
Development and validation of a model to predict the risk of recurrence in patients with laryngeal squamous cell carcinoma after total laryngectomy
Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of the most common cancers in the head and neck area, and accounts for 85–95% of all laryngeal cancers (1). It is estimated that 12,470 new cases of laryngeal cancer will be diagnosed and approximately 3,820 patients will die from the disease in the United States in 2022 (2). Approximately 60% of patients have advanced (stage III or IV) disease at the time of diagnosis (3,4). Current treatment methods for LSCC include surgery, radiotherapy, and chemotherapy (5). Total laryngectomy is used as the primary therapy for advanced laryngeal cancer (6). Several studies have suggested that undergoing a total laryngectomy for advanced-stage laryngeal cancer can improve patient survival (7,8). However, even after a total laryngectomy of the LSCC, patients are still at risk of recurrence.
A review reported that the local recurrence rate after a total laryngectomy is between 30–66% in patients with recurrent or persistent laryngeal cancer (9). Importantly, the survival of patients who have undergone a salvage total laryngectomy has been reported to be significantly lower than that of patients who do not require salvage surgery (9). Thus, it is important to identify the prognostic factors influencing the oncologic outcomes after a total laryngectomy, and to predict the likelihood of recurrence to optimize patient treatment and follow-up. Many factors have been reported to have predictive value in LSCC, including clinical (e.g., gender and age) (10), pathological (e.g., tumor stage, size, and grade) (11,12), and genetic variables (e.g., tumor protein p53 mutations and long non-coding RNA AC008440.10) (13,14). Due to the variability of clinicopathological features and tumor biology, a single feature has limited predictive effect in clinical practice. Prediction models can improve the accuracy of prediction by integrating multiple clinical variables and provide clinicians with prognostic prediction tools for individualized patients. Cui et al. developed a nomogram for predicting the risk of recurrence after curative-intend surgery in patients with LSCC (15). However, their model is mainly based on pathological data, and the inclusion of some routine inflammatory indicators related to the prognosis of LSCC patients may further improve the ability of the model. Several studies have shown that inflammation markers such as the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and lymphocyte/monocyte ratio (LMR) are independently correlated with poor outcomes in LSCC patients (16-19). However, the role of these inflammation indicators in the prediction of recurrence in LSCC patients with total laryngectomy has not been reported.
In the present study, we sought to develop and internally validate a model using systematic statistical methods to predict the risk of recurrence in LSCC patients who have undergone a total laryngectomy. We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4802/rc).
Methods
Study design and population
Patients with LCSS who underwent total laryngectomy between 2012 and 2019 at the Shanxi Province Cancer Hospital were included in this retrospective cohort study. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have been diagnosed with LCSS by histology or cytology; (II) have undergone a total laryngectomy; and (III) have complete pathological examination data and follow-up data. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had undergone an emergency tracheotomy; (II) had an active systemic inflammation (such as a pneumonia); (III) had a severe uncontrolled cardiovascular disease, or a recent history of myocardial infarction (within the last 3 months); and/or (IV) had suffered from other malignant tumors within the last 5 years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanxi Province Cancer Hospital (No. 202130) and individual consent for this retrospective analysis was waived.
Outcomes
The primary outcome of this study was the recurrence measured from the time of surgery. All the patients received regular follow-up every 3 months in the first 6 months after resection, and every 6 months thereafter; the length of follow-up was 3 years or until death. Each patient’s recurrence and survival status were recorded during the follow-ups. Recurrence was defined as a new laryngeal mass found by imaging examination and confirmed by biopsy or surgical pathology to be laryngeal cancer, or distant lymph node metastasis.
Sample size
According to previous studies (9), the recurrence rate was set as 40%, and the area under the receiver operating characteristic (ROC) curve (AUC) of the recurrence prediction model in previous studies was 0.778 as a reference (15). The sample size was calculated by PASS 15.0.5 software (NCSS, LLC, Kaysville, UT, USA), the AUC of the prediction model in this study was set as 0.90, and 114 patients were assigned to the training set with an α-error of 0.05 and a power of 0.8 (two-sided). The training set and testing set were randomly assigned in a ratio of 2:1, with a total sample size of at least 171.
Data collection and definition
Patients’ demographic and clinical data were collected, including data on their age, gender, tumor size, tumor (T) stage, node (N) stage, grade, primary site, metastatic, surgical excision margins extracapsular invasion, lymphatic vascular invasion, NLR, PLR, LMR, albumin/globulin ratio (AGR), prognostic nutritional index (PNI), the combination of the platelet count and the NLR (COP-NLR), the combined score of the plasma fibrinogen level and the NLR (F-NLR), and recurrence.
The PNI was calculated based on the albumin (Alb) and the absolute lymphocyte count (ALC) (20) using the following formula: PNI = 10 × Alb + 0.005 × ALC. The COP-NLRs were defined as follows: COP-NLR 2—patients with an elevated platelet count >300×109/L and a NLR >3; COP-NLR 1—patients with 1 abnormal value; and COP-NLR 0—patients with no abnormal value (21,22). The F-NLRs were defined as follows: F-NLR 2—patients with fibrinogen ≥341 mg/dL and a NLR ≥3.59; F-NLR 1—patients with fibrinogen ≥341 mg/dL or a NLR ≥3.59; and F-NLR 0—patients with fibrinogen <341 mg/dL and a NLR <3.59 (23).
Statistical analysis
The continuous variables were tested for normality using the Shapiro-Wilk test; the continuous variables with a normal distribution are expressed as the mean ± standard deviation (SD), and the t-test was used for comparisons between groups. The non-normal variables are expressed as median (interquartile range) [M (Q1, Q3)], and the Wilcoxon rank-sum test was used for comparisons between groups. The categorical variables are expressed as numbers and percentages, and the chi-square test (χ2) or Fisher’s test was used for comparisons between groups. The data were randomly assigned to a training set and a test set by Python at a ratio of 4:1. The random-forest model was used to construct the prediction model. The performance of the model was quantified by calculating the AUC with the 95% confidence interval (CI), and the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The AUC value of the prediction model was over 0.80, indicating good predictive performance of the model.
All the statistical analyses were two-sided, and a P value <0.05 was considered statistically significant. The baseline characteristics were analyzed by the SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). Python 3.7 software (Python Software Foundation, Delaware, USA) was used to develop the random-forest model and plot the importance of the predictors and the ROC curves.
Results
Baseline characteristics
A total of 204 LSCC patients were included in this study. The patients had a mean age of 59.89±8.90 years, and a mean tumor size of 2.93±1.01 cm. Of these patients, 193 (94.61%) were male, 11 (5.39%) were female, and 4 (1.96%) were T1 stage, 28 (13.73%) were T2 stage, 107 (52.45%) were T3 stage, and 65 (31.86%) were T4 stage. In terms of tumor grade, 28 (13.73%) patients had high differentiation, 123 (60.29%) had moderate differentiation, and 53 (25.98%) had poor differentiation. The median NLRs, PLRs, and LMRs of the patients were 2.15 (1.60, 3.14), 123.38 (96.92, 152.58), and 4.26 (3.07, 5.63), respectively. The mean AGRs and PNIs of the patients were 1.57±0.26 and 427.31±37.99, respectively. The numbers of patients with COP-NLR scores of 0, 1, and 2 were 120 (58.82%), 70 (34.31%), and 14 (6.86%), respectively. The numbers of patients with F-NLR scores of 0, 1, and 2 were 111 (54.41%), 25 (12.25%), and 68 (33.33%), respectively. At the end of the follow-up, 56 (27.45%) patients had relapsed. More detailed characteristics are shown in Table 1.
Table 1
| Variables | Total (n=204) |
|---|---|
| Age (years), mean ± SD | 59.89±8.90 |
| Gender, n (%) | |
| Male | 193 (94.61) |
| Female | 11 (5.39) |
| Tumor size (cm), mean ± SD | 2.93±1.01 |
| T stage, n (%) | |
| T1 | 4 (1.96) |
| T2 | 28 (13.73) |
| T3 | 107 (52.45) |
| T4 | 65 (31.86) |
| N stage, n (%) | |
| N0 | 129 (63.24) |
| N1 | 28 (13.73) |
| N2a | 5 (2.45) |
| N2b | 21 (10.29) |
| N2c | 21 (10.29) |
| Grade, n (%) | |
| High differentiation | 28 (13.73) |
| Moderate differentiation | 123 (60.29) |
| Poor differentiation | 53 (25.98) |
| Primary site, n (%) | |
| Glottis | 57 (27.94) |
| Supraglottis | 118 (57.84) |
| Subglottis | 13 (6.37) |
| Transglottis | 16 (7.84) |
| Surgical excision margins, n (%) | |
| Positive | 6 (2.94) |
| Negative | 198 (97.06) |
| Metastatic, n (%) | |
| Yes | 76 (37.25) |
| No | 128 (62.75) |
| Extracapsular invasion, n (%) | |
| Yes | 23 (11.27) |
| No | 181 (88.73) |
| Lymphatic vascular invasion, n (%) | |
| Yes | 17 (8.33) |
| No | 187 (91.67) |
| NLR, M (Q1, Q3) | 2.15 (1.60, 3.14) |
| PLR, M (Q1, Q3) | 123.38 (96.92, 152.58) |
| LMR, M (Q1, Q3) | 4.26 (3.07, 5.63) |
| AGR, mean ± SD | 1.57±0.26 |
| PNI, mean ± SD | 427.31±37.99 |
| COP-NLR (score), n (%) | |
| 0 | 120 (58.82) |
| 1 | 70 (34.31) |
| 2 | 14 (6.86) |
| F-NLR (score), n (%) | |
| 0 | 111 (54.41) |
| 1 | 25 (12.25) |
| 2 | 68 (33.33) |
| Group, n (%) | 148 (72.55) |
| Relapse group | 56 (27.45) |
| Non-relapse group | 148 (72.55) |
SD, standard deviation; T, tumor; N, node; NLR, neutrophil/lymphocyte ratio; M (Q1, Q3), median (interquartile range); PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; AGR, albumin/globulin ratio; PNI, prognostic nutritional index; COP-NLR, combination of the platelet count and the NLR; F-NLR, combined score of the plasma fibrinogen level and the NLR.
Comparison of differences between the training set and the test set
A total of 204 patients were randomly divided into a training set and a test set at a ratio of 4:1. The results showed that there was no statistical difference (all P>0.05) among all the characteristics between the training set and the test set (Table 2). Thus, the sampling of the training set and the test set was balanced, and the data of the test set could be used to test the training set.
Table 2
| Variables | Training group (n=163) | Testing group (n=41) | Statistic | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 59.43±8.97 | 61.71±8.50 | t=−1.470 | 0.143 |
| Gender, n (%) | – | 1.000 | ||
| Male | 154 (94.48) | 39 (95.12) | ||
| Female | 9 (5.52) | 2 (4.88) | ||
| Tumor size (cm), mean ± SD | 2.912±1.006 | 3.020±1.050 | t=−0.60 | 0.546 |
| T stage, n (%) | – | 0.436 | ||
| T1 | 3 (1.84) | 1 (2.44) | ||
| T2 | 20 (12.27) | 8 (19.51) | ||
| T3 | 85 (52.15) | 22 (53.66) | ||
| T4 | 55 (33.74) | 10 (24.39) | ||
| N stage, n (%) | – | 0.560 | ||
| N0 | 98 (60.12) | 31 (75.61) | ||
| N1 | 24 (14.72) | 4 (9.76) | ||
| N2a | 5 (3.07) | 0 (0.00) | ||
| N2b | 18 (11.04) | 3 (7.32) | ||
| N2c | 18 (11.04) | 3 (7.32) | ||
| Grade, n (%) | χ2=0.106 | 0.949 | ||
| High differentiation | 23 (14.11) | 5 (12.20) | ||
| Moderate differentiation | 98 (60.12) | 25 (60.98) | ||
| Poor differentiation | 42 (25.77) | 11 (26.83) | ||
| Primary site, n (%) | – | 0.550 | ||
| Glottis | 45 (27.61) | 12 (29.27) | ||
| Supraglottis | 92 (56.44) | 26 (63.41) | ||
| Subglottis | 11 (6.75) | 2 (4.88) | ||
| Transglottis | 15 (9.20) | 1 (2.44) | ||
| Surgical excision margins, n (%) | – | 0.602 | ||
| Positive | 6 (3.68) | 0 (0.00) | ||
| Negative | 157 (96.32) | 41 (100.00) | ||
| Metastatic, n (%) | χ2=2.386 | 0.122 | ||
| Yes | 65 (39.88) | 11 (26.83) | ||
| No | 98 (60.12) | 30 (73.17) | ||
| Extracapsular invasion, n (%) | – | 0.787 | ||
| Yes | 18 (11.04) | 5 (12.20) | ||
| No | 145 (88.96) | 36 (87.80) | ||
| Lymphatic vascular invasion, n (%) | – | 0.344 | ||
| Yes | 12 (7.36) | 5 (12.20) | ||
| No | 151 (92.64) | 36 (87.80) | ||
| NLR, M (Q1, Q3) | 2.21 (1.65, 3.10) | 2.01 (1.34, 3.67) | Z=−0.900 | 0.368 |
| PLR, M (Q1, Q3) | 124.34 (98.24, 153.03) | 122.66 (93.20, 149.68) | Z=−0.755 | 0.450 |
| LMR, M (Q1, Q3) | 4.04 (3.03, 5.48) | 5.10 (3.70, 6.18) | Z=1.885 | 0.059 |
| AGR, mean ± SD | 1.57±0.27 | 1.56±0.25 | t=0.110 | 0.910 |
| PNI, mean ± SD | 426.35±39.74 | 431.13±30.14 | t=−0.850 | 0.399 |
| COP-NLR (score), n (%) | χ2=0.133 | 0.715 | ||
| 0 | 97 (59.51) | 23 (56.10) | ||
| 1 | 55 (33.74) | 15 (36.59) | ||
| 2 | 11 (6.75) | 3 (7.32) | ||
| F-NLR (score), n (%) | χ2=4.285 | 0.117 | ||
| 0 | 94 (57.67) | 17 (41.46) | ||
| 1 | 17 (10.43) | 8 (19.51) | ||
| 2 | 52 (31.90) | 16 (39.02) | ||
| Group, n (%) | χ2=0.085 | 0.771 | ||
| Non-relapse group | 119 (73.01) | 29 (70.73) | ||
| Relapse group | 44 (26.99) | 12 (29.27) |
SD, standard deviation; T, tumor; N, node; NLR, neutrophil/lymphocyte ratio; M (Q1, Q3), median (interquartile range); PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; AGR, albumin/globulin ratio; PNI, prognostic nutritional index; COP-NLR, combination of the platelet count and the NLR; F-NLR, combined score of the plasma fibrinogen level and the NLR.
Comparison of the characteristics between the relapsed and non-relapsed patients
The training set data were divided into the relapse group and non-relapse group. A difference analysis was performed between the relapse group and non-relapse group. The results showed that there were significant differences in the PLR (Z=2.275, P=0.023), AGR (t=2.420, P=0.017), and proportion of males (P=0.002), metastatic status (χ2=5.409, P=0.020), COP-NLR (χ2=4.192, P=0.041), and F-NLR (χ2=4.242, P=0.039). These 6 factors may be associated with recurrence in LSCC patients who have undergone a total laryngectomy (see Table 3).
Table 3
| Variables | Relapse group (n=44) | Non-relapse group (n=119) | Statistic | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 61.27±10.26 | 58.75±8.38 | t=−1.60 | 0.111 |
| Gender, n (%) | – | 0.002 | ||
| Male | 37 (84.09) | 117 (98.32) | ||
| Female | 7 (15.91) | 2 (1.68) | ||
| Tumor size (cm), mean ± SD | 3.13±1.02 | 2.83±0.99 | t=−1.65 | 0.101 |
| T stage, n (%) | – | 0.799 | ||
| T1 | 1 (2.27) | 2 (1.68) | ||
| T2 | 5 (11.36) | 15 (12.61) | ||
| T3 | 21 (47.73) | 64 (53.78) | ||
| T4 | 17 (38.64) | 38 (31.93) | ||
| N stage, n (%) | – | 0.103 | ||
| N0 | 22 (50.00) | 76 (63.87) | ||
| N1 | 5 (11.36) | 19 (15.97) | ||
| N2a | 1 (2.27) | 4 (3.36) | ||
| N2b | 7 (15.91) | 11 (9.24) | ||
| N2c | 9 (20.45) | 9 (7.56) | ||
| Grade, n (%) | χ2=5.544 | 0.063 | ||
| High differentiation | 2 (4.55) | 21 (17.65) | ||
| Moderate differentiation | 27 (61.36) | 71 (59.66) | ||
| Poor differentiation | 15 (34.09) | 27 (22.69) | ||
| Primary site, n (%) | – | 0.630 | ||
| Glottis | 9 (20.45) | 36 (30.25) | ||
| Supraglottis | 27 (61.36) | 65 (54.62) | ||
| Subglottis | 3 (6.82) | 8 (6.72) | ||
| Transglottis | 5 (11.36) | 10 (8.40) | ||
| Surgical excision margins, n (%) | – | 0.661 | ||
| Positive | 2 (4.55) | 4 (3.36) | ||
| Negative | 42 (95.45) | 115 (96.64) | ||
| Metastatic, n (%) | χ2=5.409 | 0.020 | ||
| Yes | 24 (54.55) | 41 (34.45) | ||
| No | 20 (45.45) | 78 (65.55) | ||
| Extracapsular invasion, n (%) | – | 0.093 | ||
| Yes | 8 (18.18) | 10 (8.40) | ||
| No | 36 (81.82) | 109 (91.60) | ||
| Lymphatic vascular invasion, n (%) | – | 0.736 | ||
| Yes | 4 (9.09) | 8 (6.72) | ||
| No | 40 (90.91) | 111 (93.28) | ||
| NLR, M (Q1, Q3) | 2.64 (1.80, 3.69) | 2.12 (1.59, 2.92) | Z=1.927 | 0.054 |
| PLR, M (Q1, Q3) | 137.01 (108.62, 179.59) | 120.34 (96.89, 149.65) | Z=2.275 | 0.023 |
| LMR, M (Q1, Q3) | 3.88 (2.84, 5.46) | 4.11 (3.14,5.56) | Z=−0.716 | 0.474 |
| AGR, mean ± SD | 1.49±0.23 | 1.60±0.27 | t=2.42 | 0.017 |
| PNI, mean ± SD | 421.96±44.43 | 427.97±37.93 | t=0.86 | 0.393 |
| COP-NLR (score), n (%) | χ2=4.192 | 0.041 | ||
| 0 | 20 (45.45) | 77 (64.71) | ||
| 1 | 20 (45.45) | 35 (29.41) | ||
| 2 | 4 (9.09) | 7 (5.88) | ||
| F-NLR (score), n (%) | χ2=4.242 | 0.039 | ||
| 0 | 30 (68.18) | 64 (53.78) | ||
| 1 | 6 (13.64) | 11 (9.24) | ||
| 2 | 8 (18.18) | 44 (36.97) |
SD, standard deviation; T, tumor; N, node; NLR, neutrophil/lymphocyte ratio; M (Q1, Q3), median (interquartile range); PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; AGR, albumin/globulin ratio; PNI, prognostic nutritional index; COP-NLR, combination of the platelet count and the NLR; F-NLR, combined score of the plasma fibrinogen level and the NLR.
Development and validation of the random-forest model
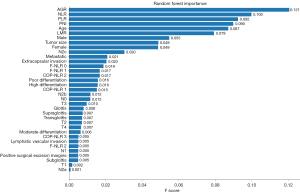
The random-forest method was used to develop a model to predict the recurrence of LSCC in patients who have undergone a total laryngectomy. Under the random-forest model, the most important predictors were the AGR, NLR, and PLR, which contributed 0.121, 0.100, and 0.092 to the model, respectively (see Figure 1).
In the training set, the AUC of the random-forest model was 0.960 (95% CI, 0.931–0.989); according to the Youden Index, a cutoff value of 0.253 was selected (see Figure 2A). The sensitivity, specificity, accuracy, PPV, and NPV of the model in the training set were 0.955 (95% CI, 0.893–1.000), 0.832 (95% CI, 0.765–0.899), 0.865 (95% CI, 0.813–0.917), 0.677 (95% CI, 0.561–0.948), and 0.980 (95% CI, 0.953–1.000), respectively. When the data of the test set was substituted into the prediction model of the training set, the AUC, sensitivity, specificity, accuracy, PPV, and NPV of the model in the test set were 0.721 (95% CI, 0.716–0.726), 0.750 (95% CI, 0.505–0.995), 0.690 (95% CI, 0.521–0.858), 0.707 (95% CI, 0.568–0.847), 0.500 (95% CI, 0.269–0.921), and 0.870 (95% CI, 0.732–1.000), respectively (see Figure 2B and Table 4).
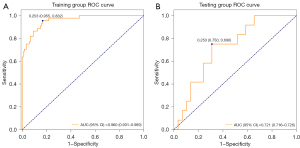
Table 4
| Variables | Training set (95% CI) | Test set (95% CI) |
|---|---|---|
| AUC | 0.960 (0.931, 0.989) | 0.721 (0.716, 0.726) |
| Accuracy | 0.865 (0.813, 0.917) | 0.707 (0.568, 0.847) |
| Specificity | 0.832 (0.765, 0.899) | 0.690 (0.521, 0.858) |
| Sensitivity | 0.955 (0.893, 1.000) | 0.750 (0.505, 0.995) |
| PPV | 0.677 (0.561, 0.948) | 0.500 (0.269, 0.921) |
| NPV | 0.980 (0.953, 1.000) | 0.870 (0.732, 1.000) |
AUC, area under the ROC curve; ROC, receiver operating characteristic; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval.
Discussion
Total laryngectomy is commonly performed in locally advanced LSCC patients or with recurrent or persistent cancer after radiation or chemoradiation treatment. However, these patients are still at risk of recurrence after a total laryngectomy. In this study, we used several easily available clinical variables to establish a random-forest model to predict the risk of recurrence of LSCC in patients who have undergone a total laryngectomy. The results showed that certain clinical indicators, including the AGR, NLR, and PLR, were the most important factors in predicting the risk of recurrence of LSCC in patients who have undergone a total laryngectomy. The AUCs of the random-forest model in the training set and the test set were 0.960 and 0.721, respectively.
Several models have been reported to predict the risk of LSCC recurrence in patients. Yang et al. established a scoring model based on the 2 independent predictors of CDGSH iron-sulfur domain 2 and N stage (24). Jover-Esplá et al. developed a risk-prediction model based on all laryngeal cancer patients, and showed that age, alcohol consumption, lymph node stage, and stage were associated with a 5-year risk of recurrence (25). Recently, Cui et al. (15) developed a nomogram to predict the recurrence risk of LSCC in patients that included 6 factors (i.e., age, tumor site, smoking, alcohol, N stage, and hemoglobin). However, few studies have reported the risk of recurrence in patients with LSCC after a total laryngectomy. In the present study, we used the random-forest method to develop a model to predict the risk of recurrence in LSCC patients who have undergone a total laryngectomy. The AUCs of our predictive model in the training set and the test set were 0.960 and 0.721, which indicated that our model was able to predict the risk of recurrence in LSCC patients after a total laryngectomy well.
Our random-forest model indicated that clinical indicators, such as the AGR, NLR, and PLR, were important predictors for predicting the risk of recurrence of LSCC in patients. Several studies have indicated that some inflammation markers, such as the NLR, PLR, and LMR, are independently correlated with poor outcomes in LSCC patients (16-19). It has been reported that cancer-associated inflammation plays an important role in tumor progression (26,27). The mechanism between inflammation and tumor progression is not clear; however, a possible explanation is that in the early stages of cancer development, various cytokines produced by cancer cells may recruit inflammatory cells that form a microenvironment, promote tumor growth, rheumatic instability, and angiogenesis (28-30). Neutrophils may secrete circulating growth factors to promote cancer cell metastasis (31). Lymphocytes play an important role in inducing cell death and inhibiting tumor cell migration and proliferation. The interaction between platelets and tumor cells can trigger the subsequent metastasis of tumor cells (32). Thus, the NLR and PLR are important factors affecting the prognosis of LSCC patients. Our results also indicated that the NLR and PLR play important roles in predicting the risk of recurrence of LSCC.
Several studies have reported that the AGR has a superior prognostic value in LSCC patients (33,34). A low AGR value indicates a poor prognosis for LSCC patients (33). Alb and globulin are two important components of serum proteins and may be related to systemic inflammation. It has been reported that a low serum Alb level reflects poor nutritional status and is an independent predictor of poor survival in many cancers (35,36). Additionally, an increase in globulin value is associated with a chronic inflammatory response and cumulative exposure to various inflammatory cytokines (37). Thus, the cumulative effect of Alb and globulin may have good prognostic value for LSCC patients. However, apart from the studies of Chen et al. (34) and Zhou et al. (33), few studies have focused on the prognostic value of the AGR in LSCC patients. In our prediction model, we found that the AGR was the most important factor for predicting the risk of recurrence of LSCC in patients. In future studies, clinicians should pay attention to the value of some basic clinical features, such as the AGR.
In the current study, a difference analysis was used to evaluate the data of the training set and the test set to ensure the reliability of the model. As described previously, the random-forest method was then used to develop a predictive model. Further, we used variables that are easily available and applicable in clinical practice to establish the model. However, this study had some limitations. First, the sample size of the females was small, which may be because the prevalence ratio of LSCC between males and females is 9.1:1 (38). Second, external validation is needed if the model is to be applied in clinical practice. Third, postoperative adjuvant treatment information such as radiotherapy and chemotherapy were not adjusted as confounders, which may affect the effect of the model.
Conclusions
A risk-prediction model to predict the recurrence risk in LSCC patients who have undergone a total laryngectomy was established, and inflammatory markers AGR, NLR, and PLR play an important role in the predictive model. This model may provide clinicians with a tool to predict recurrence risk in LSCC patients after a total laryngectomy, but external validation is needed before it is used in clinical practice.
Acknowledgments
The authors appreciate the academic support from the AME Otolaryngology Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4802/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4802/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4802/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanxi Province Cancer Hospital (No. 202130) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Unsal AA, Kılıç S, Dubal PM, et al. A population-based comparison of European and North American sinonasal cancer survival. Auris Nasus Larynx 2018;45:815-24. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Groome PA, O'Sullivan B, Irish JC, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the Surveillance, Epidemiology, and End Results areas of the United States. J Clin Oncol 2003;21:496-505. [Crossref] [PubMed]
- Falco M, Tammaro C, Takeuchi T, et al. Overview on Molecular Biomarkers for Laryngeal Cancer: Looking for New Answers to an Old Problem. Cancers (Basel) 2022;14:1716. [Crossref] [PubMed]
- Gatta G, Botta L, Sánchez MJ, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer 2015;51:2130-43. [Crossref] [PubMed]
- Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin 2017;67:31-50. [Crossref] [PubMed]
- Dziegielewski PT, O'Connell DA, Klein M, et al. Primary total laryngectomy versus organ preservation for T3/T4a laryngeal cancer: a population-based analysis of survival. J Otolaryngol Head Neck Surg 2012;41:S56-64. [PubMed]
- Grover S, Swisher-McClure S, Mitra N, et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int J Radiat Oncol Biol Phys 2015;92:594-601. [Crossref] [PubMed]
- Silverman DA, Puram SV, Rocco JW, et al. Salvage laryngectomy following organ-preservation therapy - An evidence-based review. Oral Oncol 2019;88:137-44. [Crossref] [PubMed]
- Leoncini E, Vukovic V, Cadoni G, et al. Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: a multicentre study within the INHANCE consortium. Eur J Epidemiol 2018;33:1205-18. [Crossref] [PubMed]
- Chang CF, Chu PY. Predictors of local recurrence of glottic cancer in patients after transoral laser microsurgery. J Chin Med Assoc 2017;80:452-7. [Crossref] [PubMed]
- Haapaniemi A, Väisänen J, Atula T, et al. Predictive factors and treatment outcome of laryngeal carcinoma recurrence. Head Neck 2017;39:555-63. [Crossref] [PubMed]
- Mafune A, Hama T, Suda T, et al. Homozygous deletions of UGT2B17 modifies effects of smoking on TP53-mutations and relapse of head and neck carcinoma. BMC Cancer 2015;15:205. [Crossref] [PubMed]
- Chen J, Shen Z, Deng H, et al. Long non-coding RNA biomarker for human laryngeal squamous cell carcinoma prognosis. Gene 2018;671:96-102. [Crossref] [PubMed]
- Cui J, Wang L, Tan G, et al. Development and validation of nomograms to accurately predict risk of recurrence for patients with laryngeal squamous cell carcinoma: Cohort study. Int J Surg 2020;76:163-70. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Kara M, Uysal S, Altinişik U, et al. The pre-treatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol 2017;274:535-42. [Crossref] [PubMed]
- Wang J, Wang S, Song X, et al. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. Onco Targets Ther 2016;9:7177-85. [Crossref] [PubMed]
- Magnes T, Wagner S, Kiem D, et al. Prognostic and Predictive Factors in Advanced Head and Neck Squamous Cell Carcinoma. Int J Mol Sci 2021;22:4981. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. [PubMed]
- Ishizuka M, Oyama Y, Abe A, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol 2014;110:935-41. [Crossref] [PubMed]
- Nakahira M, Sugasawa M, Matsumura S, et al. Prognostic role of the combination of platelet count and neutrophil-lymphocyte ratio in patients with hypopharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 2016;273:3863-7. [Crossref] [PubMed]
- Kuwahara T, Takahashi H, Sano D, et al. Fibrinogen and Neutrophil-to-lymphocyte Ratio Predicts Survival in Patients with Advanced Hypopharyngeal Squamous Cell Carcinoma. Anticancer Res 2018;38:5321-30. [Crossref] [PubMed]
- Yang L, Hong S, Wang Y, et al. A novel prognostic score model incorporating CDGSH iron sulfur domain2 (CISD2) predicts risk of disease progression in laryngeal squamous cell carcinoma. Oncotarget 2016;7:22720-32. [Crossref] [PubMed]
- Jover-Esplá AG, Palazón-Bru A, Folgado-de la Rosa DM, et al. A predictive model for recurrence in patients with glottic cancer implemented in a mobile application for Android. Oral Oncol 2018;80:82-8. [Crossref] [PubMed]
- Deng T, Lyon CJ, Bergin S, et al. Obesity, Inflammation, and Cancer. Annu Rev Pathol 2016;11:421-49. [Crossref] [PubMed]
- Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol 2020;15:123-47. [Crossref] [PubMed]
- Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759-71. [Crossref] [PubMed]
- Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013;108:914-23. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 2014;110:1409-12. [Crossref] [PubMed]
- Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol 2021;14:173. [Crossref] [PubMed]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576-90. [Crossref] [PubMed]
- Zhou T, Yu ST, Chen WZ, et al. Pretreatment albumin globulin ratio has a superior prognostic value in laryngeal squamous cell carcinoma patients: a comparison study. J Cancer 2019;10:594-601. [Crossref] [PubMed]
- Chen WZ, Yu ST, Xie R, et al. Preoperative albumin/globulin ratio has predictive value for patients with laryngeal squamous cell carcinoma. Oncotarget 2017;8:48240-7. [Crossref] [PubMed]
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [Crossref] [PubMed]
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223-6. [Crossref] [PubMed]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448-54. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


