
Intestinal pseudo-obstruction in systemic lupus erythematosus complicated by Castleman disease: a case report
Introduction
Intestinal pseudo-obstruction (IPO) is a serious obstructive disease caused by intestinal motility disorder. Its pathogenesis is characterized by partial or complete obstruction without any lesions blocking the intestinal lumen. The clinical manifestations can be vomiting, abdominal pain, diarrhea or constipation. Its diagnosis should exclude mechanical obstruction, ischemia, and perforation (1). IPO is a rare and serious complication of systemic lupus erythematosus. The pathogenesis of SLE complicated with IPO is still unclear. It may be related to immune damage to small blood vessels that nourish intestinal smooth muscle and nerves, as well as immune complex deposition in the wall of small blood vessels, causing chronic intestinal ischemia, leading to intestinal smooth muscle fibrosis, atrophy, and neurogenic intestinal dysfunction, even death (2,3). The prevalence of SLE complicated with IPO was about 1.96%, the in-hospital mortality rate was 7.1%, and the misdiagnosis rate was about 78% (4). Diagnosis of IPO can be challenging, especially when IPO presents as the primary symptom of SLE, which is prone to misdiagnosis and unnecessary surgical treatment (5).
SLE is a chronic autoimmune disease characterized by a variety of clinical manifestations that may affect multiple systems, often accompanied by systemic or localized lymphadenopathy. However, Castleman disease (CD), as a rare lymphoproliferative disease, can rarely accompany by SLE, and the diagnosis relies mainly on lymph node biopsy (6,7).
We report an 18-year-old female patient who presented with IPO as the initial clinical manifestations and was then diagnosed with SLE with IPO. She was diagnosed with SLE combined with CD one month after IPO symptoms. Consequently, early diagnosis and timely medical treatment are essential to avoid unnecessary surgery and achieve satisfactory results. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4461/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
An 18-year-old female patient admitted to the emergency department of Guizhou Provincial Orthopedics Hospital with abdominal pain for more than 1 month, and was transferred to our department after consultation.
Facial red rash, sensitiveness to light, and alopecia had appeared 7 months before presentation, but she didn't attach importance and treat. Her previous medical history was unremarkable. She developed abdominal pain, abdominal distension, vomiting and fever without obvious inducement 1 month ago. She was admitted to a local hospital and received abdominal computed tomography (CT) examination indicating intestinal obstruction. After symptomatic and supportive treatment, her symptoms were relieved. About 20 days later, due to reoccurrence of abdominal pain, abdominal distension, and fever, she admitted to the emergency department. Physical examination revealed a body temperature of 38.9 ℃, facial erythema (Figure 1A), enlarged bilateral axillary lymph nodes, scattered abdominal tenderness, obvious tenderness in the lower abdomen, tympanitic sound to percussion, and diminished bowel sounds of around 1–2 sounds/minute.

The patient was given further examination after the transfer to our department. The test results revealed reduced white blood cells, positive urine protein, lowered C3 and C4. Owing to the patient's previous experience of recurrent symptoms, which had been relieved after symptomatic and supportive treatment, immune system diseases were considered. Immunological tests and lymph node biopsy were performed. Blood routine tests showed a red blood cell count of 3.78×1012/L, hemoglobin of 107.0 g/L, white blood cell count of 2.98×109/L, the neutrophil ratio of 53.04%, and platelet count of 175.00×109/L. Biochemical tests showed a total protein level of 57.4 g/L, albumin of 33.9 g/L, uric acid of 393 µmol/L, and β2-microglobulin of 3.68 mg/L. Urinalysis showed protein (+++) and 24-hour urinary protein quantity was 3.19 g/L. Due to the clinical manifestations and results of the laboratory tests, a decision was made to conduct further immune-related examinations to assess the patient for immune system-related diseases. The immunoglobulin and complement tests revealed the levels of immunoglobulin A (IgA) of 2.53 g/L, immunoglobulin G (IgG) of 13.88 g/L, immunoglobulin M (IgM) of 1.29 g/L, complement C3 of 0.29 g/L, and complement C4 of 0.03 g/L. The extractable nuclear antigen polypeptide antibody (anti-ENA antibody) test was positive for anti-Sj gren syndrome A (anti-SSA) antibody, anti-Sj gren syndrome B (anti-SSB) antibody, and antiribosomal P protein. Moreover, the patient showed antinuclear antibody (ANA) homogeneity (1:640) and was positive for anti-double-stranded DNA antibody (dsDNA) and perinuclear antineutrophil cytoplasmic antibody (p-ANCA), but negative for antineutrophil cytoplasmic antibody (c-ANCA). The results of other laboratory tests were unremarkable. According to the 2019 EULAR/ACR Classification Criteria for SLE, patients ought to conform to the criteria for inclusion, which is ANA positive, followed by a total score of ≥10 points in clinical and immunological domains to be diagnosed with SLE (8). Because she had a weighted score of 25 (fever of 38.9 ℃: 2 points; white blood cell count decrease: 3 points; non-cicatricial alopecia: 2 points; subacute rash: 4 points; proteinuria: 4 points; low C3 and low C4: 4 points; positive dsDNA: 6 points) indicating a clear diagnosis of SLE.
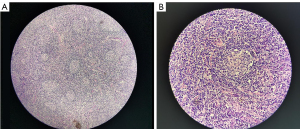
Intestinal obstruction was considered due to the symptoms of abdominal pain, abdominal distension, and nausea. A plain CT scan of the abdomen showed dilatation and gas and fluid collection in some segments of small intestines. Multiple air-fluid levels of different sizes were visible. The surrounding fatty tissue of the distal ileum was blurred. Patchy shades of liquid were seen around the adjacent intestinal tube. There were no signs of mechanical ileus. Thus, incomplete small bowel obstruction was considered (Figure 1B). Since the patient had a fever and bilateral axillary lymph node enlargement, suspicion of pulmonary infection and lymphatic system-related diseases was warranted. Next, chest CT was performed, which confirmed enlargement of multiple lymph nodes in both axillae and the mediastinum. Chest contrast-enhanced CT examination results still showed multiple enlarged lymph nodes (Figure 1C,1D), and further lymph node biopsy was performed. The results of the axillary lymph node biopsy were consistent with the typical histologic features of clear vascular CD (Figure 2A,2B). Immunohistochemistry showed the following results: CD(3+), CD20(+), CD45RO(+), Bcl-2(+), CD68 (focus +), CD38 (focus +), CD79a (+), CD34 (vascular +), CD138 (focus +), CyclinD1 (focus +), CD (focus +), CD15 (–), and Ki67 (germinal center +).
Taken together, the patient was diagnosed with IPO in SLE complicated by CD.
The patient was treated with glucocorticoids combined with immunosuppressant shock therapy, which included methylprednisolone sodium succinate (200 mg/day) intravenous infusion combined with mycophenolate mofetil (1,500 mg/day) oral shock therapy on the first, second, third, and fourth days of treatment. Methylprednisolone sodium succinate (40 mg/day) combined with mycophenolate mofetil (1,500 mg/day) was continued on the fifth to the thirteenth day of treatment. The patient’s symptoms were relieved and she was discharged on the twenty-third day. After discharge, prednisone (1 mg/kg/day), mycophenolate mofetil (1,500 mg/day, twice a day), hydroxychloroquine sulfate (0.4 g/day, twice a day) were given as maintenance treatment. She had regular outpatient follow-up and medication reduction. During the next 1 year of follow-up, she had no recurrence of abdominal pain and distension, and her urinary protein and immune indexes of outpatient reexamination gradually improved, and chest CT showed that lymph nodes had decreased. At present, the patient is still under continuous clinical follow-up and her condition is stable.
Discussion
SLE is a chronic autoimmune disease with an annual incidence of 1–10 cases per 100,000 person-years. The incidence rate is relatively high among Asians, especially Chinese, and is common in middle-aged women (9). Multiple organs are involved in SLE, such as the brain, kidneys, gastrointestinal tract, and hematologic system (10). Pseudo-obstruction is a rare and dangerous complication in SLE and is easy to be misdiagnosed. Its clinical characteristics include intestinal propulsion disturbance without mechanical obstruction. Patients often seek medical help with complaints of gastrointestinal symptoms that might recur after conventional symptomatic and supportive treatment (5,11,12). In our case, the patient repeatedly presented with abdominal pain as the initial symptom. Although abdominal pain had been relieved after symptomatic and supportive treatment for ileus, it recurred 1 month later. After reviewing relevant literature, we narrowed the potential diagnosis to SLE combined with IPO and implemented glucocorticoid and immunosuppressant therapy (13). There was no recurrence of abdominal pain during 1 year of follow-up. As a result, SLE with IPO was diagnosed (14).
CD is a rare lymphoproliferative disorder with clinical manifestations ranging from asymptomatic lymphadenopathy to severe generalized diffuse lymphadenopathy (15). The lymph node structure is basically present, and the lymph sinus is partially present or completely gone. The proliferation of lymphocytes in the sheath area appears as concentric circles or “onion skin,” surrounding single or multiple germinal centers of degenerative transformation, and eosinophilic deposition can also be seen. The proliferation of small blood vessels between the follicles is obvious, penetrating the germinal centers to form so-called lollipop follicles. Proliferated interfollicular small vessels present hyaline degeneration or fibrosis, and eosinophilic infiltration can also be (16).
Conclusions
As the initial clinical manifestation of SLE, IPO is easy to be missed diagnosed and misdiagnosed in clinical practice, thus delaying the treatment, increasing the unnecessary economic burden of the patient, and even leading to unnecessary surgical treatment. In addition, lymphadenopathy is a common phenomenon in SLE, which may be combined with CD. If lymph node biopsy is not performed, the disease may be missed. Therefore, improving the understanding of SLE and its associated diseases has certain guiding significance for clinicians in the diagnostic process and treatment of SLE.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4461/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4461/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haack H. Intestinal pseudo-obstruction. Ther Umsch 2007;64:217-20. [Crossref] [PubMed]
- Perlemuter G, Chaussade S, Wechsler B, et al. Chronic intestinal pseudo-obstruction in systemic lupus erythematosus. Gut 1998;43:117-22. [Crossref] [PubMed]
- Ceccato F, Salas A, Góngora V, et al. Chronic intestinal pseudo-obstruction in patients with systemic lupus erythematosus: report of four cases. Clin Rheumatol 2008;27:399-402. [Crossref] [PubMed]
- Zhang FJ, Zhang J, Zhou LP, et al. Intestinal pseudo-obstruction as the initial manifestation of systemic lupus erythematosus. Am J Emerg Med 2019;37:176.e1-2. [Crossref] [PubMed]
- Wang JL, Liu G, Liu T, et al. Intestinal pseudo-obstruction in systemic lupus erythematosus: a case report and review of the literature. Medicine (Baltimore) 2014;93:e248. [Crossref] [PubMed]
- Fortuna G, Brennan MT. Systemic lupus erythematosus: epidemiology, pathophysiology, manifestations, and management. Dent Clin North Am 2013;57:631-55. [Crossref] [PubMed]
- Demirkan FG, Doğan S, Kalyoncu Uçar A, et al. Systemic lupus erythematosus complicated with Castleman disease: a case-based review. Rheumatol Int 2021;41:475-9. [Crossref] [PubMed]
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019;71:1400-12. [Crossref] [PubMed]
- Ali A, Sayyed Z, Ameer MA, et al. Systemic Lupus Erythematosus: An Overview of the Disease Pathology and Its Management. Cureus 2018;10:e3288. [Crossref] [PubMed]
- Tian XP, Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment. World J Gastroenterol 2010;16:2971-7. [Crossref] [PubMed]
- Jin P, Ji X, Zhi H, et al. A review of 42 cases of intestinal pseudo-obstruction in patients with systemic lupus erythematosus based on case reports. Hum Immunol 2015;76:695-700. [Crossref] [PubMed]
- Ayari M, Nakhli A, Teyeb Z, et al. Intestinal pseudo-obstruction: Unusual presentation of systemic lupus erythematous. Clin Case Rep 2021;9:1759-62. [Crossref] [PubMed]
- Khairullah S, Jasmin R, Yahya F, et al. Chronic intestinal pseudo-obstruction: a rare first manifestation of systemic lupus erythematosus. Lupus 2013;22:957-60. [Crossref] [PubMed]
- Malaviya AN, Sharma A, Agarwal D, et al. Acute abdomen in SLE. Int J Rheum Dis 2011;14:98-104. [Crossref] [PubMed]
- Abramson JS. Diagnosis and Management of Castleman Disease. J Natl Compr Canc Netw 2019;17:1417-9. [Crossref] [PubMed]
- Wu D, Lim MS, Jaffe ES. Pathology of Castleman Disease. Hematol Oncol Clin North Am 2018;32:37-52. [Crossref] [PubMed]
(English Language Editor: J. Jones)


