
Applying contrast-enhanced ultrasound model to distinguish atypical focal adenomyosis from uterine leiomyomas
Introduction
Adenomyosis is a common gynecologic disorder that affects the quality of life of women of reproductive age. The clinical presentation is often characterized by menorrhagia and dysmenorrhea. The pathogenesis of adenomyosis is not entirely clear, but it is histologically defined as the presence of endometrial glands and mesenchyme in the myometrium (1) and is considered to be a cause of abnormal uterine bleeding according to criteria developed by the American College of Obstetricians and Gynecologists (ACOG) (2). The effects of adenomyosis on infertility are now gradually being confirmed, and its adverse effects on patients as a benign disease are increasingly being verified by more literature. Adenomyosis can be divided into focal adenomyosis and diffuse adenomyosis according to the distribution pattern of the lesions (3). Uterine leiomyomas are the most common benign tumors in women of reproductive age. The International Federation of Gynecology and Obstetrics (FIGO) classification categorizes uterine leiomyomas into nine groups (0–8) based on the relationship between the leiomyomas and the uterine wall (2).
Presently, two-dimensional (2D) ultrasound is used as the main tool to diagnose adenomyosis and leiomyomas; however, atypical focal adenomyosis is sometimes difficult to distinguish from interstitial leiomyomas of FIGO classification category 2–5 on 2D ultrasound images. Currently, the main clinical treatment modalities for uterine adenomyosis are gonadotropin-releasing hormone agonists (GnRH-a), the Levonorgestrel Intrauterine System (LNG-IUS), thermal ablation, and surgical resection (4-6). For patients diagnosed with uterine leiomyomas, uterine artery embolization (UAE) and hysterectomy are the primary treatment options (7). Patients with adenomyosis and uterine leiomyomas receive completely different treatment regimens once diagnosed. Thus, the accurate diagnosis could be critical (8,9).
Contrast-enhanced ultrasound (CEUS) is an imaging method for the real-time evaluation of tissue perfusion, allowing for both qualitative and quantitative analysis of tissue microcirculation (10). In 2016, the United States Food and Drug Administration (FDA) approved the first non-cardiac indication for microbubble contrast agents. The incidence of serious adverse events is lower than that of iodinated contrast agents and is similar to that of magnetic resonance imaging (MRI) contrast agents. Microcirculation features could be evaluated by CEUS rather through the conventional ultrasound, so that the vascular distribution characteristics of the lesions could be reflected clearer. It may provide more information for the differential diagnosis of atypical adenomyosis and uterine leiomyomas. The present study aimed to comprehensively assess the diagnostic efficacy of CEUS for the differential diagnosis of focal adenomyosis and uterine leiomyomas by looking at the different CEUS presentations versus the time-intensity curves (TICs) of both diseases and the normal uterus. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4460/rc).
Methods
Patients
Among the patients who underwent conventional ultrasound examination in our hospital from April 2021 to December 2021, the patients whose results showed myometrium lesion were prospectively enrolled in the derivation cohort. The second cohort was used as the validation cohort. According to the above inclusion and exclusion criteria, a total of 21 patients who were diagnosed as leiomyomas or adenomyosis by conventional ultrasound in our hospital from January 2022 to July 2022 were selected, including 7 patients with uterine leiomyomas and 14 with adenomyosis. CEUS was conducted for patients whose diseases were not easily distinguishable between adenomyosis and uterine leiomyomas. All subjects were evaluated and screened for eligibility by the first author before inclusion in the study. The inclusion criteria were as follows: (I) focal myometrium lesions that are difficult to diagnose on 2D ultrasound images; (II) age >18 years old; (III) no treatment prior to CEUS; (IV) lesion diameter >2 cm; and (V) cases in which the possibility of malignant tumors was excluded. The exclusion criteria were as follows: (I) routine 2D ultrasound showing typical endometrial lesions or those with confirmed malignancy; (II) patients for whom the pathological results could not be obtained; and (III) pathology suggesting the coexistence of uterine leiomyomas and adenomyosis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Shengjing Hospital of China Medical University (No. 2021PS620K) and informed consent was taken from all the patients. The main outcome indicator of this study was the diagnostic efficacy of the adenomyosis and uterine leiomyomas groups. The sample size was calculated using the PASS.15 software (NCSS, America). The formula is:
The test level α was set at 0.05 (bilateral), the area under the receiver operating characteristic (ROC) curve was set at 0.80 according to expected test results, the lower limit of the false positive rate was set at 0, the upper limit of the false positive rate was set at 1, and the certainty 1−β was 0.90. The number of cases included in the derivation cohort of each group was 17 (total =34) according to the sample size formula.
CEUS examinations
A Mindray Eagus R9 (Mindray, China) ultrasound machine with a SC6-1U convex array probe at 2.5–4.0 MHz was used. 2D ultrasound was performed by two physicians with more than 5 years of experience in gynecologic ultrasound, and measurements of uterine position, uterine size, endometrial thickness, lesion size, echogenicity, border, and location were analyzed. For patients with multiple lesions, the largest lesion was selected for observation and diagnosed in 2D ultrasound mode. Diagnosis was also performed in 2D ultrasound mode. For the purpose of the study, lesions with inconsistent diagnostic results were jointly decided.
CEUS was then performed, and the examination was conducted by an experienced physician. After selecting the largest section of the lesion and the surrounding normal myometrium, the probe was stabilized and the CEUS mode was entered. The mechanical index was adjusted to 0.06–0.08, 59 mg of SonoVue (Bracco, Italy) dry powder was mixed with 5 mL of saline, shaken to make a suspension. One-point-five millimeters of the suspension was injected into a superficial vein at the elbow, followed by a rapid push of 5 mL of saline to flush the tube. The contrast medium was injected and the timing started at the same time, and the dynamic recording lasted for 3 min. Diagnosis was made at the end of the examination by two experienced physicians who observed the imaging records. Lesions with inconsistent diagnostic findings were jointly examined after diagnosis to satisfy study purpose. The physician responsible for the diagnosis of the CEUS images was unaware of the gynecologic ultrasound diagnosis results and the final pathological findings.
Imaging analysis
Qualitative characteristics include the enhancement level based on the myometrium (high; equal; weak), the contrast enhancement pattern (first short-line vascular enhancement; peripheral to central; peripheral ring hyperenhancement), the enhanced time of the lesion based on the myometrium (early entry; simultaneous entry; late entry), post-contrast lesion border (clear; unclear), the distribution of the contrast agent (uniform; uneven), and the wash-out time based on the myometrium (early; simultaneous; late). The TIC was acquired and analyzed, and the relevant parameters were recorded and calculated, including: (I) arrive time (AT); (II) time to peak (TTP); (III) peak intensity (PI). After calculating the difference between the AT, TTP, and PI of the lesion and the normal myometrium in the two groups, the following parameters were assessed: (IV) ΔAT: the difference between the AT of the normal myometrium and the lesion; (V) ΔTTP: the difference between the TTP of the normal myometrium and the lesion; (VI) ΔPI: the difference between the PI of the lesion and the normal myometrium; and (VII) |ΔAT|, |ΔTTP|, |ΔPI|: the absolute values of ΔAT, ΔTTP and ΔPI, respectively.
The temporal variability of contrast agent entry into the lesion was defined as the difference between the time when the contrast agent started to enter the lesion and the time when the final contrast agent filled the lesion completely (Figure 1).

Reference standard
In this study, the postoperative pathological findings were considered the gold standard. For patients with multiple lesions, the pathological diagnosis of the largest lesion was selected as the reference standard, and the remaining lesions were not included in the study. Only patients with an intention to undergo surgery were included in this study because the pathological diagnosis of puncture biopsy did not provide a definitive diagnosis of uterine adenomyosis versus leiomyomas. All patients included in the study underwent surgery within 1 week after the examination. Pathological diagnosis was conducted by an experienced pathologist who was not aware of any previous diagnostic imaging findings.
Statistical analysis
All data analyses were performed using SPSS 26.0 software (IBM, America). Count data were expressed as frequencies and rates. Measurement data conforming to a normal distribution were recorded as the mean ± standard deviation, while data that did not conform to a normal distribution were recorded as the median (interquartile range).
The independent sample t-test was applied to compare between groups for measurement data with uniform variance and conforming to a normal distribution, the Mann-Whitney U test was used to compare between groups with skewed data or uneven variance, and the two-way unordered R × C Fisher’s exact probability method and chi-square test were used for count data. The agreement of the diagnosis between different observers using conventional ultrasound was tested using kappa agreement. A chi-square test was used to determine the accuracy of conventional ultrasound versus CEUS for differential diagnosis of the disease. Risk prediction models were developed using logistic regression analysis, and variable screening was performed using the forward method. The variables included in the model were compared for relative importance of factors based on standard regression coefficients. Cut-off values were determined a priori for continuous variables by the area under the ROC curve. According to the resulting Logistic regression model, the regression coefficients for each variable will be rounded to the nearest integer and will be assigned points. Therefore, each variable was assigned a score according to its correlation with the outcome obtained from the regression model. Finally, we classified the variables according to the score in order to evaluate the possible pathological type of their muscularis lesions. The Hosmer-Lemeshow goodness-of-fit test was used to assess model overfitting and calibration in the derivation cohort. The diagnostic index area under the curve (AUC) was derived by plotting ROC curves, and the optimal cut-off value was determined by applying the Youden index. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated according to the optimal cut-off point for maximizing the Youden index. P<0.05 was considered to indicate a statistically significant difference.
Results
A total of 111 patients were enrolled in this study, including 90 in the derivation cohort and 21 in the validation cohort. The basic lesion characteristics of the two cohorts are shown in Table 1. Finally, the derived cohort was pathologically confirmed to include 60 patients with adenomyosis and 30 patients with leiomyomas. The validation cohort included 7 patients with leiomyomas and 14 patients with adenomyosis. The inclusion flow chart of patients in the derived cohort is shown in Figure 2. The inclusion flow chart of patients in the validation cohort is shown in Figure 3.
Table 1
| Characteristics | Derivation cohort (n=90) | Validation cohort (n=21) | P |
|---|---|---|---|
| Age (years) | 39.57±5.64 | 38.71±5.63 | 0.534 |
| Height (m) | 1.62±0.04 | 1.65 (0.04) | 0.080 |
| Weight (kg) | 59.54±10.20 | 57.24±8.09 | 0.336 |
| BMI (kg/m2) | 22.60±3.68 | 21.24±3.10 | 0.118 |
| Uterus position (anteversion/retroversion/horizontal position) | 59/23/8 | 10/11/0 | 0.043 |
| Uterine volume (cm3) | 168.03 (168.42) | 195.69±105.50 | 0.619 |
| Endometrial thickness (cm) | 0.60 (0.42) | 0.79±0.25 | 0.082 |
| Lesion volume (cm3) | 61.47 (87.17) | 73.07±50.04 | 0.883 |
| Location of the lesion (anterior wall/posterior wall/left side wall/right side wall/bottom) | 30/50/2/2/6 | 7/9/1/0/4 | 0.104 |
| Boundary (clear/unclear) | 44/46 | 12/9 | 0.496 |
| Echo of lesion (low/high/equal) | 62/15/13 | 19/0/2 | 0.075 |
Data were expressed as mean ± standard deviation, median (interquartile range) or number. BMI, body mass index.

Diagnostic results of conventional 2D ultrasound
In the derivation cohort, out of 60 foci of adenomyosis, 32 were diagnosed and the rest were misdiagnosed as uterine leiomyomas, with a sensitivity of 53.3%. Out of 30 foci of leiomyomas, 19 were diagnosed and the rest were misdiagnosed as uterine adenomyosis, with a sensitivity of 63.33%. The diagnostic PPV (62.74%), NPV (71.79%), and kappa concordance of different observers (0.400) of conventional 2D ultrasound were also determined, and its diagnostic accuracy was 47.78%. In the validation cohort, adenomyosis was diagnosed in 9 patients (9/14) with another five patients were misdiagnosed. Five patients with uterine leiomyomas were diagnosed (5/7), and two patients were misdiagnosed with adenomyosis. The observer consensus in this cohort was 0.432. The accuracy of conventional ultrasound was 66.6%.
Qualitative analysis of CEUS in the derivation cohort
All of the included patients were free of adverse events during the conventional ultrasound examinations with CEUS. Moreover, after conventional observation for 30 minutes, none of the patients experienced any adverse events. In the adenomyosis group, 34 lesions showed short linear vascular enhancement of the lesion (56.7%; 34/60), and 26 lesions showed gradual peripheral to central enhancement (43.3%; 26/60). In the uterine leiomyoma group, nine lesions showed circumferential hyperenhancement around the lesion (30.0%; 9/30), 13 lesions showed gradual peripheral-to-center enhancement (43.3%; 13/30), and eight lesions showed short linear vascular enhancement of the lesion (26.7%; 8/30), with statistically significant differences in the enhanced morphology of the lesion between the two diseases (P<0.05). The differences in the contrast agent entry rate, post-contrast lesion border, and the distribution of the contrast agent were also statistically significant between the two groups (P<0.05) (Figures 4,5, Table 2).
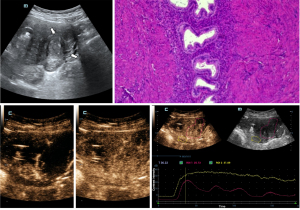

Table 2
| CEUS characteristics | Assignment | Adenomyosis group | Leiomyomas group | P |
|---|---|---|---|---|
| Enhancement mode | X1 | <0.001 | ||
| First short-line vascular enhancement | 2 | 34 | 8 | |
| Peripheral to central enhancement | 1 | 26 | 13 | |
| Peripheral ring hyperenhancement | 0 | 0 | 9 | |
| Enhanced strength | X2 | 0.383 | ||
| Weak enhancement | 2 | 27 | 17 | |
| High enhancement | 1 | 29 | 10 | |
| Equal enhancement | 0 | 4 | 3 | |
| Contrast agent entry rate | X3 | 0.048 | ||
| Early entry | 2 | 36 | 13 | |
| Late entry | 1 | 23 | 13 | |
| Simultaneous entry | 0 | 1 | 4 | |
| Contrast agent distribution | X4 | <0.001 | ||
| Uniform | 1 | 13 | 19 | |
| Uneven | 0 | 47 | 11 | |
| Boundary | X5 | <0.001 | ||
| Clear | 1 | 4 | 18 | |
| Unclear | 0 | 56 | 12 | |
| Wash-out time of the contrast agent | X6 | 0.258 | ||
| Early wash-out | 2 | 31 | 13 | |
| Late wash-out | 1 | 18 | 7 | |
| Simultaneous wash-out | 0 | 11 | 10 |
X1, enhancement mode; X2, enhanced strength; X3, contrast agent entry rate; X4, contrast agent distribution; X5, boundary; X6, wash-out time of the contrast agent. CEUS, contrast-enhanced ultrasound.
Quantitative analysis of CEUS in the derivation cohort
Statistical analysis of the adenomyosis and leiomyomas groups data revealed statistically significant differences in AT, TTP, PI, |ΔAT|, and |ΔTTP| between the two diseases (P<0.05) (Figures 4,5). The AUC was 0.882 (Figure 6), and the sensitivity and specificity were 88.33% and 73.33%, respectively when using a temporal variability of 9.5 s as the threshold value to differentiate uterine leiomyomas from adenomyosis (Table 3).

Table 3
| Quantitative indicatorsa | Assignment | Adenomyosis group | Leiomyomas group | U | P |
|---|---|---|---|---|---|
| Temporal variability of the lesions | X7 | 17.00 (19.00) | 7.5 (5.25) | 212.5 | <0.001 |
| AT | X8 | 15.30 (7.35) | 13.00 (4.33) | 644.0 | 0.028 |
| TTP | X9 | 40.48 (15.95) | 28.80 (22.53) | 654.0 | 0.035 |
| PI | X10 | 23.15 (21.13) | 31.00 (23.51) | 625.0 | 0.019 |
| ΔAT | X11 | 0.70 (3.35) | 0.00 (1.63) | 742.5 | 0.177 |
| |ΔAT| | X12 | 1.49 (1.60) | 0.75 (1.45) | 605.5 | 0.012 |
| ΔTTP | X13 | 1.55 (12.39) | 0.10 (5.85) | 784.5 | 0.323 |
| |ΔTTP| | X14 | 7.1 (10.85) | 2.55 (5.25) | 569.5 | 0.005 |
| ΔPI | X15 | 0.23 (9.93) | −0.85 (8.58) | 879.0 | 0.857 |
| |ΔPI| | X16 | 4.45 (6.32) | 3.95 (8.11) | 840.50 | 0.611 |
Data were expressed as median (interquartile range). a, quantitative analyses were conducted using the difference between normal myometrium and lesion data. TIC, time-intensity curve; AT, arrive time; TTP, time to peak; PI, peak intensity.
Multifactorial analysis of qualitative ultrasonographic features and TIC curves in the derivation cohort
Four independent risk factors for identifying focal adenomyosis were confirmed using logistic regression analysis: short linear vessels first enhanced perfusion pattern (X1), uneven contrast agent distribution (X4), unclear boundary after infecting contrast (X5), and lesion temporal variability >9.5 s (X7). The odds ratio (OR) for lesion temporal variability >9.5 s was the largest [OR =13.496, 95% confidence interval (CI): 3.159–57.655]. The sensitivity of identifying adenomyosis using this model was 98.33%, the specificity was 70.00%, the PPV was 86.76%, the NPV was 95.45%, and the accuracy was 87.78%. The AUC for the diagnosis of uterine adenomyosis using this model was 0.908 (95% CI: 0.833–0.982) (Figure 5) and the P value for the Hosmer-Lemeshow goodness-of-fit statistic with six degrees of freedom was 0.462 (Table 4). Furthermore, its kappa agreement was significantly higher compared to conventional ultrasound (0.778).
Table 4
| CEUS characteristics | Assignment | β | OR (95% CI) | P |
|---|---|---|---|---|
| Short-term vessels first enhanced enhancement mode | X1 | 1.459 | 4.301 (1.230–15.034) | 0.022 |
| Unclear boundary | X5 | 2.134 | 8.452 (1.535–46.536) | 0.014 |
| Temporal variability of lesions >9.5 s | X7 | 2.602 | 13.496 (3.159–57.655) | <0.001 |
| Uneven contrast agent distribution | X4 | 1.370 | 3.937 (0.964–16.076) | 0.056 |
| Constant | −5.288 | 0.014 | 0.000 |
X1: enhancement mode; X5: boundary; X7: temporal variability of the lesions; X4: contrast agent distribution. CEUS, contrast-enhanced ultrasound; OR, odds ratio; CI, confidence interval.
Establishment of risk prediction score and model validation
The weighted scores for each predictor were determined based on the regression coefficients, and the total score for a lesion was obtained using the sum of each weighted score (Table 5). According to the observation of the lesions, the lesions were divided into suspected uterine leiomyomas lesions (0–3 points) and suspected adenomyosis lesions (4–7 points). The score of lesions in the validation cohort were determined, the same as the final prediction results according to the model and the diagnostic efficiency of the prediction model (Table 6). Based on pathological diagnosis, the model accurately diagnosed 6 patients with uterine leiomyomas (6/7) and 12 patients with adenomyosis (12/14), with sensitivity and specificity of 85.71%, NPV of 75% and PPV of 92.3%. The area under the ROC curve was 0.898 (95% CI: 0.742–1.000) (Figure 7), and the model accuracy was 85.7%.
Table 5
| Predictors | Reference value | β | Points |
|---|---|---|---|
| Short-term vessels first enhanced enhancement mode | 1.459 | ||
| No | 0 | 0 | |
| Yes | 1 | 1 | |
| Unclear boundary | 2.134 | ||
| No | 0 | 0 | |
| Yes | 1 | 2 | |
| Temporal variability of lesions >9.5 s | 2.602 | ||
| No | 0 | 0 | |
| Yes | 1 | 3 | |
| Uneven contrast agent distribution | 1.370 | ||
| No | 0 | 0 | |
| Yes | 1 | 1 | |
Scores were obtained by β of predictors.
Table 6
| Predictors | Adenomyosis group (n=14) | Leiomyomas group (n=7) |
|---|---|---|
| Short-term vessels first enhanced enhancement mode | ||
| No | 7 | 4 |
| Yes | 7 | 3 |
| Unclear boundary | ||
| No | 2 | 6 |
| Yes | 12 | 1 |
| Temporal variability of lesions >9.5 s | ||
| No | 3 | 5 |
| Yes | 11 | 2 |
| Uneven contrast agent distribution | ||
| No | 2 | 3 |
| Yes | 12 | 4 |
| Suspected leiomyomas lesions (0–3 points) | 2 | 6 |
| Suspected adenomyosis lesions (4–7 points) | 12 | 1 |

Discussion
Adenomyosis refers to the presence of endometrial glandular tissue and mesenchyme within the myometrium. It is affected by hormonal changes that produce hemorrhage, the proliferation of myofibrous connective tissue, and eventually focal or diffuse lesions (11). Among these, the most common symptom of adenomyosis is dysmenorrhea (15–30%). In addition, adenomyosis has been reported to significantly increase the risk of endometrial and thyroid cancers (12). The notion that adenomyosis can cause infertility has been well documented (13,14), and it has been suggested that adenomyosis not only affects fertility but also pregnancy outcomes (15-17). The pathogenesis of adenomyosis is not completely clear but the most accepted mechanism is the invasion of the endometrium into the myometrium by altering or interrupting the junctional zone (JZ) (18-20).
Adenomyosis can be divided into focal adenomyosis and diffuse adenomyosis according to the distribution pattern of the lesions, and recent studies have suggested that adenomyosis can be divided into internal adenomyosis and external adenomyosis according to the pathway of invasion of the endometrium into the myometrium (21,22). The most common treatment for uterine leiomyoma is surgery, which differs from the gradual treatment pattern of adenomyosis. As the most common benign gynecologic tumor in women of childbearing age, uterine leiomyomas have a typical 2D ultrasound presentation and are always easily diagnosed by ultrasound. However, uterine leiomyomas are sometimes not completely distinguishable from focal adenomyosis on 2D images alone. Focal adenomyosis with a clear border appears very similar to leiomyomas on 2D ultrasound. The misdiagnosis rate still remains. The clinical management of adenomyosis and leiomyoma differs greatly, so it is crucial to correctly identify the appropriate treatment options of the two diseases.
The conclusions of different studies regarding the CEUS presentation of adenomyosis are not uniform, and relevant literature showing increased temporal variability of the lesion is lacking (23). A previous study has suggested that typical uterine leiomyoma is characterized by a ring of hyperenhancement around the tumor, with uniform or uneven enhancement of the entire tumor and a clear demarcation from the normal muscle layer (24).
The mechanism of formation of internal adenomyosis differs from that of external adenomyosis, as previous studies have reported that external adenomyosis does not cause changes in the JZ, but is rather due to invasion of the myometrium by external endometriotic lesions (21,25). All of the adenomyosis cases included in this study were internal adenomyosis, and all of them showed faint JZ on 2D ultrasound (external adenomyosis was not included in this study). The different subtypes of uterine leiomyoma did not behave identically under CEUS. Twenty-one patients with pathologically confirmed common uterine leiomyomas and nine patients with cellular uterine leiomyomas were included in this study, and there was no statistical difference between the two CEUS presentations, which was not consistent with previous study (26). This is probably due to the small sample size. Also, three patients had degeneration during the study, and CEUS showed no perfusion inside the leiomyomas, which were not included in this study.
In this study, we first performed a preliminary statistical analysis of the efficacy of conventional ultrasound to identify the two diseases, and focal adenomyosis was mostly seen with clearer borders, which was more difficult to identify the 2–5 types of leiomyomas, with a poor interobserver agreement (only 0.400). The qualitative analysis of this study showed that the contrast enhancement pattern, enhanced time of the lesion, post-contrast lesion border, and distribution of the contrast agent in the lesion were effective in differentiating the two diseases. However, the initial enhancement pattern of atypical cases of both diseases showed gradual enhancement from the periphery to the center, and the proportion is not low. The sensitivity and specificity of determining the type of disease by identifying the border clarity of the lesion alone were poor. A previous study has suggested that the contrast perfusion pattern of uterine leiomyomas is related to the size of the lesion, and that larger lesions (>2 cm) are more likely to exhibit circumferential hyperenhancement (27). The lesions included in this study were all lesions >2 cm in diameter, and uterine leiomyomas showed mostly low enhancement intensity. We considered that it is more clinically relevant to clearly identify lesions with larger diameter adenomyosis from those with uterine leiomyomas.
Histological analysis has shown that the endometrial stroma and glands ectopic to the myometrium in adenomyosis lead to hyperplasia of blood vessels, while the same will appear similar to the normal endometrium with varying degrees of cyclic bleeding, which appears as heterogeneous enhancement on CEUS images, and the lesions as a whole appear mostly hypoenhanced. By conducting CEUS on a patient with a normal uterus, the image of the endometrium is hypoenhanced compared to that of the myometrium. This may be correlated with the image of hypoenhancement in the lesions of patients with adenomyosis. This study selected the patient’s normal muscular layer as a control, this would eliminate the effect of individual differences on contrast perfusion. In the univariate quantitative analysis, the differences in AT, TTP, PI, |ΔAT|, and |ΔTTP| between the two diseases were statistically significant. That is, the foci of adenomyosis exhibited later enhancement than those of leiomyomas, and the time of contrast entry into the foci and the TTP differed more significantly in adenomyosis than in leiomyomas compared to the normal myometrium. However, these quantitative analysis indicators were not included in the final model when multivariate analysis was conducted. Quantitative analysis will be affected by the location of region of interest (ROI) selection. Due to the limitation of the size and location of uterine lesions, it is sometimes impossible to strictly select ROI according to the selection criteria, that is, the selected area of ROI should be the same as far as possible and on the same plane. This is also one of the limitations of this study.
In this study, the imaging analysis revealed that there was temporal variability in the filling of the lesion with contrast. The definition of temporal variability in this study was based on the difference between the time of contrast agent entry into the lesion and the time of contrast agent filling the lesion in the CEUS image. At the same time, we also observed quick entry in one part but no entry of contrast in another part. The temporal variability is more effective in directly reflecting the perfusion of the contrast than the TIC curve. The univariate analysis confirmed that the temporal variability data of the two groups of diseases were statistically different. The ROC curve was plotted and the AUC was 0.885 for identifying the two diseases. The temporal variability of the contrast medium entering the lesion was higher in adenomyosis compared to leiomyomas. As for the reasons for the increased temporal phase discrepancy seen in patients with adenomyosis, this may be related to the uneven distribution of blood vessels within the lesion in patients with adenomyosis. The team of this research suggests that the increased temporal variability of the lesion is due to the early enhancement of the vascularized area of the adenomyosis lesion, while the delayed enhancement is caused by the hypertrophied and hyperplastic myometrium around the ectopic gland compressing the vasculature at this location. There are no reports in the literature regarding the temporal phase discrepancy of CEUS lesions to differentiate uterine leiomyomas from adenomyosis.
Through logistic regression analysis, we found that short linear vessels were enhanced first, and a lesion temporal phase difference of >9.5 s, with an uneven contrast agent distribution and an unclear boundary in contrast mode, was significant in identifying focal adenomyosis. The highest OR (OR =13.496, 95% CI: 3.159–57.655) was observed for lesions with temporal differences >9.5 s. Also, the AUC for the diagnosis of uterine adenomyosis using this model was 0.908 (95% CI: 0.833–0.982), which was higher than the lesion temporal phase difference and other qualitative or quantitative indicators alone for differential diagnosis. The Hosmer-Lemeshow goodness-of-fit test was used to assess the model fit; after assessment, a better fit was observed (P=0.462). The kappa agreement between the two diseases diagnosed by CEUS was significantly improved (0.778), highlighting the advantage of CEUS in diagnosing both diseases. Compared with the traditional method, the model is of better clinical practicability and is more convenient to operate (28). We found that the diagnostic efficacy did not decrease after the risk model was transformed into a weighted scoring system, and the area under the ROC curve was 0.898 (95% CI: 0.742–1.000). Therefore, CEUS examination can be considered a novel approach to differentiate focal adenomyosis from leiomyomas, which are difficult to diagnose by conventional ultrasound. This provides new diagnostic ideas and methods for clinical application and further guides precise clinical treatment.
The present study has some limitations that should be noted. Firstly, stratified analysis of different sizes and pathological types was not performed in this study due to the limited sample size. Secondly, all patients included in this study had large lesions to a certain extent, which may lead to selection bias, because patients with large lesions were more inclined to choose surgical treatment. Further studies are needed to confirm whether smaller lesions present with similar CEUS findings. In addition, due to the limited sample size of the validation cohort included in this study, more large-sample studies are needed to further verify the accuracy of the model in the future. Finally, this was a single-center study, and it is anticipated that future studies will be expanded more deeply by collecting large multi-center case samples.
Conclusions
CEUS can effectively differentiate focal adenomyosis from uterine leiomyomas. Short linear vessels are the first to be enhanced, and a focal temporal variability >9.5 s and obscure focal boundaries in the contrast mode are specific signs of focal adenomyosis on CEUS. The focal phase difference can be used as a characteristic indicator to differentiate focal adenomyosis from uterine leiomyomas.
Acknowledgments
Funding: This study was supported by grants from the Guide Project for Key Research and Development Foundation of Liaoning Province (No. 2019JH8/10300008), the 345 Talent Project, and the Liaoning BaiQianWan Talents Program.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4460/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4460/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4460/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Shengjing Hospital of China Medical University (No. 2021PS620K) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu O, Schulze-Rath R, Grafton J, et al. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006-2015. Am J Obstet Gynecol 2020;223:94.e1-94.e10. [Crossref] [PubMed]
- Munro MG, Critchley HO, Broder MS, et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113:3-13. [Crossref] [PubMed]
- Cunningham RK, Horrow MM, Smith RJ, et al. Adenomyosis: A Sonographic Diagnosis. Radiographics 2018;38:1576-89. [Crossref] [PubMed]
- Pontis A, D'Alterio MN, Pirarba S, et al. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol 2016;32:696-700. [Crossref] [PubMed]
- Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The Role of Hysteroscopy in the Diagnosis and Treatment of Adenomyosis. Biomed Res Int 2017;2017:2518396. [Crossref] [PubMed]
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril 2018;109:406-17. [Crossref] [PubMed]
- Legendre G, Brun JL, Fernandez H. The place of myomectomy in woman of reproductive age. J Gynecol Obstet Biol Reprod (Paris) 2011;40:875-84. [Crossref] [PubMed]
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal Ultrasound for the Diagnosis of Adenomyosis: Systematic Review and Meta-Analysis. J Minim Invasive Gynecol 2018;25:257-64. [Crossref] [PubMed]
- Zhang M, Wasnik AP, Masch WR, et al. Transvaginal Ultrasound Shear Wave Elastography for the Evaluation of Benign Uterine Pathologies: A Prospective Pilot Study. J Ultrasound Med 2019;38:149-55. [Crossref] [PubMed]
- Miele V, Piccolo CL, Galluzzo M, et al. Contrast-enhanced ultrasound (CEUS) in blunt abdominal trauma. Br J Radiol 2016;89:20150823. [Crossref] [PubMed]
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: Mechanisms and Pathogenesis. Semin Reprod Med 2020;38:129-43. [Crossref] [PubMed]
- Yeh CC, Su FH, Tzeng CR, et al. Women with adenomyosis are at higher risks of endometrial and thyroid cancers: A population-based historical cohort study. PLoS One 2018;13:e0194011. [Crossref] [PubMed]
- Harada T, Khine YM, Kaponis A, et al. The Impact of Adenomyosis on Women's Fertility. Obstet Gynecol Surv 2016;71:557-68. [Crossref] [PubMed]
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod Biomed Online 2021;42:185-206. [Crossref] [PubMed]
- Mochimaru A, Aoki S, Oba MS, et al. Adverse pregnancy outcomes associated with adenomyosis with uterine enlargement. J Obstet Gynaecol Res 2015;41:529-33. [Crossref] [PubMed]
- Tamura H, Kishi H, Kitade M, et al. Complications and outcomes of pregnant women with adenomyosis in Japan. Reprod Med Biol 2017;16:330-6. [Crossref] [PubMed]
- Gruber TM, Mechsner S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021;10:1381. [Crossref] [PubMed]
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online 2017;35:592-601. [Crossref] [PubMed]
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril 2018;109:371-9. [Crossref] [PubMed]
- Rasmussen CK, Hansen ES, Dueholm M. Two- and three-dimensional ultrasonographic features related to histopathology of the uterine endometrial-myometrial junctional zone. Acta Obstet Gynecol Scand 2019;98:205-14. [Crossref] [PubMed]
- Bourdon M, Oliveira J, Marcellin L, et al. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum Reprod 2021;36:349-57. [Crossref] [PubMed]
- Chapron C, Tosti C, Marcellin L, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod 2017;32:1393-401. [Crossref] [PubMed]
- Stoelinga B, Juffermans L, Dooper A, et al. Contrast-Enhanced Ultrasound Imaging of Uterine Disorders: A Systematic Review. Ultrason Imaging 2021;43:239-52. [Crossref] [PubMed]
- Henri M, Florence E, Aurore B, et al. Contribution of contrast-enhanced ultrasound with Sonovue to describe the microvascularization of uterine fibroid tumors before and after uterine artery embolization. Eur J Obstet Gynecol Reprod Biol 2014;181:104-10. [Crossref] [PubMed]
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril 2018;109:389-97. [Crossref] [PubMed]
- Li Z, Zhang P, Shen H, et al. Clinical value of contrast-enhanced ultrasound for the differential diagnosis of specific subtypes of uterine leiomyomas. J Obstet Gynaecol Res 2021;47:311-9. [Crossref] [PubMed]
- Zhang XL, Zheng RQ, Yang YB, et al. The use of contrast-enhanced ultrasound in uterine leiomyomas. Chin Med J (Engl) 2010;123:3095-9. [PubMed]
- Wang Y, Dong T, Nie F, et al. Contrast-Enhanced Ultrasound in the Differential Diagnosis and Risk Stratification of ACR TI-RADS Category 4 and 5 Thyroid Nodules With Non-Hypovascular. Front Oncol 2021;11:662273. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


