A pilot study—is there a role for mitoxantrone pleurodesis in the management of pleural effusion due to lung cancer?
Introduction
Pleural effusion (PE) is a common clinical presentation of a wide range of diseases (1). In patients over 50 years of age, 40% of PEs are caused by malignancy (2). The most common cause of malignant pleural effusion (MPE) is lung cancer (37%) (3,4). The presence of MPE indicates disseminated disease and reduces survival (5). The goal of management of MPE is often palliation (6), as quality of life diminishes with increased amounts of PE (7).
The most frequently performed procedure and the method of choice to manage recurrent, symptomatic MPE is chemical pleurodesis with a sclerosing agent in appropriate candidates (3,6). A number of investigational sclerosing agents with varying response rates are currently available for pleurodesis, for example, tetracycline, talc (slurry or poudrage), bleomycin, doxycycline, chemotherapeutic agents (cisplatin, cytosine arabinoside, and mitoxantrone) among others (5,6). Talc is purported to be the most effective (70–100%) for preventing MPE recurrence (5,6,8). Despite its high success rate, talc is not an ideal sclerosant due to associated adverse effects following its instillation: dyspnea, fever, chest pain, atelectasis, pneumonia, arrhythmias, empyema, and acute respiratory failure, which could progress to acute respiratory distress syndrome (6).
An alternative sclerosing agent for the management of MPE is mitoxantrone. At the institution in which this study was conducted, it had been occasionally used as a potential sclerosant, in addition to the more commonly used talc, to produce pleurodesis in patients with MPE. In the literature, mitoxantrone has been reported to be safe and effective in the treatment of MPE due to different malignancies, with response rates ranging between 76% and 88% (6). The mechanism of action of mitoxantrone in causing pleurodesis has not yet been fully elucidated, but is likely due to its inflammatory and antineoplastic activity (9).
To our knowledge, there is scarce information in the literature to date about the management of MPEs with mitoxantrone pleurodesis (MP) in patients diagnosed with primary lung malignancies. Most previous studies involving mitoxantrone have included mixed study populations with a wide array of primary cancers and/or different intracavitary sites (pleural, peritoneal, or pericardial) for pleurodesis (10-20). Only a few, thus far, have focussed specifically on a select group of neoplasms of interest, such as breast cancer (20), ovarian cancer (2), or metastatic sarcoma (21), but not lung cancer. The aim of this retrospective pilot study was to describe the potential management of local intrapleural therapy with mitoxantrone for cytologically-proven MPE in a group of lung cancer patients.
Methods
Patients
A comprehensive chart review was conducted on consecutively admitted patients with PE to the Department of Post-Intensive Care at the Clinic for Respiratory Diseases “Jordanovac” in Zagreb, Republic of Croatia. Of those, 34 patients were hospitalized for pleurodesis management of symptomatic PE caused by metastases originating from a variety of malignant diseases: lung carcinoma in 26 patients (76%), mesothelioma in 2 (5.9%), non-Hodgkin lymphoma in 2 (5.9%), breast carcinoma in 2 (5.9%), ovarian carcinoma in 1 (2.9%), and hypernephroma (renal cell carcinoma) in 1 (2.9%). The final study population consisted of 21 lung cancer patients (8 women and 13 men) who underwent chemical pleurodesis with mitoxantrone between December 2003 and February 2009. The study was approved by the Institute for Pulmonary Diseases of Vojvodina, Faculty of Medicine, University of Novi Sad, Novi Sad, Serbia investigational review board. All patients signed an informed consent form in order to participate in the study.
Inclusion criteria for the study were: (I) diagnosis of lung carcinoma; (II) cytologically-proven MPE; and (III) MP for symptomatic management of MPE. In all cases, MPE was confirmed by cytological analysis for the presence of neoplastic cells in the pleural fluid evacuated during the initial thoracocentesis and/or bedside chest tube drainage. The histological tumour type was determined by cytology and/or histopathology following bronchoscopy and bronchoalveolar lavage. Tumour, node, and metastases (TNM) staging was determined by examining the CT scan and classification was performed according to the sixth edition of the TNM Classification for Lung Cancer given that the seventh edition was not available at the time this study was conducted (22).
Procedure for chemical pleurodesis
Bedside thoracostomy with a 28 F chest tube was performed by a staff thoracic surgeon. Tube insertion site, marked at ultrasound examination, was the fifth or sixth intercostal space in the midaxillary line. Adequate pleural fluid drainage was achieved by gravity alone. Chest radiographs were obtained prior to chest tube drainage as well as immediately preceding sclerotherapy. When the fluid output was less than 150 mL per day, 30 mg of mitoxantrone (Onkotrone®, Pliva, Zagreb, Republic of Croatia) mixed with 50 mL of normal saline (NaCl) was instilled through the chest tube into the intrapleural cavity. The tube was unclamped 48 hours later and removed after any remaining fluid was drained. Follow-up chest radiographs were taken at the subsequent monthly control visits (1, 2 and 3 months).
Measurements
Baseline data for each patient were recorded from the medical chart. Karnofsky Performance Status (KPS) score was assigned in each case from the documented medical history and physical examination. KPS scores were converted to Eastern Cooperative Oncology Group (ECOG, Zubrod) Equivalence Scale values. ECOG score has been reported to have better predictive ability of survival and is thus considered to be preferred to KPS (23).
The initial size of pleural effusion prior to pleurodesis, radiographically verified, was classified as: (I) moderate, extending from the diaphragm to the pulmonary hilum (1/3 to 1/2 of the hemithorax); or (II) massive, beyond the hilar region [8]. Total pleural fluid output prior to pleurodesis was calculated from the daily progress notes recorded during the hospital admission. To evaluate the success of the pleurodesis at 1, 2, and 3 months post-sclerotherapy, the radiologist’s written interpretations for each chest radiograph were recorded. Any ambiguities were clarified by examining the X-rays directly.
Sclerotherapy success based on pleural effusion levels on chest radiograph was defined as [20]: (I) complete response (CR)—no reaccumulation of pleural effusion; (II) partial response (PR)—reaccumulation of fluid above the initial post-sclerotherapy level but below the original predrainage level; and (III) progressive disease (PD)—reaccumulation to or above the original level with symptoms and requiring repeat drainage. In this study, not applicable (NA) referred to patients who died of advanced disease, and lost to follow-up (LTF), to those failing to return to the hospital for the scheduled control due to poor condition caused by advanced disease. Overall success (OS) was recorded as the sum of complete and PRs for the post-pleurodesis evaluation period.
Complications of the pleurodesis procedure and side effects of mitoxantrone instillation were obtained from the daily progress notes. Survival (in days) for each patient was calculated from the date of the MP procedure (first one, if repeated) until death. Patients (alive at the last follow-up or hospitalization and not returning later for a visit) received a telephone call by the department’s head nurse to determine survival status. The date of the final phone check-up at the end of the study period was used in the data analysis of surviving patients.
Statistical analysis
The descriptive parameters are expressed as the frequency (%), mean ± standard deviation (range), and the median (95% confidence interval, CI, or interquartile range). Kaplan-Meier (K-M) actuarial method was used to construct survival curves for the overall survival analysis. All data analyses were performed using MedCalc for Windows, Version 10.02 (MedCalc Software, Mariakerke, Belgium).
Results
In 20 patients (95.2%), the primary tumour was advanced at diagnosis (NSCLC: TNM stage IIIB and IV, and SCLC: extensive). All patients were symptomatic at the time of diagnosis, presenting with pain (chest, shoulder or back) in 66.7% of the cases, dyspnea in 80%, and cough in 75%, due to advanced tumour progression and/or the presence of PE. In 10 cases (47.6%), the diagnosis of lung cancer was made during or after work-up for the presenting symptomatic PE. The poor performance status of three patients (14.3%) precluded therapy for their underlying disease. All other patients underwent treatment according to the appropriate oncologic protocols, receiving systemic chemotherapy and/or radiotherapy (palliative and/or prophylactic) as indicated—surgical resection was not performed.
From the chest radiograph prior to chest tube drainage, moderate PE was observed in 12 patients (57.1%), while massive PE occurred in 9 (42.9%). Unilateral effusion was present in 18 patients (85.7%), bilateral effusion in three (14.3%). The mean volume of PE drained at bedside via chest tube prior to MP—the first, if two attempts were made—was 5,306±3,930 mL (range, 600 to 17,650 mL, median =4,270 mL). The mean time period between the diagnosis of MPE and MP was 33.3±70.7 days (range, 1 to 329 days; median =12 days). The chest tube remained in place for a mean interval of 11.6±5.7 days (range, 4 to 27 days; median =10.5 days). In two patients (9.5%), a long-term indwelling pleural catheter was introduced post-sclerotherapy at 139 and 333 days, respectively, to control recurrent, symptomatic MPE. For the same reason, three other patients (14.3%) required a second attempt of mitoxantrone sclerotherapy within 6, 43 and 333 days, respectively, following the first mitoxantrone instillation.
Most patients (n=17, 80.9%) did not experience any adverse effects or complications from the pleurodesis procedure. The minor side effects observed in four patients (19.0%) are shown in Table 1. There were no deaths related to bedside thoracostomy.
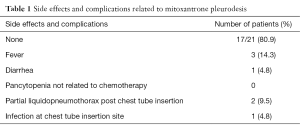
Full table
Table 2 summarizes the success rates for the 1-, 2-, and 3-month follow-up periods. The mortality rate at 1 month follow-up was 19.0% (n=4), due to rapid progression of end-stage disease; mean time period of 15±5.7 days (range, 11 to 23 days) from MP. Of the 17 patients (80.9%) alive at the 1-month follow-up, a successful outcome to MP was achieved in a total of 15 cases (88.2%).
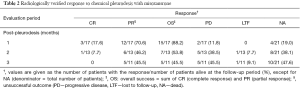
Full table
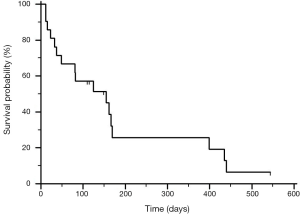
The K-M survival curve for the entire group with reference to the date of MP is plotted in Figure 1; the median survival was 155 days (5.2 months). The K-M survival curve from the date of diagnosis of primary lung carcinoma is shown in Figure 2; the median survival was 370 days (12.3 months).

Discussion
Lung cancer is the most common cause of MPE (3,4). Approximately 15% of all lung cancer patients are reported to present with MPE at diagnosis (4). In this study, 47.6% of the patients presented with MPE at diagnosis of the primary lung cancer. The higher incidence can be accounted for by the predominance of advanced stage disease (NSCLC, stage IIIB and IV) at presentation. In addition, the inclusion criteria of our study biased in favour of a higher rate of MPE as only lung cancer patients with cytologically-proven MPE requiring chemical pleurodesis for management were selected, all others were excluded.
Prognosis following detection of MPE is generally poor, with reported median survivals of 3 (lung cancer) to 12 (breast cancer) months (5,7), and 1-month mortality rates ranging from 29% to 54% (24,25). In this study, the median survival from date of MP was 5.2 months, while the 1-month mortality rate of 19% was lower than in previous studies. This discrepancy may be due to different starting points for determining survival, either from MPE diagnosis, or as in our case, from the date of the procedure itself.
Moreover, survival in MPE patients is directly related to the type and stage of primary tumour (5,7). With respect to overall median survival from diagnosis of primary lung carcinoma, our group of patients with predominantly advanced disease (NSCLC stage IIIB and IV, SCLC extensive) and MPE (n=20, 95.2%) survived almost twice as long (12.3 months) than expected for stage IIIB NSCLC patients with MPE (6.5 months) (26). The improved 1-month mortality rate and overall survival may result from better specialized care at a tertiary (referral) center for lung diseases, as in this case.
Management of MPE is often palliative and most commonly involves chemical pleurodesis with a sclerosing agent. Although talc is frequently used, there may be other equally effective and safe options depending on the institution and country in which pleurodesis is being performed. The current study was conducted to assess the feasibility of using MP in a group of lung cancer patients in order to improve the existing treatment protocol for MPE management. Most PEs are known to reaccumulate within 30 days (27). For that reason, three consecutive 1-month post-sclerotherapy intervals were used. This study, with a 1-month success rate of 88.2%, is consistent with the findings of most previous studies that mitoxantrone is an effective sclerosing agent, at least within the first 30 days of the procedure (2,8,10,16,20).
The side effects of MP in our study were mild and infrequent. This is consistent with the findings of previous studies with mitoxantrone (2,10,13,16,20). With few adverse effects, mitoxantrone may therefore be considered safe for use as a sclerosant.
There are a number of limitations to this study. The retrospective study design may have introduced several biases, including selection and referral bias. The chart review was conducted in a tertiary care center where many patients are referred for treatment at terminal stages of their disease, thus making generalization of our findings difficult. The small sample size may seem to limit this research, but as a feasibility study is justified and appropriate. Although the current practice in the United States is to reduce hospitalizations by home management with outpatient PleurX catheter drainage, pleurodesis for MPE management is still routine in Croatia and perhaps in other European countries as well. A larger prospective study is necessary in order to corroborate the response rate found here for mitoxantrone and provide relevant patient reported outcomes, including quality of life data.
Conclusions
In conclusion, our study is noteworthy for presenting the clinical usefulness and acceptable safety profile of mitoxantrone as an alternative sclerosing agent to palliate MPE in a small group of lung cancer patients in Croatia. Further research investigating MP in a larger number of lung cancer patients, preferably in a randomized controlled setting with a head-to-head comparison of talc pleurodesis, is warranted in order to determine the efficacy and safety of mitoxantrone. A closer look at the factors affecting success of the pleurodesis procedure and overall survival of lung cancer patients with MPE, as well as quality of life measures, would permit better individualized management.
Acknowledgements
The authors would like to thank Dr. Paula Peyrani for her editorial assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional ethic review board and informed consent was obtained from all patients.
References
- Rahman NM, Chapman SJ, Davies RJ. Pleural effusion: a structured approach to care. Br Med Bull 2005;72:31-47. [Crossref] [PubMed]
- Barbetakis N, Vassiliadis M, Kaplanis K, et al. Mitoxantrone pleurodesis to palliate malignant pleural effusion secondary to ovarian cancer. BMC Palliat Care 2004;3:4. [Crossref] [PubMed]
- Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med 2008;102:939-48. [Crossref] [PubMed]
- Sekine I, Sumi M, Saijo N. Local control of regional and metastatic lesions and indication for systemic chemotherapy in patients with non-small cell lung cancer. Oncologist 2008;13 Suppl 1:21-7. [Crossref] [PubMed]
- Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58 Suppl 2:ii29-38. [Crossref] [PubMed]
- Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008;83:235-50. [Crossref] [PubMed]
- Bielsa S, Salud A, Martínez M, et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med 2008;19:334-9. [Crossref] [PubMed]
- Inoue T, Ishida A, Nakamura M, et al. Talc pleurodesis for the management of malignant pleural effusions in Japan. Intern Med 2013;52:1173-6. [Crossref] [PubMed]
- Fox EJ. Mechanism of action of mitoxantrone. Neurology 2004;63:S15-8. [Crossref] [PubMed]
- van Belle AF, ten Velde GP, Wouters EF. Chemical pleurodesis with mitoxantrone in the management of malignant effusions. Eur J Cancer 1998;34:205-6. [PubMed]
- Vargas FS, Teixeira LR, Antonangelo L, et al. Acute and chronic pleural changes after the intrapleural instillation of mitoxantrone in rabbits. Lung 1998;176:227-36. [Crossref] [PubMed]
- Bjermer L, Gruber A, Sue-Chu M, et al. Effects of intrapleural mitoxantrone and mepacrine on malignant pleural effusion--a randomised study. Eur J Cancer 1995;31A:2203-8. [Crossref] [PubMed]
- Groth G, Gatzemeier U, Häussingen K, et al. Intrapleural palliative treatment of malignant pleural effusions with mitoxantrone versus placebo (pleural tube alone). Ann Oncol 1991;2:213-5. [PubMed]
- Kuhn K, Purea H, Selbach J, et al. Treatment with locally applied mitoxantrone. Acta Med Austriaca 1989;16:87-90. [PubMed]
- Maiche AG, Virkkunen P, Kontkanen T, et al. Bleomycin and mitoxantrone in the treatment of malignant pleural effusions. A comparative study. Am J Clin Oncol 1993;16:50-3. [Crossref] [PubMed]
- Morales M, Expósito MC. Intrapleural mitoxantrone for the palliative treatment of malignant pleural effusions. Support Care Cancer 1995;3:147-9. [Crossref] [PubMed]
- Musch E, Gremmler B, Nitsch J, et al. Intrapericardial instillation of mitoxantrone in palliative therapy of malignant pericardial effusion. Onkologie 2003;26:135-9. [Crossref] [PubMed]
- Norum J, Lunde P, Aasebø U, et al. Mitoxantrone in malignant pericardial effusion. J Chemother 1998;10:399-404. [Crossref] [PubMed]
- Torsten U, Opri F, Weitzel H. Local therapy of malignant pleural effusion with mitoxantrone. Anticancer Drugs 1992;3:17-8. [Crossref] [PubMed]
- Barbetakis N, Antoniadis T, Tsilikas C. Results of chemical pleurodesis with mitoxantrone in malignant pleural effusion from breast cancer. World J Surg Oncol 2004;2:16. [Crossref] [PubMed]
- Kelly J, Holmes EC, Rosen G. Mitoxantrone for malignant pleural effusion due to metastatic sarcoma. Surg Oncol 1993;2:299-301. [Crossref] [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 1996;32A:1135-41. [Crossref] [PubMed]
- Kilic D, Akay H, Kavukçu S, et al. Management of recurrent malignant pleural effusion with chemical pleurodesis. Surg Today 2005;35:634-8. [Crossref] [PubMed]
- Marom EM, Patz EF Jr, Erasmus JJ, et al. Malignant pleural effusions: treatment with small-bore-catheter thoracostomy and talc pleurodesis. Radiology 1999;210:277-81. [Crossref] [PubMed]
- Alon BN, Anson BL. Pleural effusion in patients with non-small cell carcinoma--stage IV and not T4. Lung Cancer 2007;57:123. [Crossref] [PubMed]
- Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol 2009;16:745-50. [Crossref] [PubMed]


