
Identification of six hub genes in mantle cell lymphoma patients with BTKi resistance
Introduction
Mantle cell lymphoma (MCL) is an aggressive subtype of non-Hodgkin lymphoma (NHL), accounting for 2–10% of all NHL cases (1). Despite the improvement in response to currently available therapies, patients with MCL inevitably relapse (2).
To date, Bruton tyrosine kinase inhibitors (BTKis) have been widely used in the treatment of relapse/recurrence (R/R) MCL as irreversible covalent inhibitors (3). Despite eliciting impressive responses in relapsed patients, the success of BTKi treatment has been thwarted by the development of resistance (2). Primary resistance has been observed to range from 10.2% to 35%, and acquired resistance between 17.5% and 54% among all ibrutinib-treated patients (4). The resistance is associated with a low response rate to salvage therapies, a short duration of response, and dismal overall survival (OS), which is becoming a major clinical issue in the treatment of patients with MCL (4).
In recent years, many MCL-related hub genes and clusters have been identified (5,6). Based on the WGCNA network, 12 subgenes were screened and 7 genes were associated with OS time (7). MMP9 was identified as a hub gene in MCL by protein-protein interaction (PPI) network (8). In contrast to previous analyses in MCL, our study focused on BTKi-resistant MCL mutation profile in Chinese population, which has not been reported yet, providing a real-world gene mutation analysis. In this paper, we discuss the clinical features and treatment options, and identify mutated hub genes of BTKi-resistant MCL by panel sequencing. The high-frequency mutation gene set was obtained by sequencing analysis, the prognostic related gene set was obtained by univariate Cox regression, and the hub genes were screened by intersection. Furthermore, high gene connectivity was verified, and the promising prospect of hub genes in salvage therapy was discussed. We present the following article in accordance with the REMARK reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4314/rc).
Methods
Patient data
The clinical data and tumor biopsy results of 28 BTKi-resistant MCL patients at Peking University Third Hospital between 1 August 2008 and 21 March 2021 were retrospectively collected. Any MCL patients with incomplete clinical information were excluded. Treatment decisions were based solely on consensus recommendations at the time of diagnosis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Peking University Third Hospital (No. M2022564) and individual consent for this retrospective analysis was waived. Lymphoma tissue from patients who relapsed was then sequenced by using the GP79 panel (Table 1). Clinical characteristics were collected including gender, age, immunophenotype, lactate dehydrogenase (LDH) level, extranodal involvement, Eastern Cooperative Oncology Group performance status (ECOG PS), Ann Arbor stage, MCL International Prognostic Index (MIPI) score, treatment, and response. Tumor response was classified as either complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD) according to the International Workshop Criteria. The OS was defined as the time from diagnosis to death. Progression-free survival (PFS) was defined as the time from diagnosis to either disease progression or death.
Table 1
| ARID1A | BCOR | CD58 | FAF1 | KMT2D | NOTCH2 | RB1 | TLR2 |
| ARID1B | BIRC3 | CD79A | FAS | KRAS | NRAS | RRAGC | TNFAIP3 |
| ATM | BMI1 | CD79B | FAT1 | MAP3K14 | NSD2 | S1PR1 | TNFRSF11A |
| ATP6AP1 | BRAF | CDKN2A | FOXO1 | MDM2 | P2RY8 | S1PR2 | TNFRSF14 |
| ATP6V1B2 | BTG1 | CREBBP | GNA13 | MEF2B | PIK3CA | SAMHD1 | TP53 |
| B2M | BTK | CXCR4 | HNRNPH1 | MKI67 | PIK3CD | SMARCA4 | TRAF2 |
| BAX | CARD11 | EBF1 | IKBKB | MTOR | PIM1 | SOCS1 | TRAF3 |
| BCL10 | CCND1 | EP300 | IKZF3 | MYC | POT1 | SP140 | UBR5 |
| BCL2 | CCND2 | EPHA7 | ITPKB | MYD88 | PTEN | STAT3 | XBP1 |
| BCL6 | CCND3 | EZH2 | KMT2C | NOTCH1 | PTPRD | STAT6 |
Library construction, hybrid selection, and ultra-deep next-generation sequencing
The DNA was extracted from paraffin-embedded lymphoma tissues. Indexed libraries were prepared from sheared DNA using the DNA Library Preparation Kit (Beijing Mygenostics, Beijing, China). The capture of target fragments was then undertaken using the GenCap (Beijing Mygenostics). Sequencing was performed on the DNBSEQ-T7 (MGI, Shenzhen, China) followed by the setting of paired-end 150 bp.
Sequence alignment, processing, and single nucleotide variation/indel/copy number variation detection
Sequenced reads were aligned to the human genome reference hg19 by using Burrows-Wheeler Alignment (BWA: v0.7.17;). MuTect2 (v4.2.0; Broad Institute, Cambridge, MA, USA) was applied to identify somatic mutations, small insertions, and deletions. HaplotypeCaller (v4.1.0; Broad Institute) was used to call germline single-nucleotide polymorphisms. The DNA copy number analysis was conducted using CNVkit (v0.9.2; Helen Diller Family Comprehensive Cancer Center, UCSF).
Selection, analysis and verification of hub genes
Prognostic related gene set was obtained by univariate Cox regression (P<0.1), and the hub genes were screened by intersection. A co-expression network of these hub genes via GeneMANIA (https://genemania.org/) were constructed. The hub genes connectivity was verified by CytoHubba plug-in of Cytoscape with common algorithms [Betweenness, Bottleneck, Closeness, Clustering coefficient, Degree, density of maximum neighborhood component (DMNC), EcCentricity, edge percolated component (EPC), maximal clique centrality (MCC), maximum neighborhood component (MNC), Radiality, Stress].
PPI network construction and module analysis
Search Tool for the Retrieval of Interacting Genes (STRING; http://string-db.org) (version 11.5) were used to construct a PPI network with complex regulatory relationships. Cytoscape (http://www.cytoscape.org) (version 3.8.2) was used to visualize this PPI network. Molecular complex detection technology (MCODE), a plug-in of Cytoscape, was used to analyze key functional modules. Set the selection criteria as: K-core =2, degree cutoff =2, max depth =100, and node score cutoff =0.2.
Statistical analysis
All statistical analyses were conducted with SPSS 22.0 (IBM Corp., Armonk, NY, USA) and R (version 4.1.2; https://www.r-project.org/) as appropriate. All the data were complete. The log-rank test was used to compare differences between Kaplan-Meier curves. The coefficients of the univariate Cox regression model were analyzed with the “survminer” package. Differences in qualitative variables were compared with the chi-square test. A two-tailed P value <0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 28 MCL patients with BTKi resistance were enrolled at Peking University Third Hospital in China. In our cohort, the median age at diagnosis was 61 (range, 36 to 77) years; 17 (60.7%) of patients were pathologically classified as classical MCL; 10 patients (35.7%) had experienced B symptoms; and β2 microglobulin (B2M) elevation presented in 18 (64.3%) patients. Compared with the largest published Chinese MCL data from Chinese Hematology Centers (CHC), the baseline characteristics are summarized in Table 2. Age, gender, and Ann Arbor stage were not statistically different from the CHC data (P=0.415; P=0.495; P=0.559). The ECOG PS (P=0.039), MIPI (P=0.033), LDH (P=0.001), bone marrow (BM) positive (P=0.019), and Ki67 (P=0.038) were significantly different in our BTKi cohort.
Table 2
| Characteristics | CHC1 (n=518) | BTKi (n=28) | P value2 |
|---|---|---|---|
| Age (years), median [range] | 58 [28–83] | 61 [36–77] | |
| Age, n (%) | 0.415 | ||
| ≤60 | 309 (59.7) | 14 (50.0) | |
| >60 | 209 (40.3) | 14 (50.0) | |
| Gender, n (%) | 0.495 | ||
| Male | 399 (77.0) | 20 (71.4) | |
| Female | 119 (23.0) | 8 (28.6) | |
| ECOG PS, n (%) | 0.039 | ||
| 0–1 | 459 (88.6) | 22 (78.6) | |
| >1 | 44 (8.5) | 6 (21.4) | |
| Ann Arbor stage, n (%) | 0.559 | ||
| I–II | 62 (12.0) | 2 (7.1) | |
| III–IV | 418 (80.7) | 26 (92.9) | |
| MIPI, n (%) | 0.033 | ||
| High risk | 96 (18.5) | 11 (39.3) | |
| Intermediate risk | 100 (19.3) | 9 (32.1) | |
| Low risk | 224 (43.2) | 8 (28.6) | |
| LDH, n (%) | 0.001 | ||
| Elevated | 96 (18.5) | 18 (64.3) | |
| Normal | 220 (42.5) | 10 (35.7) | |
| BM positive, n (%) | 0.019 | ||
| No | 247 (47.7) | 8 (28.6) | |
| Yes | 217 (41.9) | 20 (71.4) | |
| Ki67, n (%) | 0.038 | ||
| <30% | 205 (39.6) | 7 (25.0) | |
| ≥30% | 231 (44.6) | 21 (75.0) |
1, CHC cohort, the largest published Chinese MCL data (15); 2, chi-square test, continuous correction chi-square test, Fisher exact test. MCL, mantle cell lymphoma; BTKi, Bruton tyrosine kinase inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance score; MIPI, MCL International Prognostic Index; LDH, lactate dehydrogenase; BM, bone marrow; CHC, Chinese Hematology Centers.
Prognosis and treatment plan
After a median 22-month follow-up (range, 4 to 151 months), 11 patients died and 17 relapsed. The 1-year OS was 81.1% and 3-year OS was 63.9% (Figure 1A). The 1-year PFS was 57.14%, and 3-year PFS was 7.14%, with a 14-month median PFS (m-PFS) (Figure 1B). The 1-year PFS2 and OS2 rates were 26.0% and 81.2%, respectively, with 6-month m-PFS2 and 17-month median OS (m-OS)2 (Figure 1C,1D). After first-line treatment, 12 patients achieved CR, 10 achieved PR, 3 achieved SD, and 3 progressed (Figure 1E). Some 60.71% received ibrutinib (n=17), 17.86% received zebotinib (n=5), 14.28% received orelabrutinib (n=4), and the remainder received acalabrutinib (n=2). After a short unsatisfactory treatment response (CR/PR =17, SD/PD =11), BTK resistance occurred quickly (m-PFS2: 6 months), with 11 cases deceased by the end of follow-up.
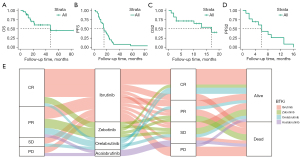
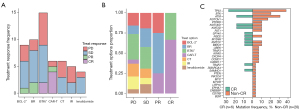
Several salvage treatments have been used in patients with BTK resistance, including B cell lymphoma-2 (BCL-2)+ (n=9), bendamustine and rituximab (BR) (n=10), BTKi+ (n=15), chimeric antigen receptor T-cell (CAR-T; n=6), chemotherapy (CT) (n=6), ibrutinib-rituximab (IR) (n=5), and lenalidomide (n=4) (Figure 2A). The BTK combined with other drugs is the most commonly used treatment. The response to BTK+ reached CR in 2 patients, SD in 7 patients, and PD in 6 patients. A total of 6 patients were treated with CAR-T and responded well with 4 CR, 1 PR, and 1 PD. Some 66% of CR benefited from CAR-T treatment, 50% of PR benefited from BR treatment, 27% and 23% of SD occurred from BTKi and BR treatment, and 31% of PD came from BTKi treatment (Figure 2B). According to the treatment effect, Patients with BTK, NOTCH1, and PTPRD mutations respond poorly to salvage therapy (Figure 2C).
The landscape of MCL-GP79
To visualize the distribution of mutations in the 79 genes for MCL patients, we depicted the landscape of MCL-BTKi-GP79 (Figure 3). A total of 38 gene mutations were detected in our cohort. Of the 28 MCL cases, all patients detected ≥1 coding mutation within the 79-panel (mean number of mutated genes per case, 3.66; range, 2 to 11). The most common mutated genes were TP53 (50%), ATM (43%), KMT2D (21%), NOTCH2 (18%), NSD2 (18%), NOTHC1 (14%), BTK (14%), BIRC3, CXCR4, and TRAF2 (11% each). Mutation frequencies greater than 5% are defined as high-frequency mutant genes.

Identification of hub genes
We further explored genes with potential prognostic effects (P<0.1) by a univariate Cox regression model (Figure 4A). Based on Cox regression, CARD11, CD79B, and MYC were associated with poor OS. The genes ATM, B2M, CARD11, CREBBP, MTOR, NOTCH2, PIK3CA, PTEN, PTPRD, and TP53 were associated with poor PFS. Additionally, the following 8 genes were associated with poor OS2: ARID1A, ARID1B, CD79B, CXCR, KRAS, STAG1, TET2, and TTN. Of these, CARD11 is related to both PFS and OS, and CD79B is related to both OS2 and OS. The results of the Cox analysis of each gene are shown in Table S1.

Among the potential prognostic-related genes, the mutation frequencies for ATM, CARD11, KRAS, NOTCH2, PTPRD, and TP53 were greater than 5%, considered as the hub genes by the intersection of the high-frequency mutation gene set and prognostic-related gene set (Figure 4B). Based on the GeneMANIA (https://genemania.org/) database, we analyzed the co-expression network and related functions of these genes (Figure 4C). A total of 20 related genes were integrated, with 7 pathways identified, including cell cycle regulation, signaling in ERBB, Notch, Ras, and TOR.
PPI network construction and analysis
Through the 12 algorithms of plug-in CytoHubba, we confirmed that hub genes rank highly in the PPI network (Table S2). The PPI network of the mutation gene was constructed using Cytoscape, which contained 36 nodes and 229 interaction pairs. According to degree algorithms, we visualized the gene connectivity (Figure 5A,5B). Through MCODE plug-in of Cytoscape, three closely connected gene modules were obtained (Figure 5C-5E). Among the six hub genes, ATM, KRAS, TP53 and NOTCH2 were enriched in model 1 and scored the highest (14.533) among the three clusters, implying a closely connection among the four hub genes (Figure 5C).

Discussion
About 5–10% of NHLs are MCL, a rare incurable B-cell lymphoma (9,10). Despite an enhanced understanding of biology and the development of effective therapeutic strategies resulting in improved survival (11), MCL patients continue to experience a poor prognosis overall and inevitable relapse (2). Currently, BTKi are widely used in the treatment of R/R MCL, with an impressive response, and uniform development of resistance (2). Once ibrutinib-treated patients relapse, the 1-year survival rate is only 22% (12,13). The resistance to BTKi is a key issue in MCL patients. A total of 28 MCL patients with BTKi resistance were included in our cohort, and their clinical characteristics, prognosis, and treatment data were collected. Gene mutations of patients were obtained by GP79 panel sequencing, with six hub genes identified.
In our cohort, the median age of onset was 61 years and the male-to-female ratio was 2.4:1 which is consistent with overall MCL patients (14). Compared with the largest published Chinese MCL data from CHC (15), our cohort had higher ECOG (P=0.039) and MIPI (P=0.033) scores, a higher percentage of elevated LDH (P=0.001), BM positive (P=0.019), and Ki67 (P=0.038), which showed statistically significant differences. Some 93% of patients were stage III–IV in our cohort, higher than the 80.7% in the CHC cohort. These data suggest that patients with BTKi resistance have less favorable initial clinical features in terms of physical situation, disease score, involvement, proliferation index, and hematologic indicators.
Based on our treatment and prognosis data, the response rate of BTKi was 60.71%, which is consistent with previous articles (55–72%) (16-18). As in previous studies, patients with BTK resistance had a very short OS. Once ibrutinib-treated patients relapse, their 1-year survival rate is only 22% (12,13). After an unsatisfactory treatment response (CR/PR =17, SD/PD =11), BTKi resistance occurred quickly (m-PFS2: 6 months), with a total of patients 11 dying, and the m-OS2 was 47 months. The median duration of response to BTKi in our cohort was 6 months, which is exactly the same as that reported by Cheah et al. [2015] (12). Our cohort suggested that BTKi-resistant patients have poor treatment response, rapid resistance occurrence, and death within a short time.
The efficacy of salvage therapy after resistance was unsatisfactory, with the longest OS of 19.2 months and the shortest OS of 3.7 months (4). After the onset of resistance, 15 patients were treated with other BTKi drugs, yielding 2 cases of CR, 7 SD, and 6 PD. Compared with other salvage therapy, BTKi+ had the highest SD/PD ratio among all kinds of rescue treatments. Two resistant patients treated with BTKi showed CR, without BTK mutation, implying the great role of BTK mutation in BTK resistance and providing clinical evidence for the use of other BTK drugs (e.g., LOXO-305) (19). The therapeutic effect of CAR-T has been verified. A total of 6 patients were treated with CAR-T (4 CR, 1 PR, and 1 PD) and 66% of CR benefited from CAR-T treatment, suggesting that CAR-T exerts a positive treatment response (20).
Tumor immune microenvironment and genes mutations are closely associated with the evolution of BTKi resistance in MCL (4). In our cohort, the most common mutated genes were TP53 (50%), ATM (43%), KMT2D (21%), NOTCH2 (18%), NSD2 (18%), NOTHC1 (14%), BTK (14%), BIRC3, CXCR4, and TRAF2 (11% each) (Figure 3).
CXCR4 has been shown to be associated with MCL drug resistance by immune microenvironment. In MCL, CXCR4 mediates homing and invasion of SOX11-positive subtype, ROS-induced bortezomib resistance and CT resistance by ERK1/2, AKT and NF-κB signal (21-23). Our mutation rate of CXCR4 (14%) is higher than previous general MCL reports, suggests the role of microenvironment in BTKi resistance (24-26). By attenuated the covalent binding affinity of ibrutinib, BTK was the first identified mutation revealing the molecular mechanisms underlying acquired ibrutinib resistance (27). Occurred in 14% of patients, BTK mutations explaining part of resistance in our cohort. Besides, our data (Figure 3) validated mutations mediated BTKi resistance in various pathways, including the classical/alternative NF-κB pathway (TRAF2 11%, BIRC3 11%, CARD11 7% and TRAF3 4%), SWI-SNF (ARID1 4% and SMARCA4 4%) and cell cycle initiation/progression (CCND1 7%) (28,29).
To further explore the association between drug resistance, mutations and prognosis, a cox regression modelling (Figure 4A) was established, and six genes (ATM, CARD11, KRAS, NOTCH2, PTPRD, and TP53) were considered as hub genes (Figure 4B) and verified by connectivity (Figure 5B).
Hub genes screened in this manuscript has been proved that correlated with patients’ clinical characteristics and prognosis, and some clinical and preclinical drugs, which provide inspiration for rescue therapy of BTKI-resistant MCL. ATM mutation is one of the most common genes mutation in previous studies and our finding (Figure 2C) (24-26). Closely related in DNA damage response (DDR) pathway, the ATM inhibitors suppressed DNA double-strand breaks repair and potentiated antitumor activity in cancer cell lines, considered as a promising target. Oral administration of ATM inhibitor (M3541 and M4076) to immunodeficient mice strongly enhanced the antitumor activity, leading to complete tumor regressions in breast cancer (30). Mutations of TP53 in MCL cells abrogated the DDR pathway more severely than those of ATM, contributing to a significantly worse prognosis (31). The TP53 mutations showed a negative effect on PFS in our data (P=0.0052, Figure 4A). Another hub gene screened in our cohort was CARD11, a critical gene in primary BTKi resistance for BCR-induced NF-κB activation, which conferred resistance to ibrutinib (4). The NOTCH2 gene is closely related to MCL resistance and increased in frequency from 33% to 50% in venetoclax-resistant MCL patients (32). In our cohort, the NOTCH2 mutation reached 18%, which was higher than the previous Chinese MCL sequencing of 6.3%, suggesting an increased frequency of NOTCH2 mutation in the BTKi resistant cohort (25). In our study, 80% of NOTCH2 mutation patients showed an ECOG PS >1, and only 47.8% of wild-type NOTCH2 patients showed ECOG PS >1, which was consistent with the poor performance status of NOTCH2 (33). The PTPRD is one of the most common recurrent lesions in nodular marginal zone lymphoma (NMZL), accounting for about 20% (34). Mutation of PTPRD in NMZL leads to loss of phosphatase activity of PTPRD with deregulation of cell cycle and increased proliferation index, which needs to be further verified in MCL (35). KRAS is the most frequent mutations in RAS/mitogen-activated protein kinase (MAPK) pathway (36). Though KRAS has a high mutation frequency in other solid tumors, it is a blind spot in MCL research (37). Until now, selective inhibitors of KRASG12C oncoproteins in phase I/II clinical trials have caused great excitement in non-small-cell lung carcinoma, cervix and gastrointestinal tumors (37,38) Although the frequency of KRAS mutations in our cohort was only 7%, T-cell transfer therapy of KRAS and oral administration of the KRAS inhibitor sotorasib provide the possibility of salvage treatment after MCL BTKi resistance (39,40).
In summary, based on 28 MCL patients’ mutation data and clinical data, prognosis and responses to salvage therapy regimen were analyzed. Patients with BTKi had less favorable clinical features at baseline. The CAR-T therapy yielded the best response among salvage treatments. Although a retrospective study with a limited number of patients, this is the first gene analysis paper for MCL in the field of BTKi resistance in a real-world sequencing setting. We screened 6 hub genes by a gene panel of 69 genes (GP79) providing a potential predictor for MCL patients with BTKi resistance.
Acknowledgments
Funding: This work was supported by the Wu Jieping Medical Foundation (No. WJPMF320.6750 to HJ), and Peking University Third Hospital Foundation (No. BYSYDL2021006).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4314/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4314/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4314/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Peking University Third Hospital (No. M2022564) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cortelazzo S, Ponzoni M, Ferreri AJM, et al. Mantle cell lymphoma. Crit Rev Oncol Hematol 2020;153:103038. [Crossref] [PubMed]
- Maddocks K. Update on mantle cell lymphoma. Blood 2018;132:1647-56. [Crossref] [PubMed]
- Bond DA, Martin P, Maddocks KJ. Relapsed Mantle Cell Lymphoma: Current Management, Recent Progress, and Future Directions. J Clin Med 2021;10:1207. [Crossref] [PubMed]
- Hershkovitz-Rokah O, Pulver D, Lenz G, et al. Ibrutinib resistance in mantle cell lymphoma: clinical, molecular and treatment aspects. Br J Haematol 2018;181:306-19. [Crossref] [PubMed]
- Bomben R, Ferrero S, D'Agaro T, et al. A B-cell receptor-related gene signature predicts survival in mantle cell lymphoma: results from the Fondazione Italiana Linfomi MCL-0208 trial. Haematologica 2018;103:849-56. [Crossref] [PubMed]
- Ferrero S, Rossi D, Rinaldi A, et al. KMT2D mutations and TP53 disruptions are poor prognostic biomarkers in mantle cell lymphoma receiving high-dose therapy: a FIL study. Haematologica 2020;105:1604-12. [Crossref] [PubMed]
- Guo D, Wang H, Sun L, et al. Identification of key gene modules and hub genes of human mantle cell lymphoma by coexpression network analysis. PeerJ 2020;8:e8843. [Crossref] [PubMed]
- Yan W, Li SX, Wei M, et al. Identification of MMP9 as a novel key gene in mantle cell lymphoma based on bioinformatic analysis and design of cyclic peptides as MMP9 inhibitors based on molecular docking. Oncol Rep 2018;40:2515-24. [Crossref] [PubMed]
- Grimm KE, O'Malley DP. Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues. Ann Diagn Pathol 2019;38:6-10. [Crossref] [PubMed]
- Roué G, Sola B. Management of Drug Resistance in Mantle Cell Lymphoma. Cancers (Basel) 2020;12:1565. [Crossref] [PubMed]
- Jain P, Wang ML. Mantle cell lymphoma in 2022-A comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol 2022;97:638-56. [Crossref] [PubMed]
- Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 2015;26:1175-9. [Crossref] [PubMed]
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015;126:739-45. [Crossref] [PubMed]
- Glimelius I, Smedby KE, Eloranta S, et al. Comorbidities and sex differences in causes of death among mantle cell lymphoma patients - A nationwide population-based cohort study. Br J Haematol 2020;189:106-16. [Crossref] [PubMed]
- Wu M, Li Y, Huang H, et al. Initial Treatment Patterns and Survival Outcomes of Mantle Cell Lymphoma Patients Managed at Chinese Academic Centers in the Rituximab Era: A Real-World Study. Front Oncol 2021;11:770988. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016;127:1559-63. [Crossref] [PubMed]
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770-8. [Crossref] [PubMed]
- Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet 2021;397:892-901. [Crossref] [PubMed]
- Newcomb R, Jacobson C. Chimeric Antigen Receptor T Cells for B-Cell Lymphoma. Cancer J 2021;27:107-11. [Crossref] [PubMed]
- Balsas P, Palomero J, Eguileor Á, et al. SOX11 promotes tumor protective microenvironment interactions through CXCR4 and FAK regulation in mantle cell lymphoma. Blood 2017;130:501-13. [Crossref] [PubMed]
- Chen Z, Teo AE, McCarty N. ROS-Induced CXCR4 Signaling Regulates Mantle Cell Lymphoma (MCL) Cell Survival and Drug Resistance in the Bone Marrow Microenvironment via Autophagy. Clin Cancer Res 2016;22:187-99. [Crossref] [PubMed]
- Kim YR, Eom KS. Simultaneous Inhibition of CXCR4 and VLA-4 Exhibits Combinatorial Effect in Overcoming Stroma-Mediated Chemotherapy Resistance in Mantle Cell Lymphoma Cells. Immune Netw 2014;14:296-306. [Crossref] [PubMed]
- Zhang J, Jima D, Moffitt AB, et al. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood 2014;123:2988-96. [Crossref] [PubMed]
- Yang P, Zhang W, Wang J, et al. Genomic landscape and prognostic analysis of mantle cell lymphoma. Cancer Gene Ther 2018;25:129-40. [Crossref] [PubMed]
- Beà S, Valdés-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A 2013;110:18250-5. [Crossref] [PubMed]
- Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov 2014;4:1022-35. [Crossref] [PubMed]
- Rahal R, Frick M, Romero R, et al. Pharmacological and genomic profiling identifies NF-κB-targeted treatment strategies for mantle cell lymphoma. Nat Med 2014;20:87-92. [Crossref] [PubMed]
- Agarwal R, Chan YC, Tam CS, et al. Dynamic molecular monitoring reveals that SWI-SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat Med 2019;25:119-29. [Crossref] [PubMed]
- Zimmermann A, Zenke FT, Chiu LY, et al. A New Class of Selective ATM Inhibitors as Combination Partners of DNA Double-Strand Break Inducing Cancer Therapies. Mol Cancer Ther 2022;21:859-70. [Crossref] [PubMed]
- Mareckova A, Malcikova J, Tom N, et al. ATM and TP53 mutations show mutual exclusivity but distinct clinical impact in mantle cell lymphoma patients. Leuk Lymphoma 2019;60:1420-8. [Crossref] [PubMed]
- Zhao S, Kanagal-Shamanna R, Navsaria L, et al. Efficacy of venetoclax in high risk relapsed mantle cell lymphoma (MCL) - outcomes and mutation profile from venetoclax resistant MCL patients. Am J Hematol 2020;95:623-9. [Crossref] [PubMed]
- Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol 2019;94:710-25. [Crossref] [PubMed]
- Bertoni F, Rossi D, Raderer M, et al. Marginal Zone Lymphomas. Cancer J 2020;26:336-47. [Crossref] [PubMed]
- Spina V, Khiabanian H, Messina M, et al. The genetics of nodal marginal zone lymphoma. Blood 2016;128:1362-73. [Crossref] [PubMed]
- Ryan MB, Corcoran RB. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol 2018;15:709-20. [Crossref] [PubMed]
- Hong DS, Fakih MG, Strickler JH, et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med 2020;383:1207-17. [Crossref] [PubMed]
- Hallin J, Engstrom LD, Hargis L, et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov 2020;10:54-71. [Crossref] [PubMed]
- Tran E, Robbins PF, Lu YC, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016;375:2255-62. [Crossref] [PubMed]
- Leidner R, Sanjuan Silva N, Huang H, et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N Engl J Med 2022;386:2112-9. [Crossref] [PubMed]
(English Language Editor: J. Jones)


