
Clinical features and long-term treatment outcome of posterior scleritis
Introduction
Posterior scleritis is an inflammatory disease that involves the sclera posterior to the ora serrata (1). Its occurrence is rare compared to that of anterior scleritis, and a report suggest that only 6.2% of patients with scleritis have posterior scleritis (2). Posterior scleritis is mainly sub-acute, occurs unilaterally, and presents with moderate to severe pain in nearly half of the patients (2). It develops frequently in young individuals and is associated with severe vision impairment, compared to anterior scleritis (2). Depending on the cause of inflammation, it may be classified into infectious and non-infectious posterior scleritis (3). Non-infectious posterior scleritis is usually of an idiopathic etiology, but is often associated with rheumatological disorders (4-6).
The diagnosis of posterior scleritis can be challenging, and a step-wise approach with suspecting and approaching posterior scleritis can help in establishing the definitive diagnosis. Serous retinal detachment, optic nerve swelling, chorio-retinal granulomas, or retinal lesions such as cotton wool spots, can be observed upon funduscopic examination (1,7). In case of posterior nodular scleritis, scleral nodules can be observed funduscopically and also using imaging tools such as B-scan ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI). Posterior scleral thickening associated with fluid surrounding the optic nerve can be seen as a characteristic “T” sign on B-scan ultrasonography, which helps in the definitive diagnosis.
Studies have mainly focused on anterior scleritis; because of the rarity of posterior scleritis, only a few large-scale studies have focused on posterior scleritis (8,9). There are even fewer reports on infectious posterior scleritis (10,11). González-López et al. analyzed the clinical characteristics of 18 patients with bilateral posterior scleritis, which is extremely rare, and reported that patients with elevated anti-nuclear antibody titers exhibited increased bilateral involvement (9). Lavric et al. reported the recurrence rate of posterior scleritis in 144 patients and its association with systemic disease, in their large-scale study, with only two patients showing an infectious etiology (4). Moreover, there are only a few case reports on posterior scleritis in Korean patients (12,13).
Therefore, we aimed to retrospectively analyze a series of cases of with posterior scleritis with respect to the etiology, clinical features, diagnosis, treatment, and visual outcome. Moreover, we have discussed our experience of two cases of infectious posterior scleritis related to Pseudomonas aeruginosa (P. aeruginosa) and coagulase-negative Staphylococci that were successfully treated with antibiotics and steroids. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-721/rc).
Methods
This was a retrospective observational study and was conducted using data of 14 patients diagnosed with posterior scleritis at Ophthalmology Department of Severance Hospital and Gangnam Severance Hospital between May 2005 and March 2020. The demographic characteristics, clinical features, and diagnostic test results of patients were analyzed. Details regarding treatment regimens and their results were collected. This study was conducted in accordance with the tenets of the Declaration of Helsinki (as revised in 2013), and was approved by the Institutional Review Board (IRB) of the Gangnam Severance Hospital retrospectively (IRB approval No. 3-2020-0304). The necessity to obtain informed consent was waived by the IRB. The inclusion criteria were patients diagnosed with either infectious or non-infectious posterior scleritis. The diagnosis of posterior scleritis was made when patients with suspected past medico-surgical history and clinical features showed the following characteristic abnormalities on imaging or blood tests: the T sign observed on B-scan ultrasound; increased choroidal thickness observed on optical coherence tomography (OCT); vascular leakage observed on fluorescein angiogram; increased choroidal thickness observed on either CT or MRI; culture results of samples taken from the lesion in cases of suspected infectious scleritis, etc. The exclusion criteria were patients with insufficient evidence of posterior scleritis and those with other ophthalmic conditions such as diabetic retinopathy, age-related macular degeneration, and glaucoma. At the first visit, the best-corrected visual acuity and intraocular pressure were measured. Vision was measured with a Snellen chart and then converted to the logarithm of the minimum angle of resolution vision for statistical analysis. Dilated funduscopic examination and OCT examination were performed. B-scan ultrasonography test confirmed whether the “T” sign was present or whether the scleral thickness had increased. Fluorescein angiography (FA) was also performed to confirm any abnormal findings suggestive of posterior scleritis, such as optic disc leakage or vascular leakage. All patients were tested for complete blood count, erythrocyte sedimentation rate, and C-reactive protein levels; additional serology tests were performed for assessing rheumatologic or infectious diseases, if needed. Patients with suspected infectious posterior scleritis were tested for bacterial and fungal culture for the suspected organism. For differential diagnosis, imaging tests such as CT and MRI, were performed, if necessary.
Statistical analysis
Descriptive statistical analyses of the patients’ demographic data were performed using SPSS v25.0 (IBM Inc., Armonk, NY, USA). The Wilcoxon’s signed rank test was performed to compare the visual changes.
Results
A total of 14 patients were treated for posterior scleritis, of which two (14.3%) were of infectious origin. The mean age was 52.6±16.4 years. Five (35.7%) patients were male and nine (64.3%) were female. Three (21.4%) patients had rheumatologic disorders, including one with systemic lupus erythematosus (7.1%), one with sero-negative spondyloarthropathy (7.1%), and one with Behçet’s disease (7.1%). One patient had a history of tuberculosis. Two patients with infectious scleritis (14.3%) had a history of eye surgery (14.3%). Culturing in pus-filled nodules revealed super-infection with both P. aeruginosa and coagulase-negative Staphylococci in those two patients (Table 1).
Table 1
| Demographic | Number (%)/mean ± SD |
|---|---|
| Age, years | 52.6±16.4 |
| Sex (male/female) | 5/9 |
| Known systemic diseases | |
| Rheumatologic diseases | 3 (21.4) |
| Systemic lupus erythematosus | 1 (7.1) |
| Sero-negative spondyloarthropathy | 1 (7.1) |
| Behçet’s disease | 1 (7.1) |
| Hypertension | 2 (14.3) |
| Diabetes | 2 (14.3) |
| Pregnancy | 2 (14.3) |
| Tuberculosis | 1 (7.14) |
| Known ocular trauma or surgery | |
| Past ocular surgery | 2 (14.3) |
| Cataract surgery | 1 (7.1) |
| Pterygium removal | 1 (7.1) |
| Types of posterior scleritis | |
| Infectious | 2 (14.3) |
| Superinfection with both Pseudomonas aeruginosa and coagulase-negative Staphylococci | 2 (14.3) |
| Non-infectious | 12 (85.7) |
SD, standard deviation.
The initial logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) of patients with posterior scleritis was 0.6±0.9. Intraocular pressure was 13.9±7.6 mmHg. The most common initial symptom of patients included conjunctival injection (n=12, 85.7%), followed by pain on eyeball movement (n=8, 57.1%) and decreased visual acuity (n=6, 42.9%). It took an average of 10.4±10.1 days for a patient to visit the outpatient clinic after initial symptom onset. Nine patients (64.3%) had inflammatory cells in the anterior chamber and eight patients (57.1%) had inflammatory cells in the vitreous cavity. During funduscopic evaluation, optic disc swelling was observed in six patients (42.9%); choroidal folding/detachment, vitreous haziness, and retinal folding in three (21.4%); and optic disc hemorrhage and epiretinal membrane in one (7.1%). However, three patients (21.4%) showed no abnormality on funduscopic examination. The mean central macular thickness was 302.9±90.8 µm, and macular edema was present in three patients (21.4%). Eight (57.14%) patients underwent FA, of which four (50.0%) had leakage. Four patients (50.0%) had optic disc leakage, whereas two (25.0%) had vascular leakage. B-scan ultrasonography confirmed an increase in the scleral thickness in all patients and the presence of “T” sign in 10 patients (71.4%). Eight patients underwent either CT or MRI, of which six patients (75.0%) showed abnormal findings in the eyeball. Five of these patients (83.3%) showed an enhancement on CT or hyperintensity on MRI, and 4 (66.7%) showed scleral wall thickening. Choroidal detachment was found in 2 patients (33.3%). Associated ocular diseases were uveitis (n=9, 64.3%), optic neuritis (n=5, 35.7%), nodular scleritis (n=4, 28.6%), idiopathic orbital inflammatory disease (n=2, 14.3%), uveal effusion (n=1, 7.1%), and non-arteritic ischemic optic neuropathy (n=1, 7.1%) (Table 2).
Table 2
| Finding | Number (%)/mean ± SD |
|---|---|
| Duration between symptoms and first visit (days) | 10.4±10.1 |
| Initial logMAR BCVA | 0.6±0.9 |
| Intraocular pressure (mmHg) | 13.9±7.6 |
| Spherical equivalent refractive error (D) | −1.6±2.0 |
| Initial symptom | |
| Injection | 12 (85.7) |
| Pain on eyeball movement | 8 (57.1) |
| Decreased visual acuity | 6 (42.9) |
| Ocular findings | |
| Cells in anterior chamber (eyes) | 9 (64.3) |
| SUN grading | 1.6±1.6 |
| Cells in anterior vitreous (eyes) | 8 (57.1) |
| Funduscopic features | |
| Optic disc swelling | 6 (42.9) |
| Vitreous haziness | 3 (21.4) |
| Retinal fold | 3 (21.4) |
| Choroidal fold/detachment | 3 (21.4) |
| No abnormality | 3 (21.4) |
| Optic disc hemorrhage | 1 (7.1) |
| Epiretinal membrane | 1 (7.1) |
| OCT features | |
| Central macular thickness (μm) | 302.9±90.8 |
| Macular edema | 3 (21.4) |
| Fluorescein angiographic leakage | 4/8 (50.0) |
| Disc leak | 4 (50.0) |
| Vascular leak | 2 (25.0) |
| B-scan ultrasonography | |
| Increased scleral thickness | 14 (100.0) |
| T-sign | 10 (71.4) |
| CT/MRI abnormal findings | 6/8 (75.0) |
| Enhancement/high signal intensity | 5/6 (83.3) |
| Thickening of sclera | 4/6 (66.7) |
| Choroidal detachment | 2/6 (33.3) |
| Combined features | |
| Uveitis | 9 (64.3) |
| Optic neuritis | 5 (35.7) |
| Nodular scleritis | 4 (28.6) |
| Idiopathic orbital inflammatory disease | 2 (14.3) |
| Uveal effusion | 1 (7.1) |
| Non-arteritic ischemic optic neuropathy | 1 (7.1) |
SD, standard deviation; logMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected visual acuity; D, diopter; SUN, the standardization of uveitis nomenclature; OCT, optical coherence tomography; CT, computed tomography; MRI, magnetic resonance imaging.
Systemic steroids were administered in 11 (78.6%) patients, and intravenous steroid pulse therapy was administered in 5 (45.5%). In four patients (28.6%), immunosuppressive agents were additionally used, including cyclosporine (Cipol-N®, Chong Kun Dang, Seoul, Republic of Korea) in two, cyclophosphamide (Alkyloxan®, JW Pharmaceutical, Seoul, Republic of Korea) in one, and methotrexate (Methotrexate®, Yuhan, Seoul, Republic of Korea) in one. Intra-vitreal dexamethasone injections (Ozurdex®, Allergan, Irvine, CA, USA) were administered in two patients (14.3%). Biological therapeutic agent adalimumab (Humira®, AbbVie Inc., North Chicago, IL, USA) was administered in one patient who was refractory to corticosteroid and immunosuppressive therapy. Pars plana vitrectomy with silicone oil injection was performed in one patient (7.1%) for exudative retinal detachment with vitreous opacity. Patients were followed up for an average of 31.6±39.2 months. Recurrence was noted in three patients (21.4%), with an average recurrence rate of 1.6±0.7 times. The final LogMAR BCVA was 0.6±1.0, which did not significantly differ from that at the first visit (P=0.878) (Table 3).
Table 3
| Variable | Number (%)/mean ± SD |
|---|---|
| Treatment | |
| Topical antibiotics and steroids | 13 (92.9) |
| Topical NSAIDs | 4 (28.6) |
| Systemic steroids | 11 (78.6) |
| Systemic NSAIDs | 2 (14.3) |
| Systemic immunosuppressants | 4 (28.6) |
| Cyclosporine | 2 (14.3) |
| Cyclophosphamide | 1 (7.1) |
| Methotrexate | 1 (7.1) |
| Systemic biologics | 1 (7.1) |
| Intravitreal dexamethasone injection | 2 (14.3) |
| Vitrectomy | 1 (7.1) |
| Recurrence (eyes, events) | 3 (21.4%, 1.6±0.7) |
| Follow-up duration (months) | 31.6±39.2 |
| Final logMAR BCVA | 0.6±1.0 (P=0.878*) |
*, comparison of initial and final visual acuity using Wilcoxon’s signed rank test. SD, standard deviation; NSAID, non-steroidal anti-inflammatory drugs; logMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected visual acuity.
Of the three patients with recurrence, one showed posterior scleritis associated with optic neuritis and idiopathic orbital inflammatory disease, which recurred despite administering intravenous steroid pulse therapy. Further recurrence was not observed after the administration of oral methotrexate. One patient in her 50s received intravenous steroid pulse therapy due to idiopathic orbital inflammatory disease, posterior scleral thickening, and choroidal detachment. Her initial visual acuity was limited to just perception of light. After 2 months of treatment, posterior scleritis with exudative retinal detachment developed, and despite intensive intravenous steroid pulse therapy and oral steroid administration, the condition did not improve; vitreous opacity developed subsequently. After vitrectomy with silicone oil tamponade, the retina re-attached, and the patient remained stable without recurrence. One patient with posterior scleritis had initially been under control with oral steroids; however, the patient had a recurrence after a few months. Improvement was observed after the administration of oral cyclosporine, with no further recurrence. All three relapsed patients did not have any rheumatologic disease.
Three patients with posterior scleritis showed no abnormalities on funduscopic examination. They visited the outpatient clinic after an average of 7.7±4.0 days after the onset of symptoms, and their initial visual acuity was 20/20. They had subjective visual deterioration, red eyes, and pain during eyeball movement. “T” sign and increased scleral thickness were observed on B-scan ultrasonography in all patients; however, only one patient showed the presence of inflammatory cells in the anterior chamber and vitreous cavity.
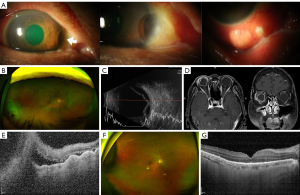
Two of the four patients with posterior scleritis associated with anterior nodules had an infectious origin; both had a history of ocular surgery. One patient had a cataract surgery 10 years ago, and visited with the symptom of decreased vision since 1 month. At the first visit, visual acuity was limited to counting fingers, and intraocular pressure was 4 mmHg. Conjunctival hyperemia and a pus-discharging scleral nodule were seen. B-scan ultrasonography confirmed the presence of choroidal detachment with choroidal effusion. From the results of the culture examination of the pus draining from the nodules, a superinfection with P. aeruginosa and coagulase-negative Staphylococci was noted. There were no signs of other systemic infections, as confirmed by head and neck, chest, and liver CT. The patient was successfully treated with intravenous administration of 3rd generation cephalosporin and ciprofloxacin. The patient’s final vision improved slightly to 20/133. The other patient had undergone resection of pterygium on the right eye 15 years ago. Because of a newly developed scleral melting, the patient received a scleral graft 2 months ago. Conjunctival hyperemia and nodules were observed. The patient’s initial visual acuity was 20/50 and intraocular pressure was 6 mmHg. Choroidal detachment was also noted. From the results of culture examination of the nodule sample, a superinfection with P. aeruginosa and coagulase-negative Staphylococci was identified. The patient was treated with intravenous administration of ampicillin/sulbactam and ciprofloxacin. The final visual acuity of the patient was 20/50, which did not differ compared to the first visit.
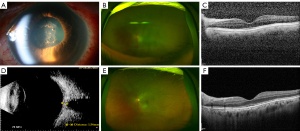
Representative pictures of non-infectious and infectious posterior scleritis are presented in Figures 1,2.
Discussion
This study demonstrated that most posterior scleritis cases are of non-infectious origin. Uveitis and anterior nodular scleritis are commonly present. However, some patients cannot be diagnosed using funduscopic and slit lamp examination alone, and additional B-scan ultrasonography may be required. Most patients can be treated using topical and systemic steroids, whereas some patients require additional immunosuppressive and biological therapeutics.
Diagnosis of posterior scleritis is sometimes challenging. Funduscopic or OCT findings involving the posterior segment, such as choroidal effusion and detachment, and cystoid macular edema, are helpful in the diagnosis (8,14,15); however, we encountered three patients who did not show abnormal funduscopic findings. B-scan ultrasonography was of diagnostic value in all patients, particularly in these three patients who showed no abnormalities on funduscopic examination. In this study, patients who did not show unusual funduscopic findings complained of subjective vision loss, injected conjunctiva, and severe pain during eye movement. Performing additional tests such as B-scan ultrasonography to identify the “T” sign and/or increased scleral thickness could help in the early diagnosis and management of posterior scleritis without abnormal fundus features.
Imaging modalities such as CT and MRI can also be useful in patients with posterior scleritis. CT or MRI was performed in eight out of 14 patients in this study, of whom six showed abnormal findings. CT findings commonly seen in posterior scleritis include eccentric enhancement of the globe wall and eccentric thickening of the sclero-uveal rim; scleral enhancement and thickening are frequently observed on MRI (16). In these patients, findings such as scleral enhancement, scleral thickening, and choroidal detachment were assessed to establish the diagnosis. However, because two patients did not show any specific findings on CT or MRI, we speculated that CT or MRI may be inconclusive. This reaffirms the significance of careful clinical examination along with the interpretation of multimodal imaging modalities including B-scan, CT, and/or MRI.
In this study, the recurrence rate was 21.4%. Two of the three patients who relapsed were stabilized after administration of immunosuppressants, following which there was no recurrence. The recurrence rate of posterior scleritis is higher in younger patients, those with Crohn’s disease, and those receiving immunosuppressive drugs such as mycophenolate mofetil (4). In our study, no cases of recurrence in patients with posterior scleritis associated with systemic rheumatologic diseases were observed. The relapse rate was also within the previously known range of 15.8–40% (4,8). Interestingly, two of the three relapsed patients received intravenous steroid pulse therapy for the accompanying idiopathic orbital inflammatory disease. Based on the relationship between idiopathic inflammatory scleritis and extraocular abnormalities, it has been hypothesized that idiopathic inflammatory scleritis falls under the spectrum of idiopathic orbital inflammatory diseases (16,17). In this study, patients with idiopathic orbital inflammatory disease had a higher relapse rate probably because the inflammation developed in an area wider than the sclera alone. Aggressive immunosuppressive therapy and careful follow-up may be helpful in successful treatment and prevention of relapse.
Two patients with infectious posterior scleritis showed pus-containing scleral nodules. Infectious scleritis is often associated with risk factors such as previous ocular surgery or trauma (18), and Pseudomonas, gram-negative bacilli, and Staphylococcus are the main causative strains. Systemic antibiotics and/or surgical treatment is often required (19). In this study, two patients diagnosed with infectious scleritis had a history of ocular surgery, and importantly, one of these developed infectious posterior scleritis after receiving a scleral graft for the treatment of scleral melting. Bacterial culture was performed, and empirical antibiotic therapy was started immediately, resulting in complete resolution of the infection and globe salvage. Misdiagnosis as non-infectious inflammatory scleritis and treatment with steroids, without the coverage of antibiotics, can worsen the infection. Hence, if patients present with a history of ocular surgery or trauma, with ocular findings suggestive of scleritis, especially with pus-containing nodules, infectious posterior scleritis should always be considered as a differential diagnosis.
This is the first study to analyze the clinical characteristics of Korean patients diagnosed with posterior scleritis. Moreover, this study comprised a relatively large group of patients, analyzing a total of 14 patients with infectious and non-infectious posterior scleritis. However, the limitations of this study include its retrospective study design, small number of patients with infectious posterior scleritis, and the large variation in the patients’ follow-up period. Since the number of patients with posterior scleritis visiting a single institution is limited, better results can be obtained in a multi-centric prospective clinical analysis of clinical features.
Conclusions
In conclusion, early diagnosis and aggressive treatment of posterior scleritis are recommended, since impaired vision does not improve significantly after treatment. Differential diagnosis of posterior scleritis may be challenging, particularly in those with the absence of typical funduscopic features. Therefore, it is advisable to include the possibility of posterior scleritis in the differential diagnosis, especially in those with poor vision, ocular pain, or severe conjunctival injection. If there are concomitant systemic or orbital diseases, aggressive treatment using immunosuppressants or biological therapeutics might be helpful in the treatment and prevention of recurrence.
Acknowledgments
Funding: This work was supported by a faculty research grant from Yonsei University College of Medicine (grant No. 2017-32-0037 to Dr. Min Kim); the National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIT) (No. 2019R1G1A1008122 to Dr. Min Kim); and the Korean Association of Retinal Degeneration (to Dr. Min Kim). The funding sources had no role in any aspect of this study or its publication.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-721/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-721/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-721/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-721/coif). MK reports that this work was supported by a faculty research grant from Yonsei University College of Medicine (grant No. 2017-32-0037); the National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIT) (No. 2019R1G1A1008122); and the Korean Association of Retinal Degeneration. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the tenets of the Declaration of Helsinki (as revised in 2013), and was approved by the Institutional Review Board (IRB) of the Gangnam Severance Hospital retrospectively (IRB approval No. 3-2020-0304). The necessity to obtain informed consent was waived by the IRB.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benson WE. Posterior scleritis. Surv Ophthalmol 1988;32:297-316. [Crossref] [PubMed]
- Gonzalez-Gonzalez LA, Molina-Prat N, Doctor P, et al. Clinical features and presentation of posterior scleritis: a report of 31 cases. Ocul Immunol Inflamm 2014;22:203-7. [Crossref] [PubMed]
- Wakefield D, Di Girolamo N, Thurau S, et al. Scleritis: Immunopathogenesis and molecular basis for therapy. Prog Retin Eye Res 2013;35:44-62. [Crossref] [PubMed]
- Lavric A, Gonzalez-Lopez JJ, Majumder PD, et al. Posterior Scleritis: Analysis of Epidemiology, Clinical Factors, and Risk of Recurrence in a Cohort of 114 Patients. Ocul Immunol Inflamm 2016;24:6-15. [Crossref] [PubMed]
- Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, et al. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology 2012;119:43-50. [Crossref] [PubMed]
- Dammacco R, Guerriero S, Alessio G, et al. Natural and iatrogenic ocular manifestations of rheumatoid arthritis: a systematic review. Int Ophthalmol 2022;42:689-711. [Crossref] [PubMed]
- McCluskey PJ, Watson PG, Lightman S, et al. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1999;106:2380-6. [Crossref] [PubMed]
- Kumar A, Ghose A, Biswas J, et al. Clinical profile of patients with posterior scleritis: A report from Eastern India. Indian J Ophthalmol 2018;66:1109-12. [Crossref] [PubMed]
- González-López JJ, Lavric A, Dutta Majumder P, et al. Bilateral Posterior Scleritis: Analysis of 18 Cases from a Large Cohort of Posterior Scleritis. Ocul Immunol Inflamm 2016;24:16-23. [Crossref] [PubMed]
- Yanagida C, Usui Y, Sakai JI, et al. An unusual case of Behcet disease with posterior scleritis: A case report. Medicine (Baltimore) 2019;98:e16886. [Crossref] [PubMed]
- Taravella MJ, Johnson DW, Petty JG, et al. Infectious posterior scleritis caused by Pseudallescheria boydii. Clinicopathologic findings. Ophthalmology 1997;104:1312-6. [Crossref] [PubMed]
- Moon SY, Yoon WT, Park SP. Recurrent Unilateral Vogt-Koyanagi-Harada Disease with Posterior Scleritis. Korean J Ophthalmol 2015;29:352-4. [Crossref] [PubMed]
- Lim JW, Park JH. Intravitreal bevacizumab (avastin) as an adjuvant for the treatment of posterior scleritis. Korean J Ophthalmol 2011;25:282-4. [Crossref] [PubMed]
- Dong ZZ, Gan YF, Zhang YN, et al. The clinical features of posterior scleritis with serous retinal detachment: a retrospective clinical analysis. Int J Ophthalmol 2019;12:1151-7. [Crossref] [PubMed]
- Lane J, Nyugen E, Morrison J, et al. Clinical Features of Scleritis Across the Asia-Pacific Region. Ocul Immunol Inflamm 2019;27:920-6. [Crossref] [PubMed]
- Diogo MC, Jager MJ, Ferreira TA CT. AJNR Am J Neuroradiol 2016;37:2334-9. [Crossref] [PubMed]
- Gordon LK. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye (Lond) 2006;20:1196-206. [Crossref] [PubMed]
- Ahn SJ, Oh JY, Kim MK, et al. Clinical features, predisposing factors, and treatment outcomes of scleritis in the Korean population. Korean J Ophthalmol 2010;24:331-5. [Crossref] [PubMed]
- Guerrero-Wooley RL, Peacock JE Jr. Infectious Scleritis: What the ID Clinician Should Know. Open Forum Infect Dis 2018;5:ofy140. [Crossref] [PubMed]


