
Effect of Bushen Antai recipe on pyroptosis mechanism of subclinical hypothyroidism decidual cells in early pregnancy
Introduction
Subclinical hypothyroidism (SH) is a common endocrine disorder during pregnancy. Due to physiological changes during pregnancy, the prevalence is significantly higher in pregnant woman than in nonpregnant women. Thyroid hormone is a key hormone that promotes growth and development, neural differentiation and maturation, and maintains the normal metabolism of the body. A previous study has shown that untreated SH during pregnancy seriously threatens the health of the mother and the fetus (1). The nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome is closely related to placental inflammation-related diseases, and its abnormal activation can lead to the activation of Caspase-1 and the maturation of gasdermin D (GSDMD), inducing pyroptosis and accompanying inflammatory response, disrupting the physiological balance of the maternal-fetal interface, and leading to the occurrence of various adverse pregnancy events (2,3). In SH, elevated serum reactive oxygen species (ROS) may abnormally activate the NLRP3 inflammasome (4,5). However, there are no relevant reports on the occurrence of pyroptosis in subclinical hypothyroid decidual cells during pregnancy. According to the principle of prevention first and the traditional Chinese medicine (TCM) theory of “kidney governs reproduction”, this study used a rat model of SH during pregnancy to explore the therapeutic effect and mechanism of Bushen Antai recipe (BAR) on SH in early pregnancy and the relationship between SH and decidual cell pyroptosis during pregnancy, aiming to provide a certain scientific and theoretical basis for the study of the mechanism of BAR in the treatment of SH. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4079/rc).
Methods
This study was a randomized controlled animal study. A protocol was prepared before the study without registration.
Animals
A total of 90 8-week-old specific-pathogen free (SPF) Sprague Dawley (SD) rats were used in this study, including 60 females with a body weight of 220g±20 g, and 30 males with a body weight of 250±20 g (purchased from Changsha Tianqin Biotechnology Co., Ltd., license number: SCXK [Hunan] 2019-0014). The rats were raised in the SPF animal laboratory of the Clinical Research Center of the Affiliated Hospital of Guizhou Medical University. The indoor relative humidity was 60–70%, the temperature was 24 ℃, and the rats had free access to food and water. A 12-hour day-night alternating light cycle was used, and the cages were cleaned and disinfected regularly. This study was approved by the Ethics Review Committee of Laboratory Animals of Guizhou University of Traditional Chinese Medicine (approval No. 20210092). The animal experiments strictly abided by the “Manual for the Management and Use of Laboratory Animals” and the relevant standards of the “Guiding Opinions on Treating Laboratory Animals Kindly” issued by the Ministry of Science and Technology of the People’s Republic of China.
Drugs
Levothyroxine (L-T4) sodium tablets (Merck Pharmaceutical Co., Ltd., Darmstadt, Germany; import drug registration number: H20171152; specification: 50 µg/tablet) were converted into 0.95 µg/mL solution with distilled water in preparation for injection. The tablets were ground and dissolved in distilled water to prepare 0.45 µg/mL suspension. Calcium acetate (Kunming Bangning Pharmaceutical Co., Ltd., China) and BAR were purchased from the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (prescription composition: 20 g dodder seed, 10 g dipsaci radix, 10 g mulberry parasite, 10 g Ejiao, 8 g Amomum, 6 g Rehmannia glutinosa, 3 g ginseng). To prepare the concoction for nourishing the “kidney”, the medicinal materials were processed according to the national processing standards: the dodder seeds, succulents, mulberry parasitoids, Amomum, Rehmannia glutinosa, and ginseng were soaked in water for 30 minutes 6–8 times, then decocted twice, 1.5 hours each time, and the residue was discarded and the juice extracted. The alcoholized liquid and gelatinized liquid of donkey-hide gelatin were combined, filtered, refrigerated, and concentrated until there was no liquid. Distilled water was then added to control the pH at about 6.5 to 7 and to prepare a medicinal liquid with a crude drug concentration of 2 g/mL, which was sterilized for later use.
Agents
The following agents were used: rat interleukin-18 (IL-18) and interleukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) kits (Bioswamp, Wuhan, China; batch numbers RA20058 and RA20020, respectively); rat thyroid-stimulating hormone (TSH) and free thyroxine (FT4) ELISA kits (CUSABIO, Wuhan, China; batch numbers CSB-E05115r, C0104070199, CSB-E05079r, and C0104060198, respectively); radioimmunoprecipitation assay (RIPA) lysate and bicinchoninic acid (BCA) protein concentration assay kit (Beyotime, Shanghai, China; catalog numbers S1873 and P0013B, respectively); tetramethylethylenediamine (TEMED) electrophoresis reagent [China National Pharmaceutical Group Co., Ltd. (Sinopharm), Shanghai, China; catalog number 80125336]; sodium dodecyl sulfate (SDS) reagent (BioFroxx, Heidelberg, Germany; catalog number 3016642); phenylmethylsulfonyl fluoride (PMSF) protease inhibitor (Aladdin, Shanghai, China; catalog number P105539); polyvinylidene fluoride (PVDF) membrane (Millipore, Burlington, MA, USA; catalog number IPVH00010); rabbit-derived NLRP3 polyclonal antibody, rabbit-derived Caspase-1 polyclonal antibody, and rabbit-derived GSDMD polyclonal antibody (Affinity, Biosciences, Cincinnati, OH, USA; catalog numbers DF7438, AF5418, and AF4012, respectively); rabbit-derived glyceraldehyde 3-phosphate dehydrogenase (GAPDH) polyclonal antibody (Goodhere Biotech, Hangzhou, China; catalog number AB-P-R001); horseradish peroxidase (HRP)-labeled goat anti-rabbit immunoglobulin G (IgG) secondary antibody (Boster, Wuhan, China; catalog number BA1054); and enhanced chemiluminescence (ECL) substrate solution (APPLYGEN, Beijing, China; catalog number P1050). The rest of the reagents were commonly used laboratory specifications, and the water was purified water.
Instruments
The following instruments were used: ultrapure water machine (Shenzhen Yiliyuan Water Treatment Equipment Co., Ltd., China; model YL-100BU); vertical ultra-low temperature storage box (Haier Group, Qingdao, China; model DW-86L386); chromatography experiment freezer and automatic enzyme labeling instrument (BIOBASE, Jinan, China; model YC-2/EL10A); vortex mixer and horizontal shaker (Haimen Kylin-Bell Instrument Manufacturing Co., Ltd., Qilin, China; model XW-80A/TS-1); microplate constant temperature shaking instrument (Hangzhou Miu Instruments Co., Ltd., Hangzhou, China; model ST70-2); automatic plate washer (Beijing Top Analytical Instrument Co., Ltd., Beijing, China; model DEM-3); electrophoresis apparatus power supply, vertical electrophoresis tank, and electrophoresis apparatus (Beijing Liuyi Instrument Factory, Beijing, China; models DYY-7C, DYCZ-24DN, and DYCZ-40, respectively); pH meter (Mettler-Toledo GmbH, Giessen, Germany; model LP115); electronic balance (Sartorius Instrument System Co., Ltd., Beijing, China; model CPA); magnetic stirrer (Jintan Zhongda Instrument Factory, Jiangsu Province, China; model T8-1); and centrifuge (Hunan Xiangyi Laboratory Instrument Development Co., Ltd., Changsha, China; model HI650).
Experiment
Animal grouping and model replication
In accordance with previous studies (6,7), 60 female rats were randomly and equally divided into blank control group, model group, L-T4 group, high-dose BAR group, medium-dose BAR group, and low-dose BAR group (n=10). All rats were fasted for 12 hours before the operation. The rats then received 10% chloral hydrate (Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) (3 mL/kg) via intraperitoneal injection as anesthesia. Rats in the blank control group underwent pseudothyroidectomy, which only exposed the thyroid without damaging it, and were given ordinary feed and distilled water after the operation. The remaining 50 rats underwent total thyroidectomy and after the operation, given ordinary feed and water with 0.1% calcium acetate added. The physical condition of the rats was observed every morning and evening after the operation. After 1 month of recovery, the blank control group was given subcutaneous injection of 0.9% normal saline (1 mL/d), and the rats in the other groups were given L-T4 (0.95 µg/100 g/d). The SH rat model was prepared by subcutaneous injection. The injection time was fixed every day. After 3 weeks, blood was collected from the femoral vein of the rats to test thyroid function. Compared with the blank control group, the serum TSH levels of the other groups were increased, and there was no statistical difference in FT4 levels, indicating that the nonpregnant subclinical hypothyroid rats were successfully established. Afterwards, female rats and male rats were mated in cages at a ratio of 2:1. The next morning, vaginal smears from the female rats were observed under a microscope for sperm or vaginal plugs to confirm successful conception, with the time marked as pregnancy E0. The number of successful models in each group was as follows: 8 in the blank control group, 8 in the model group, 7 in the L-T4 group, 8 in the high-dose group, 7 in the medium-dose group, and 7 in the low-dose group.
Drug treatment
At 9:00 am every day during the early stage of pregnancy (E0–E7) (8), the blank control group and model group were given normal saline by gavage at a dose of 10 mL/kg, the L-T4 group was administered with L-T4 at a concentration of 0.45 µg/mL, L-T4 suspension was administered by gavage at a dose of 4.5 µg/kg, and BAR decoction with concentrations of 24, 12, and 6 g/kg per day were administered by gavage to the high-, medium-, and low-dose groups, respectively. The low dose for rats was equivalent to the dose for 70-kg adults [the equivalent dose for rats was calculated according to the formula: dose for rats (mg/kg) =6.3 × dose for adults (mg/kg)].
Specimen collection and preservation
After the final administration, all rats were fasted for 12 hours and then given 10% chloral hydrate (3 mL/kg) via intraperitoneal injection as anesthesia. Blood was collected, left standing at 4 ℃ for 30 minutes, centrifuged at 3,000 r/minute for 15 minutes, and the serum was separated and aliquoted into 1.5-mL Eppendorf tubes. After blood collection, the abdominal cavity was quickly exposed, and the pregnant rat uterus was isolated. The uterine wall was bluntly separated from the uterine horn to remove the embryos and placenta at the implantation site. The decidual tissue at the implantation site was scraped and washed with precooled phosphate-buffered saline (PBS), and the decidual tissue was then sucked up with a pipette and put into a sterilized cryopreservation tube. The rats were sacrificed by decapitation after removing the pregnant uterus.
Indicator detection
The content of TSH, FT4, IL-1β, and IL-18 in rat serum was detected by ELISA in strict accordance with the instructions of the ELISA kit. The frozen decidual tissue was prepared into tissue homogenate with lysate, and Western blot was used to detect the protein expression levels of NLRP3, Caspase-1 and GSDMD in decidual tissue homogenates. Due to the low quality of the decidua of some pregnant rats, the samples were degraded during repeated detection. Finally, the decidual tissue of 6 pregnant rats in each group was selected for Western blot detection. In this study, the relationship between BAR and pyroptosis was evaluated by GSDMD.
Data processing and statistical analysis
IBM SPSS statistics 26.0 statistical software was used for data analysis, and continuous data are expressed as mean ± standard deviation. For normally distributed data, t-test was used for comparison between 2 groups, and one-way analysis of variance (ANOVA) was used for comparison between multiple groups. Levene’s test was used for homogeneity of variance, least significant difference (LSD) t-test was used for homogeneity of variance, and Dunnett’s test was used when variance was not uniform. Nonparametric test was used if data were not normally distributed. P<0.05 was the standard for statistical significance.
Results
The effect of BAR on serum TSH, FT4, IL-1β, and IL-18 levels of rats in each group
There was no statistically significant difference in serum FT4 among the rats in each group (P>0.05). Compared with the control group, the level of serum TSH in the model group was significantly higher (P<0.05). TSH in the low-dose BAR group was also significantly higher than that in the blank control group (P<0.05). Compared with the model group, after L-T4 or high, medium and low doses of BAR intervention treatment, serum TSH was significantly decreased, and the difference was statistically significant (P<0.05). Overall, TSH levels among dose groups were comparable (P>0.05), although the low-dose group was significantly higher than the high-dose group (P<0.05). There was no statistical difference in TSH levels between the high- and medium-dose groups and the L-T4 group (P>0.05). Details are listed in Table 1.
Table 1
| Groups | FT4 (pmol/L) | TSH (μIU/mL) | IL-1β (pg/mL) | IL-18 (pg/mL) |
|---|---|---|---|---|
| Blank control group (n=10) | 6.96±0.98 | 0.63±0.07 | 390.05±67.62 | 812.93±76.99 |
| Model group (n=10) | 6.23±0.39 | 1.04±1.44ac | 500.80±78.95a | 941.08±110.94a |
| L-T4 group (n=10) | 6.97±0.86 | 0.67±0.08b | 375.20±100.90b | 818.48±69.29b |
| BAR low dose (n=10) | 6.23±0.55 | 0.83±0.14abc | 472.05±52.42 | 992.48±108.13ac |
| BAR medium dose (n=10) | 6.85±0.75 | 0.72±0.13bd | 447.17±101.34d | 955.01±196.39ac |
| BAR high dose (n=10) | 6.94±0.77 | 0.63±0.12bd | 393.52±74.62bd | 800.12±78.55bd |
| F value | 1.832 | 15.016 | 3.003 | 4.209 |
| P value | 0.129 | <0.01 | 0.022 | <0.01 |
Data are presented as mean ± standard deviation. a, compared with the blank control group, P<0.05; b, compared with the model group, P<0.05; c, compared with the high-dose group, P<0.05; d, compared with the L-T4 group, P>0.05. FT4, free thyroxine; TSH, thyroid-stimulating hormone; IL-1β, interleukin-1β; IL-18, interleukin-18; L-T4, levothyroxine; BAR, Bushen Antai recipe.
Compared with the blank control group, IL-1β and IL-18 in the model group were significantly increased (P<0.05). Compared with the model group, IL-1β and IL-18 in the L-T4 group and the high-dose BAR group were significantly decreased (P<0.05). The medium- and low-dose groups were significantly decreased (P<0.05), and IL-1β had no statistical difference (P>0.05). There was no significant difference with the L-T4 group (P>0.05).
Effects of BAR on NLRP3, Caspase-1 and GSDMD protein levels in the decidua of rat uterus in each group
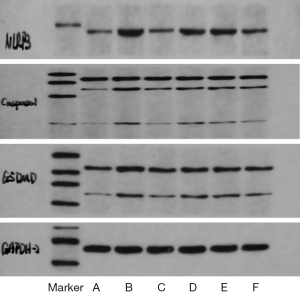
Compared with the blank control group, the expression of NLRP3, Caspase-1, and GSDMD proteins in the decidua of the uterus of pregnant rats of the model group was significantly higher (P<0.01). Compared with the model group, the protein contents of NLRP3 and GSDMD in the decidual tissue of rats in each intervention group were significantly decreased (P<0.05). High- and medium-dose BAR and L-T4 significantly decreased the expression of Caspase-1 in membranous tissue (P<0.05), while the difference in level of Caspase-1 between the low-dose group and the model group was not significant (P>0.05). The expression levels of NLRP3, Caspase-1, and GSDMD showed a certain dose-effect relationship. Compared with the medium- and low-dose groups, the high-dose group had the best reduction effect, and the difference was statistically significant (P<0.05). The protein expression levels of NLRP3, Caspase-1, and GSDMD in decidual tissue were similar in the medium- and low-dose groups, with no statistically significant difference (P>0.05). There was no statistical difference in the protein expression levels of NLRP3, Caspase-1, and GSDMD (P>0.05). Details are listed in Table 2 and illustrated in Figure 1.
Table 2
| Groups | NLRP3 (μg/mL) | Caspase-1 (μg/mL) | GSDMD (μg/mL) |
|---|---|---|---|
| Blank control group (n=6) | 0.227±0.101 | 0.226±0.070 | 0.613±0.043 |
| Model group (n=6) | 0.772±0.120ac | 0.742±0.077ac | 1.020±0.100ac |
| L-T4 group (n=6) | 0.383±0.145b | 0.408±0.095b | 0.702±0.089b |
| BAR low dose (n=6) | 0.596±0.080bc | 0.642±0.124c | 0.883±0.082bc |
| BAR medium dose (n=6) | 0.595±0.118bc | 0.597±0.116bc | 0.803±0.056abc |
| BAR high dose (n=6) | 0.432±0.208bd | 0.404±0.100bd | 0.688±0.095bd |
| F value | 16.312 | 22.253 | 21.001 |
| P value | <0.01 | <0.01 | <0.01 |
Data are presented as mean ± standard deviation. a, compared with the blank control group, P<0.01; b, compared with the model group, P<0.05; c, compared with the high-dose group, P<0.05; d, compared with the L-T4 group, P>0.05. NLRP3, nucleotide-binding oligomerization domain-like receptor protein 3; L-T4, levothyroxine; GSDMD, gasdermin D; BAR, Bushen Antai recipe.
Discussion
SH during pregnancy refers to an endocrine disorder in which insufficient synthesis or release of thyroid hormones during pregnancy results in serum TSH higher than the normal range and FT4 at normal levels. During pregnancy, the placenta secretes a large number of hormones, which places the hypothalamus-pituitary-thyroid axis in a state of stress. As pregnant women experience physiological metabolic changes, they are especially prone to thyroid secretion disorders. A previous study has shown that the occurrence rate of SH during pregnancy is 3–5%, and there is an increasing trend (9). Thyroid hormones are essential for maintaining the development of fetal systems. However, fetal thyroid function does not begin to be established until 12–14 weeks of gestation, so the fetus’s need for thyroid hormones in early pregnancy is completely dependent on maternal supply (10). Therefore, it is very important to ensure that patients with SH during pregnancy receive treatment as soon as possible in the first 12 weeks of pregnancy. Western medicine mainly uses exogenous thyroid hormone replacement therapy, but hormone therapy is prone to drug-induced hyperthyroidism or hypothyroidism and side effects. However, TCM has the advantages of multisystem and multitarget overall regulation, has fewer side effects, and is safer. In view of this, it is useful to explore the efficacy and mechanism of TCM in the treatment of SH during pregnancy.
According to the clinical manifestations and pathogenesis of adverse pregnancy outcomes caused by SH, TCM attributes the disease to the categories of “fetal leakage”, “uneasy fetal movement”, “slippery tire”, “consumption of labor”, and “gall disease”. In this case, the disease is located in the “kidney” (11). The TCM classic “Fu Qing Governs Women’s Subjects” states that “the kidney water is sufficient and the fetus is safe, and the kidney water is deficient and the fetus moves”. The “kidney” is the innate foundation, storing the essence of the whole body and absorbing the qi of the five Zang organs. If the innate qi endowment of the “kidney” is insufficient and the kidney essence is deficient, the five Zang organs will lose nourishment, function will be weakened, and disease will occur. If the warmth of the “kidney” is insufficient, it will be transported and transformed. The organ where the thyroid axis is located may be hypofunctional due to insufficient nourishment, resulting in dystrophy of the uterus and eventually fetal atrophy and even threatened abortion (12). Therefore, based on the TCM theory of “prevention before disease, and prevention of change after disease”, for SH in early pregnancy, the treatment should tonify the “kidney”, nourish the blood, and repair the fetus.
BAR is composed of dodder, Sangjiji, Tukuduan, Ejiao, Amomum, Rehmannia glutinosa, and ginseng, of which dodder is the monarch, or main, medicine, tonifying the kidney and nourishing essence. Sangjie, Tsubishi, and Rehmannia can strengthen the function of dodder to nourish the liver and kidney, strengthening fetal qi and solidifying Chongren. Donkey-hide gelatin nourishes yin and blood and makes Chongren blood prosperous, solidifying the fetal qi. Amomum glutinosa and ginseng tonify and promote qi and prevent miscarriage. The compatibility of various medicines can synergistically play the role of invigorating the kidney and prevent miscarriage. In this study, it was found that after intervention of high, medium and low doses of BAR, serum TSH of rats was significantly lower than that of the model group. The results were consistent with some previous studies (13,14), indicating that BAR could effectively reduce TSH, improve the thyroid function of patients with SH during pregnancy, and promote the endocrine balance of pregnant women with SH.
The decidual tissue of the uterus plays an indispensable role in the maintenance of pregnancy, and its abnormal inflammation can affect the implantation and development of the embryo and the process of endometrial decidualization, eventually leading to a variety of adverse pregnancy events (15,16). The NLRP3 inflammasome is a high-molecular-weight protein complex that coordinates the inflammatory response. It is composed of NLRP3 protein, apoptosis-associated speck-like protein (ASC), and Caspase-1 (17), which activates NLRP3 inflammation. The danger signals of the body are very extensive, including lysosomal cleavage and ROS generation. ROS activate the NLRP3 inflammasome through assembly, and then activate Caspase-1. Pro-IL-1β, pro-IL-18, and other inflammatory precursors are cleaved to promote the maturation and release of downstream inflammatory factors IL-1β and IL-18, while GSDMD protein is cleaved to expose the GSDMD-N-terminal domain. Pore formation induces pyroptosis, which eventually leads to lytic cell death (18,19). The results of this study showed that the expression levels of NLRP3, Caspase-1, GSDMD, and serum IL-1β and IL-18 in the decidual tissue of pregnant rats in the model group were significantly higher than those in the blank control group, indicating that the decidual cells of the pregnant rats with SH during pregnancy were significantly increased. Pyroptosis occurred and there was a high inflammatory response in pregnant rats, which may be an important reason for the adverse pregnancy outcomes of SH during pregnancy. After the intervention of “kidney”-tonifying, the serum levels of IL-1β and IL-18 of pregnant rats in the high-dose group were significantly decreased, and the expression levels of NLRP3, Caspase-1, and GSDMD in the decidual tissue of the pregnant rats in the medium- and high-dose groups were also significantly decreased. These results suggested that BAR could effectively inhibit the pyroptosis of decidual cells and improve the balance of inflammatory indexes. Therefore, the cause of poor pregnancy outcomes in SH in early pregnancy may be closely related to decidual cell pyroptosis. BAR could promote the return of thyroid hormones to normal in early pregnancy and also effectively reduce and inhibit decidual cell pyroptosis and inflammation level in pregnant rats. It is speculated that these are the possible mechanisms through which BAR improves pregnancy outcomes in patients with SH during pregnancy.
This study had some limitations. As an animal model study, a model corresponding to TCM dialectics could not be established, but the effect of TCM prescriptions on SH during pregnancy could be objectively observed. However, there may be significant differences between clinical hypothyroidism in rats and humans, and the current research results cannot be inferred to humans, which should be observed in future clinical studies.
In conclusion, BAR could improve the TSH level of SH during pregnancy, inhibit the pyroptosis of decidual cells, decrease the expression of inflammatory factors, and relieve the decidual tissue damage of rats with SH during pregnancy. This study enriched understanding of the mechanism of BAR in the treatment of this disease and provided a preliminary basis for the TCM treatment of SH during pregnancy. Based on our study and previous studies, it is recommended that BAR be used in selected patients.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 82060881), Guizhou Science and Technology Support Program Project (Qianke Joint [2021] Support General 021), and Special Project on Cultivation and Innovation of New Academic Seedlings in Guizhou University of Traditional Chinese Medicine (Talents of Guizhou Science and Technology Cooperation Platform [2018]5576-02).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4079/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4079/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4079/coif). All authors report that this study was supported by the National Natural Science Foundation of China (No. 82060881), Guizhou Science and Technology Support Program Project (Qianke Joint [2021] Support General 021), and Special Project on Cultivation and Innovation of New Academic Seedlings in Guizhou University of Traditional Chinese Medicine (Talents of Guizhou Science and Technology Cooperation Platform [2018]5576-02). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Review Committee of Laboratory Animals of Guizhou University of Traditional Chinese Medicine (approval No. 20210092). The animal experiments strictly abided by the “Manual for the Management and Use of Laboratory Animals” and the relevant standards of the “Guiding Opinions on Treating Laboratory Animals Kindly” issued by the Ministry of Science and Technology of the People’s Republic of China.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu P, Chu R, Pan S, et al. Impact of TPOAb-negative maternal subclinical hypothyroidism in early pregnancy on adverse pregnancy outcomes. Ther Adv Endocrinol Metab 2021;12:20420188211054690. [Crossref] [PubMed]
- Sano M, Shimazaki S, Kaneko Y, et al. Palmitic acid activates NLRP3 inflammasome and induces placental inflammation during pregnancy in rats. J Reprod Dev 2020;66:241-8. [Crossref] [PubMed]
- Fang X, Wang Y, Zhang Y, et al. NLRP3 Inflammasome and Its Critical Role in Gynecological Disorders and Obstetrical Complications. Front Immunol 2020;11:555826. [Crossref] [PubMed]
- Novakovic TR, Dolicanin ZC, Djordjevic NZ. Effects of maternal subclinical hypothyroidism on amniotic fluid cells oxidative status. Reprod Toxicol 2018;78:97-101. [Crossref] [PubMed]
- Evavold CL, Hafner-Bratkovič I, Devant P, et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell 2021;184:4495-4511.e19. [Crossref] [PubMed]
- Ercin ME, Erdil G. Effect of single-dose depot leuprolide acetate on embryonal implantation: an experimental rat model. Gynecol Endocrinol 2020;36:611-4. [Crossref] [PubMed]
- Koullali B, Zhang Y, Peterson A, et al. Cervical Augmentation with an Injectable Silk-Based Gel: Biocompatibility in a Rat Model of Pregnancy. Reprod Sci 2020;27:1215-21. [Crossref] [PubMed]
- Camilleri C, Beiter RM, Puentes L, et al. Biological, Behavioral and Physiological Consequences of Drug-Induced Pregnancy Termination at First-Trimester Human Equivalent in an Animal Model. Front Neurosci 2019;13:544. [Crossref] [PubMed]
- Zhang F, Lin X, Liu A, et al. Maternal Subclinical Hypothyroidism in Rats Impairs Spatial Learning and Memory in Offspring by Disrupting Balance of the TrkA/p75NTR Signal Pathway. Mol Neurobiol 2021;58:4237-50. [Crossref] [PubMed]
- Thompson W, Russell G, Baragwanath G, et al. Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: A systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018;88:575-84. [Crossref] [PubMed]
- Yang GY, Luo H, Liao X, et al. Chinese herbal medicine for the treatment of recurrent miscarriage: a systematic review of randomized clinical trials. BMC Complement Altern Med 2013;13:320. [Crossref] [PubMed]
- Springer D, Jiskra J, Limanova Z, et al. Thyroid in pregnancy: From physiology to screening. Crit Rev Clin Lab Sci 2017;54:102-16. [Crossref] [PubMed]
- Kou YQ, Li JF, Wang LZ. The efficacy of Bushen Yangxue Antai Decoction in the treatment of threatened abortion complicated with hypothyroidism and its effects on pregnancy hormone levels and thyroid function. Chinese Journal of Experimental Traditional Medical Formulae 2018;24:175-9.
- Xu L, Chen Y, Bian WH, et al. Clinical study on the effects of invigorating spleen, nourishing kidney and warming yang method on TSH and TPOAb in subclinical hypothyroidism in early pregnancy. Chinese Archives of Traditional Chinese Medicine 2019;37:2042.
- Ng SW, Norwitz GA, Pavlicev M, et al. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int J Mol Sci 2020;21:4092. [Crossref] [PubMed]
- Saito Reis CA, Padron JG, Norman Ing ND, et al. High-mobility group box 1 is a driver of inflammation throughout pregnancy. Am J Reprod Immunol 2021;85:e13328. [Crossref] [PubMed]
- Wang L, Hauenstein AV. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol Aspects Med 2020;76:100889. [Crossref] [PubMed]
- Moretti J, Blander JM. Increasing complexity of NLRP3 inflammasome regulation. J Leukoc Biol 2021;109:561-71. [Crossref] [PubMed]
- Bai B, Yang Y, Wang Q, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis 2020;11:776. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


