
Development and validation of a novel model based on hand knob score and white matter injury on MRI to predict hand function in children with cerebral palsy
Introduction
Cerebral palsy (CP) is a group of permanent movement and posture development disorders causing activity limitations that are attributed to non-progressive disturbances occurring in the developing fetal or infant brain. With a prevalence rate of 0.15–0.40%, CP currently affects approximately 17 million patients worldwide. CP is also one of the most frequent causes of motor disability in children (1). According to the American Classification of Permanent Functional Disability, human upper extremity function accounts for 60% of total body function, while hand function accounts for 90% of upper extremity function. Hand function is the most influential component of motor function and is essential for daily activities, communication, and independent social competence. At least two-thirds of children with CP have unilateral or bilateral upper extremity dyskinesia. Limited hand function in childhood is considered one of the strongest predictors of the ability to participate in daily activities as children with CP reach adulthood (2).
The manual ability classification system (MACS) is currently the most commonly used method for grading hand function in children with CP (3). However, it is highly subjective in manual grading (4), which is not favorable for objective assessment or comprehensive understanding of hand function and is not conducive to the development of a scientific and rational training program or to pertinently enhancing hand function training. The assessment of MACS is completed by physicians, physicists or people who take care of children with cerebral palsy by observing the daily movement level of children with cerebral palsy with the naked eye. The assessment process is highly subjective, and the assessment results are greatly influenced by the experience of the assessor. Thus, identifying more objective evaluation indicators of hand function is an important clinical issue that needs to be resolved.
Neuroimaging studies have confirmed that hand dysfunction often involves multiple circuits of motor function (5-7). The hand motor area is mainly restricted to a specific part of the precentral gyrus called the hand knob, and lesions in this area are associated with hand dysfunction (8-10). Moreover, hand knob infarction can lead to isolated chiral paralysis (9,11). In a study on the relationship between the morphological changes of the hand knob and hand function in glioma patients, Liang et al. identified a link between the morphological changes of the hand knob and hand dysfunction and proposed that the width, height, and shortest distance from the tumor to the hand knob are three anatomical biomarkers associated with preoperative hand motor impairment (6,8).
Therefore, it follows that hand knob morphology correlates with hand function, and the hand knob is an important control center for hand movement (12). At present, few studies have linked the structural indicators and morphological alterations of hand knobs with hand function in children with CP. This study aimed to analyze the relationship between hand knob morphology, structural indicators, and hand function in children with CP, and to construct an objective hand function evaluation model and visualize it as a nomogram. We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4112/rc).
Methods
Participants
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee for biomedical research of the Affiliated Hospital of Zunyi Medical University (No. KLLY-2020-102). Children with CP and their guardians were informed of the corresponding rights and obligations. Their guardians signed the informed consent form.
Children who visited the Affiliated Hospital of Zunyi Medical University and were clinically diagnosed with CP from May 2019 to September 2021 were consecutively collected. A total of 70 children with CP were finally enrolled in this study. We did not perform formal sample size calculations because all cohort studies are ongoing studies. Also, there are no generally accepted approaches to estimate the sample size requirements for derivation and validation studies of prediction models.
Inclusion criteria
Children aged between 2 and 12 years with clinically diagnosed CP.
Exclusion criteria
(I) Children with contraindications to magnetic resonance imaging (MRI) examination; (II) those with a history of upper extremity surgery; (III) children with a history of botulinum treatment on upper limbs within 6 months; (IV) those with a history of neurological disease: trauma, tumor, infection; (V) absence or incomplete clinical data; (VI) children with brain developmental malformation that made hand knob structure impossible to assess; and (VII) MR images with motion artifacts.
On the day of the MRI examination, the hand function of each child was evaluated by the child rehabilitation physician according to MACS without the MR results. In this study, children with MACS I, II, and III were defined as the mild hand function injury group, and those with MACS IV and V were defined as the severe hand function injury group.
Scanning parameters and precautions
To reduce the motion artifacts in MR images, all children who could not cooperate with MR examination autonomously were sleep deprived by their guardians the day before the examination. Children that were still unable to fully cooperate with MR examination were given 10% chloral hydrate (0.25–0.5 mL/kg, maximum dose 10 mL/day) orally 30 minutes before the scan, or an enema with phenobarbital injection (3–5 mg/kg). The parents were informed of the potential risks of chloral hydrate and phenobarbital, and the children were closely observed for adverse reactions. The examined children were tightly wrapped in a small quilt on the examination table to avoid getting cold. Their heads were fixed bilaterally with sponges to reduce motion artifacts. The child was required to be fully awake before being allowed to leave.
MR scans were acquired with a MRI scanner (3.0T HDxt, GE Healthcare, Milwaukee, WI) and an 8-channel cranial phased array coil. The MRI scan included axial T2 weighted image (T2WI), axial T2-fluid-attenuated inversion recovery (T2-FLAIR), and three-dimensional T1 weighted image (3D-T1WI) sequences (Table 1). The axial scanning positioning line was parallel to the anterior commissure-posterior commissure (AC-PC) line.
Table 1
| Sequence | TR (ms) | TE (ms) | FOV (mm) | SL (mm) |
|---|---|---|---|---|
| T2WI | 4,480 | 120 | 256×256 | 3 |
| T2-FLAIR | 7,500 | 140 | 240×240 | 2 |
| 3D-T1WI | 7.8 | 3.0 | 256×256 | 1 |
T2WI, T2 weighted image; T2-FLAIR, T2-fluid-attenuated inversion recovery; 3D-T1WI; three-dimensional T1 weighted image; TR, repeating time; TE, echo time; FOV, field of view; SL, slice thickness.
Evaluation of hand knob morphology and measurement of structural indexes
All 3D-T1 images were imported into RadiAnt software (https://www.radiantviewer.com) and translated and rotated so that the axial image was parallel to the AC-PC line.
According to the literature, there are five hand knob morphological variations in the axial plane: omega shape, epsilon shape, medially asymmetric epsilon, laterally asymmetric epsilon, and null (13). However, we identified a novel morphologic variation during the hand knob morphology evaluation, which had more than three fissures and exhibited multiple bulges, so we named it the multi-peaks shape (Figure 1).

The hand knob structure index included the hand knob width (the distance between the clefts on both ends of the hand knob), the hand knob height (the vertical distance along the line from the highest point of the hand knob to the width of the hand knob), the distance of the hand knob from the midline (the vertical distance of the hand knob midpoint to the brain midline), the hand knob cortical thickness (the distance from the surface of the hand knob cortex to the gray-white matter demarcation), and the hand knob white matter height (hand knob height minus hand knob cortical thickness) (Figure 2).

To reduce bias, two imaging physicians with extensive diagnostic experience (A and B) independently completed the morphological evaluation of the hand knob and structural index measurements. One week later, physician A conducted a re-evaluation and measurement. Relevant clinical information, such as age, gender, and symptoms, were not mentioned in the evaluation.
The MRI classification system (MRICS) of CP proposed by the Surveillance of Cerebral Palsy in Europe (SCPE) consists of five groups: maldevelopment, predominant white matter injury, predominant grey matter injury, miscellaneous, and normal finding (14). The maldevelopment group was excluded because the assessment and measurement of the hand knob were not effective. Finally, we classified the MR lesion types of children with CP into five groups: predominant white matter injury, predominant grey matter injury (including basal ganglia and/or thalamus lesions, cortical-subcortical lesions, etc.), white matter and gray matter injury, cerebellar injury, and normal findings.
Statistical analysis
Data were analyzed using IBM SPSS Statistics software (version 18.0; SPSS Inc., Chicago, IL) and R statistical software (version 3.6.3) (https://www.r-project.org). We used the sampling software package for randomization, the glmnet package for ridge regression, the pROC package for plotting receiver operating characteristic (ROC) curves, and the rms package for plotting nomograms and calibration curves. The Kappa consistency test and intraclass correlation coefficient (ICC) were used for consistency testing to analyze the consistency and stability within and between evaluators.
Construction and validation of the models
The study subjects were randomly sampled at a 2:1 ratio into training and validation sets, with MACS as a stratification variable in the random sampling.
Hand knob score model
To overcome the collinear relationship between the left- and right-hand knob index variables, MACS was treated as the outcome variable and the hand knob index was considered an independent variable and normalized in the training set. Using five-fold cross-validation, the corresponding one with the smallest variance was selected λ-values, a logistic ridge regression model was constructed, and the linear predictive values for the individual samples were calculated using the model coefficients. P<0.05 was considered statistically significant.
Clinical features model
Univariate and multivariate logistic regression models were built, with MACS as the outcome variable and clinical features (including age, gestational age, gender, clinical type of CP, and type of MRI lesion) as the independent variables. Selected factors with P<0.05 in the univariate logistic regression model were included in the multivariate logistic regression model, and the stepwise regression method was used to obtain the best model. Akaike’s information criterion (AIC) was chosen as the selection criterion for the model, and a lower AIC value indicated a better corresponding model. The goodness of fit was tested using the Hosmer-Lemeshow test.
A combined model of the hand knob score and clinical features
The MACS was used as the outcome variable, the hand knob score and clinical features were used as independent variables to establish univariate and multivariate logistic regression models, and stepwise regression was used to obtain the best model.
We plotted the ROC curves and calculated the area under the curve (AUC), sensitivity, specificity, positive predictive value, and negative predictive value for the training and validation sets, respectively. The best model was selected to establish a nomogram for assessing the degree of hand function impairment, and the Bootstrap method (the original data were repeatedly sampled 1,000 times) was used to internally validate the model and to draw a calibration curve.
Results
Demographic data
A total of 84 children with CP were finally enrolled in this study. To ensure the reliability of data, we excluded patients who had missing information on the key predictors. Among the 84 CP children, we excluded three due to unclear clinical type, three cases due to an unclear MACS, six cases due to failure of 3D-T1 sequence scan, one case due to severe artifacts of MR image, and one case due to cerebral cortex developmental deformity (Table 2).
Table 2
| Characteristics | CP children (n=70) |
|---|---|
| Age (years) | 7.3 (5.7–9.6) |
| Gestational age | |
| Term | 39 (55.7) |
| Preterm | 24 (34.3) |
| Unknown | 7 (10.0) |
| Gender | |
| Male | 40 (57.1) |
| Female | 30 (42.9) |
| Clinical types | |
| Spastic hemiplegia | 18 (25.7) |
| Spastic diplegia | 21 (30.0) |
| Spastic quadriplegia | 21 (30.0) |
| Dyskinetic | 4 (5.7) |
| Ataxia | 3 (4.3) |
| Mixed | 3 (4.3) |
| Hand function | |
| Mild impairment (MACS I, II, III) | 53 (75.7) |
| Severe impairment (MACS IV, V) | 17 (24.3) |
The data are shown as n (%) or medians (first and third quartiles). CP, cerebral palsy; MACS, manual ability classification system.
Stability and consistency of the hand knob assessment measurement
The morphological character assessment of the hand knob showed high intra-group consistency (right cerebral hemisphere: Kappa 0.856, P<0.001; left cerebral hemisphere: Kappa 0.864 P<0.001) and high inter-group consistency (right cerebral hemisphere: Kappa 0.806, P<0.001; left cerebral hemisphere: Kappa 0.813, P<0.001). The structural index measurements, including hand knob width, hand knob height, the distance of the hand knob from the midline, hand knob cortical thickness, and hand knob white matter height, showed high intra-group and inter-group consistency (Table 3).
Table 3
| Region | Consistency | Structure index | N | ICC | P |
|---|---|---|---|---|---|
| Right cerebral hemisphere | Intra-group | Width | 70 | 0.885 | <0.001 |
| Height | 70 | 0.851 | <0.001 | ||
| Distance from the midline | 70 | 0.892 | <0.001 | ||
| Cortical thickness | 70 | 0.779 | <0.001 | ||
| Inter-group | Width | 70 | 0.823 | <0.001 | |
| Height | 70 | 0.801 | <0.001 | ||
| Distance from the midline | 70 | 0.814 | <0.001 | ||
| Hand knob cortical thickness | 70 | 0.762 | <0.001 | ||
| Left cerebral hemisphere | Intra-group | Width | 70 | 0.892 | <0.001 |
| Height | 70 | 0.804 | <0.001 | ||
| Distance from the midline | 70 | 0.877 | <0.001 | ||
| Cortical thickness | 70 | 0.767 | <0.001 | ||
| Inter-group | Width | 70 | 0.812 | <0.001 | |
| Height | 70 | 0.804 | <0.001 | ||
| Distance from the midline | 70 | 0.846 | <0.001 | ||
| Cortical thickness | 70 | 0.760 | <0.001 |
ICC, intraclass correlation coefficient.
Construction and validation of the model
Hand knob score = −1.110 + 0.090 × the right distance from the hand knob to the midline (mm) + 0.011 × the right hand knob white matter height (mm) + 0.003 × right height of hand knob (mm) −0.170 × the left distance from the hand knob to the midline (mm) −0.116 × the left width of hand knob (mm) −0.032 × the left cortical thickness of hand knob (mm) −0.021 × the right cortical thickness of hand knob (mm) −0.009 × the right width of hand knob (mm) −0.001 × the left height of hand knob (mm). A logistic regression model with the hand knob score as the independent variable showed that the hazard of severe impairment of hand function was 85.344-fold for each unit increase in the hand knob score (OR =85.344, 95% CI: 2.257–3,227.226) (Table 4).
Table 4
| Factor | r | SE | Z | P | OR (95% CI) |
|---|---|---|---|---|---|
| Hand knob score | 4.447 | 1.853 | 2.399 | 0.016* | 85.344 (2.257–3,227.226) |
*, P<0.05, the difference is statistically significant. r, regression coefficient; SE, standard error; OR, odds ratio; CI, confidence interval.
The MRI lesion type was the statistically significant variable in univariate analysis and was included in multivariate logistic regression analysis. The results showed that the white matter injury type had a 0.048-fold risk of developing severe impairment of hand function compared with the other lesions type (OR =0.048, 95% CI: 0.009–0.272) (Table 5).
Table 5
| Factor | r | SE | Z | P | OR (95% CI) |
|---|---|---|---|---|---|
| Intercept | 0.357 | 0.493 | 0.724 | 0.469 | 1.429 (0.544–3.753) |
| MRI lesion type | |||||
| Others | 1 | ||||
| White matter injury | −3.031 | 0.882 | −3.438 | <0.001* | 0.048 (0.009–0.272) |
*, P<0.05, the difference is statistically significant. Others: including predominant grey matter injury, white matter and gray matter injury, cerebellar injury, and normal findings. r, regression coefficient; SE, standard error; MRI, magnetic resonance imaging; OR, odds ratio; CI, confidence interval.
The MRI lesion type and hand knob score were included in the multivariate logistic regression model, and a combined model of the hand knob score and clinical features was constructed. The OR values of the hand knob score and white matter injury were 8.633 and 0.052, respectively (Table 6).
Table 6
| Factor | r | SE | Z | P | OR (95% CI) |
|---|---|---|---|---|---|
| Intercept | 2.562 | 1.322 | 1.937 | 0.053 | 12.956 (0.970–172.971) |
| MRI lesion type | |||||
| Others | 1 | ||||
| White matter injury | −2.953 | 0.934 | −3.159 | 0.002* | 0.052 (0.008–0.326) |
| Hand knob score | 2.156 | 1.245 | 1.732 | 0.083 | 8.633 (0.753–99.017) |
*, P<0.05, the difference is statistically significant. Others: including predominant grey matter injury, white matter and gray matter injury, cerebellar injury, and normal findings. r, regression coefficient; SE, standard error; MRI, magnetic resonance imaging; OR, odds ratio; CI, confidence interval.
The ROC analysis results showed that the AUC of the hand knob score model for predicting the degree of hand function impairment was 0.752 (0.586–0.919) in the training set, that of the clinical features model was 0.819 (0.691–0.948), and that of the hand knob score and clinical features combined model was 0.880 (0.743–1.000). The AUC of the hand knob score model in the validation set was 0.765 (0.544–0.985), the clinical features model was 0.782 (0.561–1.000), and the combined model was 0.894 (0.724–1.000).The combined model discriminated better than the hand score model and the clinical features model, and no significant differences between the AUCs of the three models were observed (P>0.05) (Figure 3).

The best combined model of hand knob score and clinical features was selected using the AIC and visualized as a nomogram. The nomogram finally incorporated two assessment items: the hand knob score and white matter injury (Figure 4).
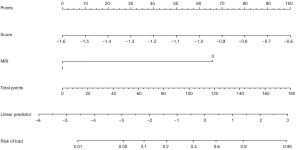
The calibration curve demonstrated that the predicted probability of the degree of hand function injury of the combined model of hand knob score and clinical features showed good agreement with the actual occurrence probability (Figure 5).

Discussion
In the hand knob score model, each one-unit increase in the hand knob score equated to an 85.344-fold increase in the risk of severe hand function impairment. In the clinical features model, the white matter injury type had a 0.048-fold risk of developing severe hand function impairment compared with the other lesion types. In the combined model of the hand knob score and clinical features, the OR values of the hand knob score and white matter injury were 8.633 and 0.052, respectively. The ROC analysis results showed that the AUC of the training set for the hand knob score model was 0.752, that of the clinical features model was 0.819, and that of the hand knob score clinical features combined model was 0.880. The combined model discriminated better than the other two models, and there were no significant differences between the AUCs of the three models (P>0.05).
Hand knob score model
Hand knob formation primarily involves a process of cortical folding that occurs during the second to the third trimester of gestation. Head injury from various causes in children with CP during this period can result in altered neuronal density, which affects neuronal migration and leads to variations in cortical folding. The distance of the hand knob from the midline of the left cerebral hemisphere serves as the strongest protective factor, and the greater this distance, the less severe the hand function impairment. The structures between the hand knob and the brain midline are mainly white matter in the corona radiata region as well as parts of the gyral structures, including the cingulate gyrus, which is mainly located in the medial aspect of the cerebral hemispheres. Reduced white matter volume or gyral atrophy can lead to a lesser distance of the hand knob from the midline.
Moreover, the width of the hand knob in the bilateral cerebral hemispheres is also a protective factor. Different structural regions of the hand knob play different roles in hand movement; caudal knobs are involved in the control of hand movement, whereas rostral knobs are involved in the choice of hand movement (15,16). Functional imaging study has shown that the motion of the hand is activated along the contour of the hand knob (17). Therefore, we have reason to believe that the increase of the width of the hand knob enhances the control and selection ability of the hand knob to participate in hand movement, thereby improving the ability of CP children’s hands to grasp and release the target object and increasing the coordination ability of both hands. In the hand knob score model, the cortical thickness of the hand knob in the bilateral cerebral hemispheres is the protective factor of hand function, and the height of white matter is the risk factor. The white matter height of the hand knob is calculated by subtracting the thickness of the cortex from the height of the hand knob. The height of the hand knob has the least influence on hand function, and then we have reason to believe that the thicker the hand knob cortex, the less impaired the hand function. Imaging studies of infants and young children suggest that cortical thickness may peak at 1–2 years, and by 2 years of age, the average cortical thickness is approximately 97% of that in adults (18-20).
The present study analyzed children with CP who were older than 2 years of age when the brain development is approaching that of adults. Previous study has found that the significant differences in the morphological characteristics of cortical thickness, cortical curvature, and sulcus depth are related to the functions of movement, cognition, vision, and communication (21). It has also been demonstrated that the stronger an individual’s willingness to move the hand, the thicker the cortical thickness of the hand knob (22). Humans and animal studies have shown that the thickness of the motor cortex increases with physical training (23), and these increases are related to more synapses per neuron (24). Although this increase in synapses may be associated with more downward driving, it can also increase the sensory regulation of motor cortical activity through connections to the thalamus or indirect connections to the somatosensory cortex. The increase of this sensory connection may lead to individuals with a thicker motor cortex having more motor decision-making sensitivity and increasing their motor agility, coordination ability, and other motor behaviors. The occurrence and development of many neurological and mental diseases are related to cortical thickness changes (25,26). Changes in the cortical thickness are associated with Parkinson’s disease, Huntington’s disease, multiple sclerosis, and lateral sclerosis, and dyskinesia is a prominent symptom of these diseases (27-29).
Clinical features model
In the clinical features model, the risk of severe hand function injury in CP children with white matter injury was 0.048 times that in other injury types, so white matter injury was found to be a strong predictor of mild hand function injury. Previous study has found that white matter injury is the most common type of cranial MRI injury in children with CP, and those with white matter injury are predominantly spastic-type CP, usually with mild motor impairment (30). Neuroimaging study has found that mild hand dysfunction is significantly associated with periventricular white matter lesions in children with hemiplegia, and those who present with periventricular white matter changes have mostly mild hand dysfunction (MACS grades I and II) (31). Individuals with gray matter lesions and basal ganglia and/or thalamus involvement are associated with more severe hand function impairment (32). Also, lesion location and extent are more strongly correlated with hand function in children with cortical and deep gray matter lesions than in children with periventricular white matter lesions (33). Basal ganglia involvement has a significant impact on sensorimotor control of grasping objects in children with CP; one reason for this is the critical role of these structures in sensorimotor integration, and this role cannot be compensated for by other brain structures (34).
In our clinical prediction model, we classified head injury primarily as white matter injury versus other injury types, and the results were consistent with several previous studies that demonstrated that white matter injury was a strong predictor of mild hand function impairment. In our clinical prediction model, we classified head injury mainly as white matter injury and other injury types, which was consistent with the conclusions of many previous studies that showed that white matter injury is a strong predictor of mild hand function impairment.
Combined model of the hand knob score and clinical features
The predicted performance of the combined model on the degree of hand function injury was better than that of the hand knob score model and the clinical features model, which indicated that the measurement of hand knob structural indexes based on MRI images and the evaluation of the type of brain injury could be a relatively effective predictor of the hand function injury in children with CP. Our visualization of the combined model of the hand knob score and clinical features as a nomogram enables individualized assessment of the degree of hand function impairment in children with CP and also enhances the objectivity of clinical assessment. The degree of hand function impairment predicted by this model is in good agreement with the probability of the actual degree of hand function impairment in children with CP.
Limitations
The present study also has some limitations that should be noted. Firstly, the sample size was relatively small and this study was not studied separately for the different typing of CP children, which may have weakened our findings. However, considering the difficulty in collecting such patients and the fact that the results can be explained to some extent, we still presented our findings. Also, since some children with CP were diagnosed relatively late, some children were treated with varying degrees of intervention; thus, effects from the intervention treatments could not be completely excluded. Lastly, this was a single-center study and lacked external validation.
Conclusions
The combined model of the hand knob scores and clinical features is capable of effectively assessing hand function in children with CP with good calibration. The hand knob score may be an objective biomarker for hand function assessment in children with CP.
Acknowledgments
We thank the children and their families who participated in this study.
Funding: This work was supported by the Science and Technology Supporting Program of Guizhou Province (grant No. qiankehezhicheng [2020]4Y122), the Young Outstanding Scientific and Technological Talent of Guizhou Province (grant No. Qiankehepingtairencai [2021]5620), the Key Basic Research Program of Guizhou Province (grant No. Qiankehejichu-ZK[2022]zhongdian 051), the Talent Program for Future Famous Clinical Doctors of Zunyi Medical University (No. rc220211205), and the Chongqing Medical Scientific Research Project (Joint project of the Chongqing Health Commission and Science and Technology Bureau, No. 2020MSXM081).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4112/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4112/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4112/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee for biomedical research of the Affiliated Hospital of Zunyi Medical University (No. KLLY-2020-102). Children with CP and their guardians were informed of the corresponding rights and obligations. Their guardians signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr Dis Treat 2020;16:1505-18. [Crossref] [PubMed]
- Park H, Choi JY, Yi SH, et al. Relationship between the more-affected upper limb function and daily activity performance in children with cerebral palsy: a cross-sectional study. BMC Pediatr 2021;21:459. [Crossref] [PubMed]
- Burgess A, Boyd R, Ziviani J, et al. Stability of the Manual Ability Classification System in young children with cerebral palsy. Dev Med Child Neurol 2019;61:798-804. [Crossref] [PubMed]
- Klingels K, Meyer S, Mailleux L, et al. Time Course of Upper Limb Function in Children with Unilateral Cerebral Palsy: A Five-Year Follow-Up Study. Neural Plast 2018;2018:2831342. [Crossref] [PubMed]
- Jaspers E, Byblow WD, Feys H, et al. The Corticospinal Tract: A Biomarker to Categorize Upper Limb Functional Potential in Unilateral Cerebral Palsy. Front Pediatr 2015;3:112. [PubMed]
- Kim H, Kim J, Lee HJ, et al. Optimal stimulation site for rTMS to improve motor function: Anatomical hand knob vs. hand motor hotspot. Neurosci Lett 2021;740:135424. [Crossref] [PubMed]
- Wu F, Zhao H, Zhang Y, et al. Morphologic Variants of the Hand Motor Cortex in Developing Brains from Neonates through Childhood Assessed by MR Imaging. AJNR Am J Neuroradiol 2022;43:292-8. [Crossref] [PubMed]
- Jingshan L, Shengyu F, Xing F, et al. Morphometry of the Hand Knob Region and Motor Function Change in Eloquent Area Glioma Patients. Clin Neuroradiol 2019;29:243-51. [Crossref] [PubMed]
- Finkelsteyn AM, Saucedo MA, Miquelini LA, et al. Ischemic stroke of the "hand knob area": A case series and literature review. J Clin Neurosci 2019;65:100-5. [Crossref] [PubMed]
- Dubbioso R, Madsen KH, Thielscher A, et al. The Myelin Content of the Human Precentral Hand Knob Reflects Interindividual Differences in Manual Motor Control at the Physiological and Behavioral Level. J Neurosci 2021;41:3163-79. [Crossref] [PubMed]
- Hoei T, Kawahira K, Shimodozono M, et al. Repetitive facilitative exercise under continuous electrical stimulation for recovery of pure motor isolated hand palsy after infarction of the "hand knob" area: A case report. Physiother Theory Pract 2022; Epub ahead of print. [Crossref] [PubMed]
- Simone L, Viganò L, Fornia L, et al. Distinct Functional and Structural Connectivity of the Human Hand-Knob Supported by Intraoperative Findings. J Neurosci 2021;41:4223-33. [Crossref] [PubMed]
- Caulo M, Briganti C, Mattei PA, et al. New morphologic variants of the hand motor cortex as seen with MR imaging in a large study population. AJNR Am J Neuroradiol 2007;28:1480-5. [Crossref] [PubMed]
- Himmelmann K, Horber V, De La Cruz J, et al. MRI classification system (MRICS) for children with cerebral palsy: development, reliability, and recommendations. Dev Med Child Neurol 2017;59:57-64. [Crossref] [PubMed]
- Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature 2016;536:171-8. [Crossref] [PubMed]
- Amiez C, Petrides M. Functional rostro-caudal gradient in the human posterior lateral frontal cortex. Brain Struct Funct 2018;223:1487-99. [PubMed]
- Pizzella V, Tecchio F, Romani GL, et al. Functional localization of the sensory hand area with respect to the motor central gyrus knob. Neuroreport 1999;10:3809-14. [Crossref] [PubMed]
- Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci 2018;19:123-37. [Crossref] [PubMed]
- Wang F, Lian C, Wu Z, et al. Developmental topography of cortical thickness during infancy. Proc Natl Acad Sci U S A 2019;116:15855-60. [Crossref] [PubMed]
- Remer J, Croteau-Chonka E, Dean DC 3rd, et al. Quantifying cortical development in typically developing toddlers and young children, 1-6 years of age. Neuroimage 2017;153:246-61. [Crossref] [PubMed]
- Pagnozzi AM, Dowson N, Fiori S, et al. Alterations in regional shape on ipsilateral and contralateral cortex contrast in children with unilateral cerebral palsy and are predictive of multiple outcomes. Hum Brain Mapp 2016;37:3588-603. [Crossref] [PubMed]
- Umesh A, Kutten KS, Hogan PS, et al. Motor cortical thickness is related to effort-based decision-making in humans. J Neurophysiol 2020;123:2373-81. [Crossref] [PubMed]
- Voss P, Thomas ME, Cisneros-Franco JM, et al. Dynamic Brains and the Changing Rules of Neuroplasticity: Implications for Learning and Recovery. Front Psychol 2017;8:1657. [Crossref] [PubMed]
- Oby ER, Golub MD, Hennig JA, et al. New neural activity patterns emerge with long-term learning. Proc Natl Acad Sci U S A 2019;116:15210-5. [Crossref] [PubMed]
- Gao Y, Nie K, Mei M, et al. Changes in Cortical Thickness in Patients With Early Parkinson's Disease at Different Hoehn and Yahr Stages. Front Hum Neurosci 2018;12:469. [Crossref] [PubMed]
- Suh JS, Schneider MA, Minuzzi L, et al. Cortical thickness in major depressive disorder: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2019;88:287-302. [Crossref] [PubMed]
- Tereshchenko A, Magnotta V, Epping E, et al. Brain structure in juvenile-onset Huntington disease. Neurology 2019;92:e1939-47. [Crossref] [PubMed]
- Turner MR, Grosskreutz J, Kassubek J, et al. Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurol 2011;10:400-3. [Crossref] [PubMed]
- Engl C, Tiemann L, Grahl S, et al. Cognitive impairment in early MS: contribution of white matter lesions, deep grey matter atrophy, and cortical atrophy. J Neurol 2020;267:2307-18. [Crossref] [PubMed]
- Franki I, Mailleux L, Emsell L, et al. The relationship between neuroimaging and motor outcome in children with cerebral palsy: A systematic review - Part A. Structural imaging. Res Dev Disabil 2020;100:103606. [Crossref] [PubMed]
- Hadžagić Ćatibušić F, Užičanin S, Bulja D, et al. Hand function in children with unilateral spastic cerebral palsy. Med Glas (Zenica) 2019;16:66-70. [PubMed]
- Baranello G, Rossi Sebastiano D, Pagliano E, et al. Hand function assessment in the first years of life in unilateral cerebral palsy: Correlation with neuroimaging and cortico-spinal reorganization. Eur J Paediatr Neurol 2016;20:114-24. [Crossref] [PubMed]
- Mailleux L, Simon-Martinez C, Klingels K, et al. Structural Brain Damage and Upper Limb Kinematics in Children with Unilateral Cerebral Palsy. Front Hum Neurosci 2017;11:607. [Crossref] [PubMed]
- Feys H, Eyssen M, Jaspers E, et al. Relation between neuroradiological findings and upper limb function in hemiplegic cerebral palsy. Eur J Paediatr Neurol 2010;14:169-77. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


