Thoracic duct cyst of posterior mediastinum: a “challenging” differential diagnosis
Introduction
The thoracic duct is the largest lymphatic vessel of the lymphatic system. It originates from the cisterna chyli in the abdomen, traverses the diaphragm at the aortic aperture and penetrates through the posterior and superior mediastinum (1). Thoracic duct cysts, which may be of congenital or degenerative origin, are extremely rare lesions usually located at the neck area and less often at the mediastinum (2). We report a case of a thoracic duct cyst, located just above the diaphragm, that was treated with surgical excision and emphasize the “challenging” diagnosis, since such cysts can be easily mistaken for neurogenic tumors or bronchial cysts.
Case presentation
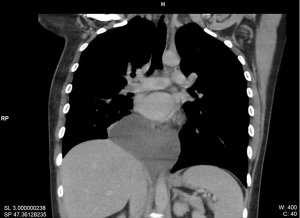
During a regular follow-up examination of a 28-year-old female, without pathologic medical history, a chest X-ray revealed a retrocardial mass lesion located at the left lower lung field with no other thoracic anomaly. Physical examination and laboratory findings were normal. Chest computed tomography demonstrated a large cystic formation 13×7 cm2 located at the base of the chest. This formation had clear boundaries and homogeneous hydric densities, penetrating the area between esophagus and the aorta (Figure 1). Judging by the mediastinal audit, no swollen lymph nodes or pathological dimensions to any of the large vessels were noted. The pulmonary parenchyma presented normal and no focal lesions were observed. The bronchial tree was unrestrained at its central junctions. No pleural effusion or pleural thickening was observed.
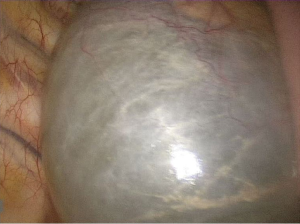
The patient, under general anesthesia, underwent video-assisted thoracic surgery (VATS) excision of the cyst. Intraoperatively, a large cyst filled with liquid was recognized at the base of the right thoracic cavity (Figure 2). The liquid of the cyst was aspirated and sent for cytological examination. The cyst was gradually and thoroughly detached from the esophagus, the aorta and the right lower lobe. The remaining thoracic duct was ligated with multiple nitinol clips and followed by chest tube drainage.
The patient, extubated in a hemodynamically stable condition and was transferred to the ward. The post-operative course was uneventful. The patient was discharged from the hospital in the third (3rd) post-op day.
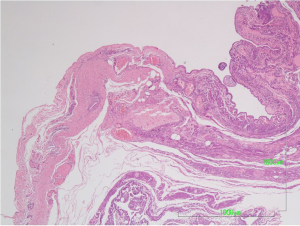
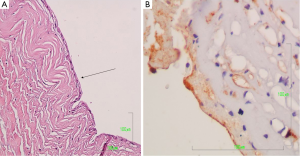
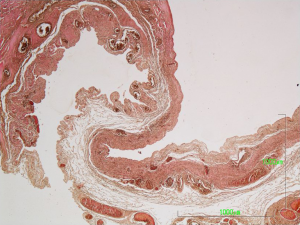
The surgical specimen was sent for pathologic examination and the content of the cyst for cytologic examination. Microscopic examination of the fluid content showed bloody material with abundant foamy histiocytes and inflammatory cells. On gross inspection, the lesion measuring 4.5×3.5×0.8 cm3, was unilocular cystic, with smooth and whitish surface. Microscopic examination revealed that the cyst wall consisted of fibrous connective tissue with scattered lymphocytic aggregates and small vessels (Figure 3). The wall was lined by flat or low cuboidal cells with uniform, bland nuclei (Figure 4A). The endothelial origin of lining cells was confirmed with the positivity for the endothelial marker CD34 (Figure 4B). No atypia neither mitosis was observed. The fibrous cyst wall was revealed with Van Gieson histochemical stain for connective tissue (Figure 5). The given diagnosis according to the histopathological, histochemical and immunohistochemically findings was that of a thoracic duct cyst.
Two years after the surgical procedure, all follow-up imaging findings are normal and our patient is well and healthy.
Discussion
Thoracic duct cysts are rare. The first reported case was found during an autopsy examination by Carbone in 1892 and the first ante mortem description of this disease was made by Emerson in 1950 (3). Although the pathogenesis of thoracic duct cysts remains obscure, it is thought to be related to congenital or degenerative defects of the wall of the thoracic duct and obstruction of lymphoid flow (4). These cysts are usually asymptomatic, although they sometimes cause clinical symptoms such as chest pain, dyspnea, dysphagia, cough or backache due to pressure of the surrounding structures (5). The preoperative diagnosis of thoracic duct cysts can be difficult. Magnetic resonance imaging (MRI), especially T2-weighted images, is an accurate “diagnostic tool” for demonstrating the anatomic boundaries and the cystic nature of these lesions. However, it is less helpful in distinguishing these lesions among “mimickers”, such as pericardial, pleural mesothelial, bronchial, esophageal duplication or neurogenic cysts (6). The recommended treatment remains surgical resection although direct puncture sclerotherapy was recently reported to be an optional method (7,8). However, the long term effect still needs to be evaluated. Chylothorax, which is the most common postoperative complication after surgical procedure, can be avoided by ligating all the brunches of thoracic duct (9). Fine-needle aspiration is thought to be an alternative option; however there is still controversy about its diagnostic value (7). Final diagnosis is established with microscopic examination of the surgical specimen. The lining of the wall consists of uniform flat or low cuboidal cells of endothelial nature, whereas the lining of pericardial and pleural cysts is mesothelial and that of bronchial origin is columnar pseudostratified epithelium. Differential diagnostic problems can be solved by using specific immunohistochemically markers that reveal the endothelial nature of the lining cells (10). Thoracic duct cysts are totally benign and carry an excellent prognosis after adequate surgical excision. No cases of malignant transformation have been reported so far (11). In summary, our patient was incidentally diagnosed with a mediastinal tumor during a regular follow-up examination. Initially, the possibility of a thoracic duct cyst was not considered, due its “unexpected” anatomical location. These therefore, benign lesions are diagnostically challenging with distinct clinical significance, since they can be easily mistaken for other, more common mediastinal tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Neas JF. The lymphatic system. In: Martini FH, Timmons MJ, Tallitsch B, editors. Human anatomy. 4th ed. Old Tappan (NJ): Pearson Education/Benjamin Cummings, 2003.
- Pramesh CS, Deshpande MS, Pantvaidya GH, et al. Thoracic duct cyst of the mediastinum. Ann Thorac Cardiovasc Surg 2003;9:264-5. [PubMed]
- Emerson GL. Supradiaphragmatic thoracic duct cyst: an unusual mediastinal tumor. New Engl J Med 1950;242:575-8. [Crossref]
- Mortman KD. Mediastinal thoracic duct cyst. Ann Thorac Surg 2009;88:2006-8. [Crossref] [PubMed]
- Turkyilmaz A, Eroglu A. A giant thoracic duct cyst: an unusual cause of dysphagia. J Thorac Cardiovasc Surg 2007;134:1082-3. [Crossref] [PubMed]
- Nakano T, Okamoto H, Maruyama S, et al. Three-dimensional imaging of a thoracic duct cyst before thoracoscopic surgery. Eur J Cardiothorac Surg 2014;45:585. [Crossref] [PubMed]
- Gill MT, Lian TS, Thibodeaux JD, et al. Cervical thoracic duct cyst: Importance of preoperative suspicion for appropriate management of left-sided neck mass. Ear Nose Throat J 2012;91:E13-5. [PubMed]
- Kadkhodayan Y, Yano M, Cross DT 3rd. Direct puncture sclerotherapy of a thoracic duct cyst presenting as an enlarging left supraclavicular mass. BMJ Case Rep 2013;2013.
- Kwak MY, Bae CH. Thoracic Duct Cyst in Mediastinum - A case report -. Korean J Thorac Cardiovasc Surg 2011;44:83-5. [Crossref] [PubMed]
- Petrova TV, Mäkinen T, Mäkelä TP, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 2002;21:4593-9. [Crossref] [PubMed]
- Kumar A, Ramakrishnan TS, Sahu S. Primary cervical thoracic duct cyst: a case report and review of the literature. Ear Nose Throat J 2014;93:E17-21. [PubMed]