervical cancer patients is 10–13 months, and that of 2nd-line treatment after disease progression is only 5–9 months (5). Even with targeted therapy, the median OS of 2nd-line treatments cannot be effectively improved. Thus, patients with advanced cervical cancer require a 1st-line treatment regimen with a better curative effect to improve their prognosis.
In multiple clinical trials represented by KEYNOTE-028 and KEYNOTE-158, immune checkpoint inhibitors have been shown to have superior anti-tumor activity in the treatment of recurrent and metastatic cervical cancer (6,7). For patients with PD-L1-positive recurrent or metastatic cervical cancer, the National Comprehensive Cancer Network Cervical Cancer Guidelines (version 1, 2022) recommends pembrolizumab combined with chemotherapy as the 1st-line treatment, and the results of the KEYNOTE-826 clinical study confirm that in the 1st-line treatment of advanced cervical cancer, the combination of pembrolizumab and chemotherapy significantly prolonged the PFS and OS of patients compared to the placebo (8). The Phase-III trial results of the KEYNOTE-826 clinical study confirmed that the combination of pembrolizumab and chemotherapy with bevacizumab further improved the survival rate of PD-L1-positive patients compared to chemotherapy alone (9). However, the Phase-III trial of the KEYNOTE-826 clinical study did not discuss the timing of the combination of chemotherapy and PD-1 inhibitors; rather, our study analyzed the difference in efficacy between early and late combinations.
The results of the EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 clinical study presented at the 2021 European Society of Medical Oncology Congress showed that with a median follow-up of 18.2 months, the chemotherapy group had a significantly shorter median OS than the cemiplimab group (8.5 vs. 12.0 months, respectively). There was also a significant improvement in the ORR (16.4% in the cemiplimab group and 6.3% in the chemotherapy group), confirming that PD-1 inhibitors alone can achieve a better local control rate and prolong the survival time compared to chemotherapy alone.
In our study, the median follow-up time was 23.4 months (95% CI: 22.19–24.62 months), the 24-month OS rate was 73.1%, and the median OS was not reached. Our study showed that the combination therapy group had better survival than the combination therapy of previous studies. A previous study by Cheng et al. found that the ORR and PFS of patients with advanced cervical cancer in combination with immune checkpoint inhibitors ranged from 0–65.9% and 2.9–13.8 months, respectively (10). In our study, the ORR was 44.7% and the median PFS was 10.3 months, which is consistent with the findings of Cheng et al. (10). Thus, the combination of PD-1 inhibitors and chemotherapy can improve the survival of patients compared to PD-1 inhibitors or chemotherapy alone.
The multivariate analysis in this study showed that the timing of PD-1 inhibitor administration combined with chemotherapy was an independent factor affecting PFS (HR 0.34, 95% CI: 0.20–0.55, P<0.001). Most previous studies on immunotherapy in cervical cancer have used monotherapy (11-13), and the efficacy of immune checkpoint inhibitor monotherapy in cervical cancer failed to overcome the bottleneck of traditional regimens. Recent ongoing and planned clinical studies are gradually administering combination therapies to determine if a higher effective rate can be achieved. For example, immunotherapy combined with concurrent chemoradiotherapy (14,15), immunotherapy combined with targeted therapy (16,17), double immunotherapy (18), and other treatment strategies have achieved good therapeutic effects. However, few studies have investigated the timing of combined therapy.
The results of this study showed that the early combination of PD-1 inhibitors and chemotherapy resulted in a better PFS than the late combination. The possible reasons for this are as follows. First, the immune-related mechanisms may enhance the survival benefit of concomitant chemotherapy (19). A study has shown that patients with cervical cancer treated with immune checkpoint inhibitors have a higher tendency of immune infiltration by cluster of differentiation (CD)3, CD4, and CD8 T cells during chemotherapy (20). In vitro experiments have also shown that PD-1 inhibitors combined with cisplatin prevent a decrease in T cell dependence (21). Thus, early combination therapy may activate the tumor immune state. Second, early combination therapy may change the characteristics of the tumor immune microenvironment (TIME) and affect efficacy (22). A study has shown that chemotherapy can lead to the death of immunogenic cells, thereby releasing neoantigens (23). This may lead to injury-associated molecular patterns and activate dendritic cells (24-26). Dendritic cells then activate cytotoxic T cells, allowing T cells to migrate to the TIME and ultimately activate T cell-mediated immunity (27). Thus, compared to late combination therapy, early combination therapy of PD-1 inhibitors and chemotherapy may activate T cell-mediated immunity earlier and have a better effect.
In addition, previous studies found that histological type (squamous cell carcinoma and adenocarcinoma) had no effect on OS. Similarly, we found that histological type was not a prognostic factor for PFS. The CheckMate 358 trial explored the anti-tumor activity and safety of nivolumab in virus-related cancers (28). The ORR of the patients enrolled in CheckMate 358 trial was 26.3%, and the median PFS and median OS were 5.1 and 21.9 months, respectively. The subgroup analysis showed that the ORR was 20% in the PD-L1-positive group and the median OS was 19.9 months. This indicated that PD-L1 positivity did not improve prognosis, which was consistent with the findings of our present study that PD-L1 expression was not a prognostic factor.
Our study found that PD-1 inhibitors combined with chemotherapy is safe in the treatment of advanced cervical cancer. The overall incidence of adverse events in this study was 56.5%, and the incidence of grade ≥3 adverse events was 12.9%, which is consistent with the findings of previous studies (5,29,30). In this study, chemotherapy-related adverse events mainly included thrombocytopenia, neutropenia, anemia, diarrhea, and vomiting, and some patients were treated with local radiotherapy, which might have aggravated these adverse reactions. The immune-related adverse events mainly included hypothyroidism, immune-related pneumonia, and reactive cutaneous capillary endothelial proliferation. Compared to PD-1 inhibitors alone or chemotherapy alone, their combination did not increase the incidence of adverse events, regardless of whether the combination was early or late. The symptoms associated with these adverse events were relieved or resolved following symptomatic treatment or the temporary discontinuation of the drug. Thus, our findings suggest that the combination of PD-1 inhibitors and chemotherapy for advanced cervical cancer is safe.
This study had several limitations. First, it was based on a retrospective analysis and had a small sample size, which may have led to a bias in the data. Second, the follow-up time in this study was short, and the median OS was not reached. Thus, a longer follow-up time is required so that OS can be used as the next validation endpoint. Third, due to economic problems and the implementation of strategies to prevent the spread of coronavirus disease 2019 (COVID-19), some patients did not regularly receive chemotherapy combined with PD-1 inhibitor therapy. A higher clinical benefit may be expected if patients receive combination therapy on a regular basis. Thus, the conclusions of this study need to be further confirmed by prospective, multicenter, large-scale, randomized controlled clinical studies to guide clinical practice.
Conclusions
In conclusion, the combination of PD-1 inhibitors and chemotherapy shows excellent efficacy in the treatment of advanced cervical cancer, and the adverse reactions are safe and controllable, which provides additional options for the treatment of such patients. The early combination of chemotherapy and PD-1 inhibitors is more effective than the late combination of chemotherapy and PD-1 inhibitors, which provides a new path for exploring the optimal application timing of PD-1 inhibitors in the treatment of advanced cervical cancer.
Acknowledgments
The authors would like to thank all the patients and physicians at the centers involved in this study.
Funding: This work was supported by the Henan Province Medical Science and Technology Research Project (No. LHGJ20200191).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4298/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4298/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4298/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This multicenter, retrospective, real-world study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of The Affiliated Cancer Hospital of Zhengzhou University (approval No. 2021-KY-0053-003). The Ethics Committee of other hospitals (The First Affiliated Hospital of Henan University of Science and Technology, Huaihe Hospital of Henan University, and The First Affiliated Hospital of Henan University) were informed and agreed the study. All the patients who met the inclusion criteria signed an informed consent form before enrolment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350:535-40. [Crossref] [PubMed]
- Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol 2016;214:22-30. [Crossref] [PubMed]
- Santin AD, Deng W, Frumovitz M, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol 2020;157:161-6. [Crossref] [PubMed]
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017;390:1654-63. [Crossref] [PubMed]
- Frenel JS, Le Tourneau C, O'Neil B, et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J Clin Oncol 2017;35:4035-41. [Crossref] [PubMed]
- Chung HC, Ros W, Delord JP, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019;37:1470-8. [Crossref] [PubMed]
- Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N Engl J Med 2021;385:1856-67. [Crossref] [PubMed]
- Nishio S, Yonemori K, Usami T, et al. Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: Results from KEYNOTE-826. Cancer Sci 2022; Epub ahead of print. [Crossref] [PubMed]
- Cheng M, Wang H, Zhao Y, et al. Efficacy and Prognostic Factors for Response to PD-1 Inhibitors in Advanced Cervical Carcinoma: A Retrospective Study. Drug Des Devel Ther 2022;16:887-97. [Crossref] [PubMed]
- Tamura K, Hasegawa K, Katsumata N, et al. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial. Cancer Sci 2019;110:2894-904. [Crossref] [PubMed]
- Choi MC, Kim YM, Lee JW, et al. Real-World Experience of Pembrolizumab Monotherapy in Patients with Recurrent or Persistent Cervical Cancer: A Korean Multi-Center Retrospective Study (KGOG1041). Cancers (Basel) 2020;12:3188. [Crossref] [PubMed]
- O'Malley DM, Oaknin A, Monk BJ, et al. Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer. Gynecol Oncol 2021;163:274-80. [Crossref] [PubMed]
- Mayadev J, Nunes AT, Li M, et al. CALLA: Efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. Int J Gynecol Cancer 2020;30:1065-70. [Crossref] [PubMed]
- Mayadev J, Zamarin D, Deng W, et al. Anti-PD-L1 (atezolizumab) as an immune primer and concurrently with extended-field chemoradiotherapy for node-positive locally advanced cervical cancer. Int J Gynecol Cancer 2020;30:701-4. [Crossref] [PubMed]
- Huang X, He M, Peng H, et al. Genomic profiling of advanced cervical cancer to predict response to programmed death-1 inhibitor combination therapy: a secondary analysis of the CLAP trial. J Immunother Cancer 2021;9:e002223. [Crossref] [PubMed]
- Xu Q, Wang J, Sun Y, et al. Efficacy and Safety of Sintilimab Plus Anlotinib for PD-L1-Positive Recurrent or Metastatic Cervical Cancer: A Multicenter, Single-Arm, Prospective Phase II Trial. J Clin Oncol 2022;40:1795-805. [Crossref] [PubMed]
- Umeda Y, Yoshikawa S, Kiniwa Y, et al. Real-world efficacy of anti-PD-1 antibody or combined anti-PD-1 plus anti-CTLA-4 antibodies, with or without radiotherapy, in advanced mucosal melanoma patients: A retrospective, multicenter study. Eur J Cancer 2021;157:361-72. [Crossref] [PubMed]
- Lippens L, Van Bockstal M, De Jaeghere EA, et al. Immunologic impact of chemoradiation in cervical cancer and how immune cell infiltration could lead toward personalized treatment. Int J Cancer 2020;147:554-64. [Crossref] [PubMed]
- Meng Y, Liang H, Hu J, et al. PD-L1 Expression Correlates With Tumor Infiltrating Lymphocytes And Response To Neoadjuvant Chemotherapy In Cervical Cancer. J Cancer 2018;9:2938-45. [Crossref] [PubMed]
- Kroon P, Frijlink E, Iglesias-Guimarais V, et al. Radiotherapy and Cisplatin Increase Immunotherapy Efficacy by Enabling Local and Systemic Intratumoral T-cell Activity. Cancer Immunol Res 2019;7:670-82. [Crossref] [PubMed]
- Someya M, Tsuchiya T, Fukushima Y, et al. Prediction of treatment response from the microenvironment of tumor immunity in cervical cancer patients treated with chemoradiotherapy. Med Mol Morphol 2021;54:245-52. [Crossref] [PubMed]
- Suzuki Y, Mimura K, Yoshimoto Y, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012;72:3967-76. [Crossref] [PubMed]
- Deng L, Liang H, Xu M, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014;41:843-52. [Crossref] [PubMed]
- Gajewski TF, Corrales L, Williams J, et al. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv Exp Med Biol 2017;1036:19-31. [Crossref] [PubMed]
- McGee HM, Jiang D, Soto-Pantoja DR, et al. Targeting the Tumor Microenvironment in Radiation Oncology: Proceedings from the 2018 ASTRO-AACR Research Workshop. Clin Cancer Res 2019;25:2969-74. [Crossref] [PubMed]
- van den Ende T, van den Boorn HG, Hoonhout NM, et al. Priming the tumor immune microenvironment with chemo(radio)therapy: A systematic review across tumor types. Biochim Biophys Acta Rev Cancer 2020;1874:188386. [Crossref] [PubMed]
- Naumann RW, Hollebecque A, Meyer T, et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. J Clin Oncol 2019;37:2825-34. [Crossref] [PubMed]
- Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol 2010;28:3562-9. [Crossref] [PubMed]
- Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J Clin Oncol 2015;33:2129-35. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)





