
A retrospective cohort study of polatuzumab vedotin combined with immunochemotherapy in Chinese patients with relapse/refractory diffuse large B cell lymphoma
Introduction
Non-Hodgkin lymphoma (NHL) is the most common hemolymphatic malignancy in adults. Diffuse large B-cell lymphoma (DLBCL), the most common subtype of NHL, accounts for approximately 30–40% of all NHL cases worldwide, versus 37% in China. It is estimated that about 35,000 new DLBCL cases are diagnosed each year in China (1).
The current standard first-line treatment option for DLBCL is the R-CHOP regimen (rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride, vincristine, and prednisone); however, only 50–70% of patients are cured by first-line regimens. In addition, 15–25% of patients are resistant to any treatment regimen, and 20–30% cases relapse after achieving complete remission (2). Only a very small number of these relapsed/refractory (R/R) DLBCL cases can be cured by salvage chemotherapy and autologous stem cell transplantation (ASCT) (3). Meanwhile, most patients have a poor prognosis and short survival. In refractory DLBCL, the overall (ORR) and complete (CR) response rates after subsequent treatment are about 26% and 7%, respectively, with a median overall survival (OS) of only 6.3 months (4).
Currently, there are no standard and effective therapies for R/R DLBCL cases ineligible for transplantation or those showing relapse after transplantation. The efficacy of bendamustine and rituximab (BR) combination has been validated in prospective studies of R/R cases ineligible for transplantation, but patient prognosis remains dismal; in addition, the efficacy of immunotherapy, for example, application of new drugs and chimeric antigen receptor T-cell immunotherapy (CAR-T), still needs validation (5). Therefore, there is a huge need for treatments with significantly improved efficacy to extend survival in patients with R/R DLBCL with acceptable safety and tolerability profiles.
Polatuzumab vedotin (pola) is an antibody drug conjugate (ADC) that contains a humanized immunoglobulin G1 (IgG1) anti-human CD79b monoclonal antibody (Mab) and a potent anti-mitotic agent, mono-methyl auristatin E (MMAE), linked through a protease labile linker (MC-VC-PABC) (6). The expression of the cell surface antigen CD79b is restricted to all mature B cells except plasma cells. It is expressed in the majority of B-cell-derived malignancies, including nearly all NHLs (7). Pola combined with rituximab and bendamustine (pola-BR) has been approved in the United States and Europe successively for the treatment of adult patients with R/R DLBCL. However, innovative treatments in RR DLBCL in China are in urgent need. But pola has not been approved in China, and no reported clinical trial has examined its efficacy in the Chinese population. The aim of this study was to preliminarily evaluate the efficacy and safety of the pola-BR regimen in patients with R/R DLBCL by reviewing and analyzing the clinical data of compassionate use of pola, providing a reference for clinical treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4593/rc).
Methods
Data source
This multicenter, retrospective study analyzed the data from a Chinese compassionate use program (CUP) of pola (pola CUP) Four Chinese medical centers were involved, including the First Affiliated Hospital of Hainan Medical University (Haikou), Henan Cancer Hospital (Zhengzhou), Jiangsu Cancer Hospital (Nanjing), and the First Affiliated Hospital of Nanchang University (Nanchang). The study was approved by the institutional ethics committee of Jiangsu Cancer Hospital (approval ID: 2019-062-02). Informed consent was waived by the ethics committee due to the retrospective nature of the study. First Affiliated Hospital of Hainan Medical University, Henan Cancer Hospital, and the First Affiliated Hospital of Nanchang University were informed and agreed with the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patients
All patients with R/R DLBCL who participated in the pola CUP program from 11 December 2019 were enrolled. The eligibility criteria for pola CUP were as follows: (I) histologically confirmed DLBCL; (II) exhaustion of all therapeutic options for DLBCL and treatment with ≥2 prior lines of therapy including R-CHOP (or similar regimen for first-line DLBCL treatment); (III) ineligibility for bone marrow transplantation (BMT; allogenic or autologous); (IV) no previous treatment with BR; and (V) no grade ≥2 peripheral neuropathy (PN). Refractoriness was defined as progressive disease or no response <6 months from the start of prior standard therapy (refractory); relapse was defined as disease relapse after initial response ≥6 months from the start of prior therapy. This study is a retrospective observational study without any formal statistical assumptions, and all the patients who meet the eligibility criteria were included in the study.
Dosage and administration
Patients received up to 6 cycles of pola in combination with either rituximab (R) or BR, per the investigator’s judgement. A cycle was typically 21 days. Treatments were administered sequentially in the order specified below (Figure 1): (I) rituximab at 375 mg/m2 IV on day 1 of each cycle; (II) polatuzumab vedotin at 1.8 mg/kg IV on day 2 of cycle 1, then day 1 of each subsequent cycle; (III) bendamustine at 90 mg/m2 on days 2 and 3 of cycle 1, then days 1 and 2 of cycles 2–6.
Assessments
Objective tumor response was assessed according to the Lugano Response Criteria (8) by local investigators, using positron emission tomography-computed tomography (PET-CT), computed tomography (CT), or bone-marrow biopsy. The efficacy was recorded as CR, partial response (PR), stable disease (SD), or progressive disease (PD). Confidence intervals (CIs) for response rates were calculated by the Clopper-Pearson method. The best overall response (BOR) was defined as the best response recorded from the start of the treatment to disease progression. Clinical assessments were performed every 2–3 cycles during the treatment and every 2–3 months after treatment completion until disease progression.
Adverse events (AEs), serious adverse events (SAEs), and AEs of special interest (AESI) occurring during treatment or within 120 days after treatment were assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v5.0; https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm). In this program, AESI included potential drug-induced liver injury, tumor lysis syndrome (TLS), secondary malignancy, grade 2–5 PN, grade 3–5 infusion-related reaction (IRR), grade 3–5 neutropenia, and hepatitis B reactivation.
Statistical analysis
For demographic and baseline characteristics, continuous variables will be summarized using mean, standard deviation, median, and range, and categorical variables will be summarized using proportions, as appropriate. The population for efficacy analyses comprise all patients received at least one dose of Pola. For BOR and ORR at the end of treatment, the proportion of responding patients will be used as an estimate and 95% CI will be calculated using Clopper-Pearson method. For PFS, the median will be estimated by the Kaplan-Meier method and the 95% CI for the median will be calculated by the Brookmeyer-Crowley method.
Results
Patients
A total of 28 R/R DLBCL patients with at least 1 efficacy assessment were analyzed. A total of 12 patients received the pola-BR regimen, including 3 who crossed from the pola-R cohort in cycle 3 or 4; 16 patients received the pola-R regimen.
The baseline demographic characteristics of the study population are shown in Table 1. The median age was 59 (range, 24 to 78) years. The median number of prior therapeutic lines was 3 (range, 2 to 6). All participants had been treated with rituximab, and 82.1% were refractory to the last prior therapy. At treatment initiation, 53.6% of patients had stage IV disease, and Eastern Cooperative Oncology Group performance status (ECOG PS) scores were 0–1 in 71.4% of patients. There were 3 patients (10.7%) who had been previously treated by ASCT, and 7 (25.0%) had received prior CAR-T therapy.
Table 1
| Characteristic | Number (n=28) |
|---|---|
| Male gender, n (%) | 22 (78.6) |
| Age (years), median [range] | 59 [24–78] |
| Lines of prior therapy, median [range] | 3 [2–6] |
| 2, n (%) | 5 (17.9) |
| 3, n (%) | 15 (53.6) |
| 4, n (%) | 4 (14.3) |
| 5, n (%) | 1 (3.6) |
| 6, n (%) | 3 (10.7) |
| Refractory to last prior therapy, n (%) | 23 (82.1) |
| Ann Arbor stage at diagnosis, n (%) | |
| I | 0 |
| II | 3 (10.7) |
| III | 10 (35.7) |
| IV | 15 (53.6) |
| ECOG PS 0–1, n (%) | 20 (71.4) |
| GCB subtype, n (%) | 11 (39.3) |
| Non-GCB subtype, n (%) | 17(60.7) |
| DHL, n (%) | 1 (4.3)a |
| DEL, n (%) | 11 (40.7)b |
| Prior CAR-T therapy, n (%) | 7 (25.0) |
| Prior ASCT therapy, n (%) | 3 (10.7) |
a, FISH was performed for 23 samples; b, DEL status was assessed in 27 samples. ECOG PS, Eastern Cooperative Oncology Group performance status; GCB, germinal center B-cell-like; DHL, double hit lymphoma; DEL, double expression lymphoma; CAR-T, chimeric antigen receptor T-cell immunotherapy; ASCT, autologous stem cell transplantation; FISH, fluorescence in situ hybridization.
The cell-of-origin (COO) assay was performed in all cases by the immunohistochemistry (IHC)‐based Hans algorithm. The COO distribution rates were 60.7% (17/28) and 39.3% (11/28) for non-germinal center B-cell-like (non-GCB) and GCB, respectively. Double expression lymphoma (DEL) status was assessed in 27 samples, and 23 specimens were examined by fluorescence in situ hybridization (FISH). Totally 40.7% (11/27) DEL and 4.3% (1/23) double hit lymphoma (DHL) were identified.
Overall, 32.1% (9/28) of patients completed all 6 cycles. At the clinical cut-off date (30 September 2020), 28.6% (8/28) were still under treatment; 3 patients withdrew consent due to personal reasons.
Efficacy
Efficacy data and exposure time for each patient are shown in Figure 2. Best response (CR or PR) was achieved at the first assessment in most cases (84.2%, 16/19). The investigator-assessed responses of the entire cohort and treatment regimen subgroups are summarized in Table 2. In the entire cohort, the BOR rate was 67.9% (95% CI: 47.7% to 84.1%), including 25.0% CRs and 42.9% PRs. Five patients (17.9%) achieved SD and 4 (14.3%) had PD. The BOR rate of the pola-BR cohort was higher than that of the pola-R cohort (83.3% vs. 56.3%). The efficacy in biomarker subgroups is presented in Table 3. The BOR rates were 70.6% and 54.5% (6/11) in non-GCB subtype and DEL patients, respectively. Even in the prior CAR-T therapy failure cohort (n=7), 2 patients achieved CR and 2 had PR.
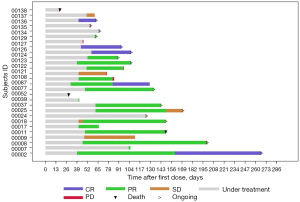
Table 2
| Outcome | All patients (n=28) | Pola-BR (n=12) | Pola-R (n=16) |
|---|---|---|---|
| Best response, n (%) | |||
| CR | 7 (25.0) | 2 (16.7) | 5 (31.3) |
| PR | 12 (42.9) | 8 (66.7) | 4 (25.0) |
| SD | 5 (17.9) | 1 (8.3) | 4 (25.0) |
| PD | 4 (14.3) | 1 (8.3) | 3 (18.8) |
| BOR rate, % (95% CI) | 67.9 (47.7–84.1) | 83.3 (51.6–97.9) | 56.3 (29.9–80.3) |
Pola-BR, polatuzumab vedotin combined with bendamustine-rituximab; Pola-R, polatuzumab vedotin combined with rituximab; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; BOR, best overall response; CI, confidence interval.
Table 3
| Outcome | Non-GCB | DEL |
|---|---|---|
| n | 17 | 11 |
| BOR rate, n (%) | 12 (70.6) | 6 (54.5) |
| CR rate, n (%) | 5 (29.4) | 5 (45.5) |
Non-GCB, non-germinal center B-cell-like; DEL, double expression lymphoma; BOR, best overall response; CR, complete response.
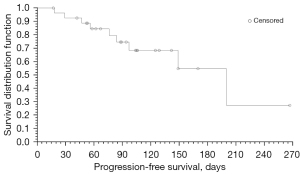
Progression-free survival (PFS) was summarized as median survival time estimated by the Kaplan-Meier method with 95% Greenwood’s CI (Figure 3). In all, there were 9 (32.1%) PFS events, and 19 (67.9%) patients were censored. With a median follow-up of 94.5 days, the estimated median PFS of all patients was 200 [95% CI: 97 to not evaluable (NE)] days.
Safety
The AEs of all grades and grade 3–4 are presented in Table 4. Totally, 75.0% of patients (21/28) experienced at least 1 AE (any grade), and 35.7% had at least 1 grade 3–4 AE. A higher proportion of patients in the pola-BR cohort compared with the pola-R cohort experienced AEs of any grade (91.7% vs. 62.5%). However, the incidence of grade 3–4 AEs was similar in both groups (33.3% vs. 37.5%). Most AEs were hematological, and the most common (≥10% in all patients) were thrombocytopenia (32.1%), neutropenia (28.6%), anemia (10.7%), fever (14.3%), and PN (10.7%). The vast majority of patients recovered after symptomatic treatment or observation.
Table 4
| AEs | All patients (n=28), n (%) | Pola-BR (n=12), n (%) | Pola-R (n=16), n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | |||
| Hematological | ||||||||
| Thrombocytopenia | 9 (32.1) | 5 (17.9) | 4 (33.3) | 3 (25.0) | 5 (31.3) | 2 (12.5) | ||
| Neutropenia | 8 (28.6) | 6 (21.4) | 3 (25.0) | 3 (25.0) | 5 (31.3) | 4 (25.0) | ||
| Anemia | 3 (10.7) | 3 (10.7) | 1 (8.3) | 1 (8.3) | 2 (12.5) | 1 (6.3) | ||
| Febrile neutropenia | 2 (7.1) | 2 (7.1) | 1 (8.3) | 1 (8.3) | 1 (6.3) | 1 (6.3) | ||
| Leukopenia | 1 (3.6) | 1 (3.6) | – | – | 1 (6.3) | 1 (6.3) | ||
| HLH | 1 (3.6) | 1 (3.6) | 1 (8.3) | 1 (8.3) | – | – | ||
| Non-hematological | ||||||||
| IRR | 1 (3.6) | – | – | – | 1 (6.3) | – | ||
| Fever | 4 (14.3) | – | 1 (8.3) | 1 (8.3) | 3 (18.8) | – | ||
| PN | 3 (10.7) | – | 1 (8.3) | 1 (8.3) | 2 (12.5) | – | ||
| Anorexia | 2 (7.1) | – | 2 (16.7) | 2 (16.7) | – | – | ||
| Lung infection | 1 (3.6) | – | – | – | 1 (6.3) | – | ||
| Anorectal infection | 1 (3.6) | – | 1 (8.3) | 1 (8.3) | – | – | ||
| Dyspnea | 1 (3.6) | 1 (3.6) | – | – | 1 (6.3) | 1 (6.3) | ||
| Ileus | 1 (3.6) | 1 (3.6) | – | – | 1 (6.3) | 1 (6.3) | ||
| Bone pain | 1 (3.6) | – | – | – | 1 (6.3) | – | ||
| Hypersomnia | 1 (3.6) | – | 1 (8.3) | 1 (8.3) | – | – | ||
| Hematuria | 1 (3.6) | – | – | – | 1 (6.3) | – | ||
| Mucositis oral | 1 (3.6) | – | 1 (8.3) | 1 (8.3) | – | – | ||
| Hypokalemia | 1 (3.6) | 1 (3.6) | – | – | 1 (6.3) | 1 (6.3) | ||
| Alopecia | 1 (3.6) | – | – | – | 1 (6.3) | – | ||
| Skin rash | 1 (3.6) | – | – | – | 1 (6.3) | – | ||
| Any AE | 21 (75.0) | 10 (35.7) | 11 (91.7) | 4 (33.3) | 10 (62.5) | 6 (37.5) | ||
AE, adverse event; Pola-BR, polatuzumab vedotin combined with bendamustine-rituximab; Pola-R, polatuzumab vedotin combined with rituximab; IRR, infusion-related reaction; HLH, hemophagocytic lymphohistiocytosis; PN, peripheral neuropathy.
There were SAEs reported in 4 patients, including 1 each who died due to PD in cycle 1, experienced grade 4 hemophagocytic lymphohistiocytosis in cycle 3, developed dyspnea in cycle 1, and had an ileus in cycle 4. All SAE cases recovered after supportive treatment except the death case. We observed AESI in 6 patients with grade 3–4 neutropenia. A patient dropped out due to grade 4 thrombocytopenia. Grade 1 PN occurred in 3 cases, manifesting as finger numbness.
Discussion
In patients with R/R DLBCL, salvage therapy followed by high-dose chemotherapy and ASCT may offer a second chance of cure, and only 30–40% of patients who proceed to transplantation are cured (4,9). Meanwhile, the majority of R/R patients are ineligible for ASCT due to age, co-morbidities, and/or chemotherapy-insensitive disease. The outcomes of those transplantation-ineligible patients remain poor, with median OS of approximately 6 months (4,10,11). There are no curative options, and the standard of care after the second-line chemotherapy remains to be improved.
Pola obtained accelerated approval by the U.S. Food and Drug Administration in June 2019 based on a phase II randomized controlled study (GO29365), which showed that-BR confers an additional 23.5% CR rate improvement in R/R DLBCL versus BR alone (40% vs. 17.5%) (12). The updated survival result showed that the pola-BR arm achieved a significantly prolonged OS compared to the BR arm (12.4 vs. 4.7 months) (13). However, pola is not approved in China, and there is still a gap in efficacy and safety data for pola in Chinese patients.
It is gratifying that the compassionate use of pola has been approved and implemented in China since 2019. The unauthorized product would be provided to patients with R/R DLBCL who have exhausted all therapeutic options and undergone at least 2 prior lines of therapy in this program.
By retrospectively analyzing the clinical data of the first 28 patients enrolled in this CUP in all 4 institutions in China, we primarily confirmed the efficacy and tolerability of the pola-BR regimen in heavily treated relapsed and refractory cases. Furthermore, the inclusion criteria of CUP were not very strict, and patients in urgent clinical need but not meeting the inclusion and exclusion criteria of ongoing pola clinical trials could also be enrolled. Therefore, these data could complement findings from clinical trials of pola.
Compared with the approved study, the baseline characteristics showed that the patients of this cohort had a worse prognostic profile. The median number of prior treatment lines was 3 (range, 2 to 6), yet patients in the GO29365 study received a median of 2 lines of prior treatment (12). Totally, 82.1% (23/28) of cases in this study were refractory to the last therapy, which was much higher than the 75% determined in the approved study. This may partly explain the lower best CR rate of 25% versus 50% in the clinical study (12).
Furthermore, 57.1% (16/28) of the patients in this study only received the pola-R regimen without bendamustine due to tolerability or economic reasons. The previous Romulus study revealed that the ORR of the pola-R combined regimen in the treatment of R/R DBLCL was only 53.8%, for a CR rate of 20.5% (14). Similarly, in this study, the BOR rates of these 2 cohorts were 56.3% (pola-R) and 83.3% (pola-BR). However, in the German CUP cohort, the improved best ORR (53% vs. 40%) of pola-R versus pola-R-chemo did not translate into differences in OS (P=0.84). Therefore, the benefit of added chemotherapy in this heavily treated population with late-stage disease requires further investigation.
The non-GCB subtype and DEL status are generally considered high-risk factors for DLBCL, leading to poor prognosis and difficulty in treatment. In this study, 17 patients with non-GCB subtype achieved a BOR rate of 70.6%, and a CR rate of 29.4%. Among 11 patients with DEL status, 54.5% (6/11) responded to pola-BR treatment, including 45.5% (5/11) who achieved CR. Hence, subgroup analyses revealed that the pola-based regimen is also effective in high-risk patient populations. Similar results were observed in the Taiwan CUP cohort, which showed non-GCB patients had even better ORR (69.2% vs. 30.8%) and OS (NR vs. 6.23 months) compared with the GCB subgroup (15).
Notably, the present study cohort also included 7 patients administered pola for disease relapse after CAR-T therapy; of these, 4 (57.1%) responded, including 2 each who achieved CR and PR. This corroborates findings by the German CUP cohort reporting a BOR rate of 58.3% in patients after CAR-T failure (16).
Due to the short follow-up period, only 9 progression events (9/28, 32.1%) occurred until the data cutoff time; indeed, outcome data in this analysis were not mature, and longer follow-up is required for a more accurate median PFS time. Considering the limitations of the relatively small sample size and short follow-up time, subsequent long-term follow-up and more mature data are warranted. Nevertheless, these preliminary results are encouraging, and indicate the promising efficacy and tolerable safety of pola-based therapy in Chinese patients.
Acknowledgments
We are grateful to the patients and their families, study investigators, study coordinators, and support staff. The authors also appreciate the academic support from the AME Lymphoma Collaborative Group.
Funding: This study was supported by the National Nature Science Foundation of China (Nos. 81960043, and 81970183) and the Thousand Talents Plan in the Central Plains of China - Leading Talents in Basic Research.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4593/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4593/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4593/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional ethics committee of Jiangsu Cancer Hospital (approval ID: 2019-062-02). Informed consent was waived by the ethics committee due to the retrospective nature of the study. First Affiliated Hospital of Hainan Medical University, Henan Cancer Hospital, and the First Affiliated Hospital of Nanchang University were informed and agreed with the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do? Hematology Am Soc Hematol Educ Program 2016;2016:366-78. [Crossref] [PubMed]
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:498-505. [Crossref] [PubMed]
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800-8. Erratum in: Blood 2018;131:587-8. [Crossref] [PubMed]
- Malecek MK, Watkins MP, Bartlett NL. Polatuzumab vedotin for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma. Expert Opin Biol Ther 2021;21:831-9. [Crossref] [PubMed]
- Shingleton JR, Dave SS. Polatuzumab Vedotin: Honing in on Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol 2020;38:166-8. [Crossref] [PubMed]
- Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 2009;114:2721-9. [Crossref] [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184-90. [Crossref] [PubMed]
- Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica 2013;98:1726-31. [Crossref] [PubMed]
- Czuczman MS, Trněný M, Davies A, et al. A Phase 2/3 Multicenter, Randomized, Open-Label Study to Compare the Efficacy and Safety of Lenalidomide Versus Investigator's Choice in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin Cancer Res 2017;23:4127-37. [Crossref] [PubMed]
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol 2020;38:155-65. [Crossref] [PubMed]
- Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv 2022;6:533-43. [Crossref] [PubMed]
- Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 2019;6:e254-e265. [Crossref] [PubMed]
- Wang YW, Tsai XC, Hou HA, et al. Polatuzumab vedotin-based salvage immunochemotherapy as third-line or beyond treatment for patients with diffuse large B-cell lymphoma: a real-world experience. Ann Hematol 2022;101:349-58. [Crossref] [PubMed]
- Liebers N, Duell J, Fitzgerald D, et al. Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Adv 2021;5:2707-16. [Crossref] [PubMed]
(English Language Editor: J. Jones)



