
Systemic lupus erythematosus with disseminated aspergillosis misdiagnosed as lupus encephalopathy: a case report and literature review
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that involves multiple organs and systems and often requires long-term immunosuppressive therapy due to its disease characteristics. In general speaking, the most common cause of the visual loss was ocular pathologies. However, the literature suggests that the incidence of SLE immune-mediated uveitis is extremely low, at only 0.1–4.8% (1). Besides the immune-mediated uveitis, there is a wide differential diagnosis to consider, such as central retinal artery occlusion (CRAO), intraocular infections, intracranial tumor, glaucoma, or drug-related retinopathy, among other conditions (2-4). Compared with uveitis, some of the other causes can lead to a variety clinical presentation, not limited to the eye. The diversity and low specificity of clinical manifestation of retinopathy leads to frequent misdiagnosis or missed diagnosis. Particularly, invasive infection. This paper reports a rare case of disseminated infection by aspergillosis which was misdiagnosed as lupus encephalopathy. Patients with SLE presenting sudden vision lost with intracranial and intrathoracic space-occupying lesions are distinctly rare clinically. It is difficult and challenging to make a differential diagnosis of this case. At the same time, we further discussed how to make early diagnosis and the clinical experiences for the management of similar cases. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4362/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 40-year-old woman was diagnosed with SLE 1.5 years prior. In December 2020, medication self-discontinuation led to SLE recurrence, which manifested as high fever, autoimmune hepatitis (total bilirubin: 241 µmol/L), hemolytic anemia (minimum hemoglobin: 46 g/L, reticulocytes: 10.8%), and thrombocytopenia (minimum platelet count: 31×109/L), for which she was administered glucocorticoids (maximum methylprednisolone 80 mg/d) and rituximab (100 mg*twice, 0-day and 14-day) for disease control. The patient’s condition remained stable and the hormones were gradually withdrawn. In May 2021, she experienced a sudden loss of vision in both eyes without dizziness, headache, nausea, vomiting, or limb hemiplegia at that time, but with fatigue and lethargy. The patient’s body temperature was not measured. The ophthalmology department of the local hospital administered a peribulbar injection of 40 mg methylprednisolone for suspected acute retinitis but no improvement was observed. Head magnetic resonance imaging (MRI) and lung computed tomography (CT) revealed space-occupying lesions and the patient was diagnosed with lupus encephalopathy at another hospital. Intravenous injection of dexamethasone (10 mg *3 days) also did not improve the patient’s vision. The patient was then transferred to our hospital. On admission, she showed good limb mobility, was independently ambulatory, and had a mild low-grade fever. The patient showed multiple ulcers and leukoplakia in her oral cavity, bilateral drug-induced pupil dilation, diminished light response, and clear breath sounds in both lungs and lower lungs. Her bilateral lower limbs were negative for the Babinski sign. Her muscle strength was grade IV for the right upper limb and grade V for the remaining limbs. The patient was negative for signs of meningeal irritation.
Among auxiliary tests, routine blood and high-sensitivity C-reactive protein (CRP) tests showed a white blood cell (WBC) count of 14.5×109/L, neutrophil percentage of 87.6%, hemoglobin concentration of 135 g/L, platelet count of 171×109/L, and high-sensitivity CRP concentration of 12.5 mg/L. Liver and kidney function tests were normal. The patient was positive for cytomegalovirus (CMV) DNA and antinuclear antibody (ANA) 1:1,000, as well as anti-RNP antibody +++, and negative for anti-double-stranded DNA anti-body or SM anti-body. The immunoglobulin + complement IgG concentration was 12.50 g/L, IgA was 4.56 g/L, C3 was 1.14 g/L, and C4 was 0.21 g/L.
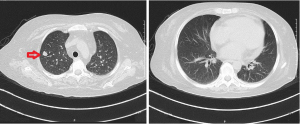
Ophthalmic examination showed intermediate and posterior uveitis (Figures 1,2). Contrast-enhanced head MRI showed multiple abnormal foci in the bilateral cerebrum and cerebellum, which were suspected to be infectious lesions (fungal or tuberculosis), as well as the formation of multiple small abscesses (Figure 3). Lung plain CT scan revealed solid nodules in the right upper lobe and fibrosis of the bilateral lower lobes. A small amount of pericardial effusion was observed (Figure 4).
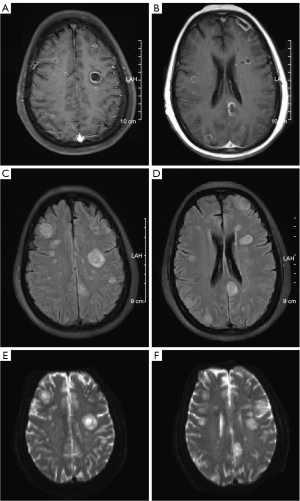
As the patient had been receiving long-term immunosuppressive therapy, had multiple lesions, an SLE Disease Activity Index (SLEDAI) score of 0, and a lack of space-occupying lesions on head MRI performed 3 months prior in another hospital, on the day of admission the patient’s current condition was suspected to have occurred due to infection. The hormone dosage was reduced to methylprednisolone [20 mg intravenous guttae once daily (ivgtt qd)], followed immediately by lumbar puncture. The specimens were sent for blood and cerebrospinal fluid (CSF) tests and broad-spectrum antibiotics were administered for the full coverage of gram-negative bacteria, gram-positive bacteria, and fungi (meropenem 1.0 g ivgtt q8h, voriconazole 200 mg ivgtt q1h, and linezolid 0.6 g ivgtt q12h). The test results revealed a CSF pressure of 215 mmH2O. The CSF examination showed a colorless, clear sample negative for Pandy’s test, with a WBC count of 1×106/L and a red blood cell (RBC) count of 0/L. The blood globulin G level was >600 pg/mL, showed positivity for Aspergillus antigen (+), and an I value of 2.26. CSF next-generation sequencing (NGS) revealed CMV, while evaluation for background microorganisms showed 12 bands of Aspergillus fumigatus. The patient samples were negative for autoimmune encephalitis antibodies. Evaluation of antibodies associated with demyelinating disease revealed the following: AQP4(−), MOG(−), GFAP(−), and MBP(−). The CMV PCR showed 1.49×103 copies/µL.
As the CSF tests did not reveal any reliable etiological evidence, the ophthalmology department was contacted to perform a vitreous tap. During the operation, a large amount of white flocculent deposit was observed intraocularly and the retina was completely obscured (Figure 5). Unfortunately, the patient’s vision did not recover after careful removal of the floccules to expose the macula. Vitreous fluid NGS indicated 688 Aspergillus sequences.
Based on the combination of medical history and laboratory test findings, disseminated aspergillosis was considered, including intracranial infection, Aspergillus-induced pneumonia, and acute uveitis. Linezolid injection was gradually discontinued and voriconazole (200 mg q12h) and ganciclovir (0.2 g q12h) were administered intravenously for anti-infective treatment. The glucocorticoids were gradually withdrawn to oral methylprednisolone (Medrol 8 mg qd). During this treatment period, vitrectomy was performed separately for the left and right eyes, as well as intravitreal injection of a prepared voriconazole solution and pan-retinal photocoagulation therapy in both eyes.
Outcome and follow-up
The patient’s bilateral vision could not be restored and only the globe structure was preserved. The ganciclovir and voriconazole injections were discontinued after 1 month and the patient was switched to oral voriconazole tablets (200 mg q12h) which they are taking to the present day. The lung lesions were completely absorbed after 2 months of treatment. At the time of this report, the anti-fungal treatment had been carried out for more than 10 months. Repeat examination showed that the head lesions had basically all been absorbed (Figure 6), with no uveitis recurrence. The timeline of this case is shown in Figure 7.

Discussion
The diagnosis of SLE-related eye involvement requires a comprehensive analysis of patient medical history and vitreous and/or aqueous humor culture, or blood culture in certain endogenous cases. The occurrence of acute uveitis in patients with SLE is frequently linked to autoimmune mediation. The patient in the present case report was misdiagnosed with immune-mediated uveitis in the ophthalmology department of another hospital and high-dose glucocorticoid treatment was administered erroneously, which significantly impeded infection control. Although the survival rate of SLE has greatly improved in recent years, its all-cause mortality is prominently associated with infection, especially sepsis (5).
Based on a literature review, we believe that the following causes required identification in the present case:
- CRAO: CRAO is a serious retinal vasculopathy and a typical presentation of SLE-induced retinopathy that can manifest as a sudden painless loss of vision (6). The pathogenesis of CRAO remains poorly understood. CRAO may be caused by immune complex-mediated vasculitis and fibrin-dominant thrombosis, manifesting as cotton-wool spots, dye leakage with perivascular exudates on fundus fluorescein angiography, retinal hemorrhage, or microaneurysms (7). A previous study reported that CRAO was associated with antiphospholipid antibodies and that patients were usually positive for lupus anticoagulants or anti-β2 glycoprotein IgG (8). This may be due to SLE-associated antiphospholipid syndrome which is significantly associated with SLE activity (6-8). Therefore, CRAO cannot explain the pulmonary lesions of the patient in the present case. Furthermore, the patient’s vision did not improve despite immunosuppressive and anticoagulation therapies.
- Intracranial tumor: the patient in the present case was a woman of child-bearing age who presented with rapid disease progression and no space-occupying lesions on head MRI performed 3 months before disease onset. Thus, if malignant tumors were suspected in this case, the focus should be on the identification of non-gestational choriocarcinoma (NGC). NGC is a rare germ cell tumor that accounts for <0.6% of all gestational tumors and has a poor metastatic prognosis (9). Reports of NGC with brain metastasis are even rarer. Gestational choriocarcinoma (GC) has high morbidity and mortality rates in cases with brain metastasis. However, NGC was not supported in the present case as the patient had normal levels of human chorionic gonadotropin (HCG) and no space-occupying lesions at the globe or optic nerve. Therefore, no metastatic tumor of any origin could explain the occurrence of uveitis.
- Acute optic neuritis: optic neuropathy is rare in SLE, with a prevalence of approximately 1% (10). It can manifest as acute retrobulbar optic neuritis, papillitis, anterior ischemic optic neuropathy, posterior ischemic optic neuropathy, or slowly progressive vision loss. The pathogenesis of SLE-associated optic neuropathy is thought to differ from that of idiopathic optic neuritis, which can manifest as thrombosis, vascular occlusive events, focal axonal necrosis, or immune vascular inflammation. Unlike CRAO, acute optic neuritis can occur at any stage of SLE and is not absolutely correlated with disease activity. Laboratory tests often indicate elevated IgG and anti-double-stranded DNA, but not necessarily a decrease in complements. Fundus examination findings are usually unremarkable, while head MRI may reveal T2 hyperintensities at the optic nerve (10,11). Optic neuritis could not explain the intravitreal empyema and lung lesions in the present case, while significant differences were observed in the head MRI findings. Therefore, optic neuritis was not considered.
- Drug-related retinopathy: up to 11–15% of glaucoma and 13% of cataracts are caused by long-term glucocorticoid use (3). Several guidelines recommend hydroxychloroquine (HCQ) as an essential medication for SLE (12,13). Its retinal toxicity is related to drug dose and disease duration. More importantly, the most serious adverse reaction caused by HCQ is irreversible macular degeneration leading to vision loss (14). In addition, a small number of cases with ocular complications due to methotrexate (MTX), cyclophosphamide (CYC), and cyclosporine (CsA) have been reported (2) and should not be overlooked by clinicians.
In conclusion, infectious causes should always be at the top on the list of differential diagnoses when people with SLE accompanying by uveitis or multiple system damage. The cause of multiple systemic damage is not only limited to immune disorder but also infection. The diagnosis is based on post history, clinical findings, SLE activity index and bacteriological examination. The risk of organ biopsies increases in patients with SLE. Compared with the internal organ biopsies, the bacterial culture of the vitreous fluid may aid in the diagnosis of infectious endophthalmitis and appears to be much safer. Incorrectly using of high-dose glucocorticoids without careful consideration, sometimes can cause a worsening of disease.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81974549) and the Administration of Traditional Chinese Medicine of Zhejiang Province, China (No. 2021ZB050).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4362/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4362/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kedves M, Kósa F, Kunovszki P, et al. Large-scale mortality gap between SLE and control population is associated with increased infection-related mortality in lupus. Rheumatology (Oxford) 2020;59:3443-51. [Crossref] [PubMed]
- Dammacco R. Systemic lupus erythematosus and ocular involvement: an overview. Clin Exp Med 2018;18:135-49. [Crossref] [PubMed]
- Carli L, Tani C, Querci F, et al. Analysis of the prevalence of cataracts and glaucoma in systemic lupus erythematosus and evaluation of the rheumatologists' practice for the monitoring of glucocorticoid eye toxicity. Clin Rheumatol 2013;32:1071-3. [Crossref] [PubMed]
- Dave VP, Pappuru RR, Pathengay A, et al. Aspergillus Endophthalmitis: Clinical Presentations and Factors Determining Outcomes. Asia Pac J Ophthalmol (Phila) 2020;9:9-13. [Crossref] [PubMed]
- Gao N, Li MT, Li YH, et al. Retinal vasculopathy in patients with systemic lupus erythematosus. Lupus 2017;26:1182-9. [Crossref] [PubMed]
- Narang S, Giran M, Lehl SS, et al. Bilateral combined retinal vascular occlusion as a presenting feature in a case of systemic lupus erythematosus. Rheumatology (Oxford) 2021;60:4949-50. [Crossref] [PubMed]
- Chandran K, Shenoy SB, Kulkarni C, et al. Bilateral simultaneous Central Retinal Artery Occlusion (CRAO) in a patient with Systemic Lupus Erythematosus (SLE). Am J Ophthalmol Case Rep 2020;19:100833. [Crossref] [PubMed]
- Au A, O'Day J. Review of severe vaso-occlusive retinopathy in systemic lupus erythematosus and the antiphospholipid syndrome: associations, visual outcomes, complications and treatment. Clin Exp Ophthalmol 2004;32:87-100. [Crossref] [PubMed]
- Duong J, Ghanchi H, Miulli D, et al. Metastatic Nongestational Choriocarcinoma to the Brain: Case Report and Proposed Treatment Recommendations. World Neurosurg 2018;115:170-5. [Crossref] [PubMed]
- Lin YC, Wang AG, Yen MY. Systemic lupus erythematosus-associated optic neuritis: clinical experience and literature review. Acta Ophthalmol 2009;87:204-10. [Crossref] [PubMed]
- Man BL, Mok CC, Fu YP. Neuro-ophthalmologic manifestations of systemic lupus erythematosus: a systematic review. Int J Rheum Dis 2014;17:494-501. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 2021;100:S1-S276. [Crossref]
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736-45. [Crossref] [PubMed]
- Braslow RA, Shiloach M, Macsai MS. Adherence to Hydroxychloroquine Dosing Guidelines by Rheumatologists: An Electronic Medical Record-Based Study in an Integrated Health Care System. Ophthalmology 2017;124:604-8. [Crossref] [PubMed]






