
Increased serum uric acid level is associated with better outcome after endovascular treatment for acute ischemic stroke—a prospective cohort study
Introduction
Endovascular treatment (EVT) is effective for patients with an acute ischemic stroke (AIS) from a large vessel occlusion (LVO) (1-3). Although successful recanalization leads to the restoration of cerebral circulation, these patients may still have a poor functional outcome (4,5), which might be related with advanced age, higher baseline National Institutes of Health Stroke Scale (NIHSS) (6) score, delayed puncture to reperfusion, and the use of general anesthesia (7). Cerebral ischemia-reperfusion injury may be partly responsible for this undesirable result. Reperfusion injury after revascularization may be related to the inflammatory process with the release of free radicals once the occluded artery is re-opened (8).
Serum uric acid (SUA) is an end-product of the metabolism of purines and is an important endogenous free radical scavenger (9). While in the process of SUA production, reactive oxygen species are generated, increasing oxidative stress (10) and exerting proinflammatory effects such as the stimulation of some cytokines (11). The benefit of SUA against the risk of stroke and other cardiovascular events is unclear from epidemiological studies (12-14). Two meta-analyses indicated that hyperuricemia could modestly increase the risk of both stroke and mortality (15,16). Two prospective studies with conflicting results have been published since then (17,18). As higher SUA levels often coexist with other metabolic syndrome risk factors, almost all of the studies point out that elevated SUA level is an independent predictor of stroke in stroke-free people. However, 2 studies have revealed a protective effect of SUA in brain ischemia at the preclinical level (19,20). For instance, SUA reduced the exacerbated oxidative stress in hyperperfused rats after an ischemic stroke (21). However, there is no consensus about the relationship between SUA levels and stroke outcomes in AIS patients (22,23), especially after EVT (24,25). Furthermore, whether there is a dose-response relationship between SUA level and risk of stroke is unknown.
Our study aimed to explore the effect of SUA on functional outcomes at 90 days in LVO-related AIS patients with EVT and identify whether a dose-response correlation exists. We hypothesized that higher baseline SUA level could have a positive effect on functional outcomes at 90 days in these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4494/rc).
Methods
Study design and population
The RESCUE-RE (Registration Study for Critical Care of Acute Ischemic Stroke after Recanalization) registry is an ongoing, prospective, observational cohort study involving 18 comprehensive stroke centers across China (Registration number: ChiCTR1900022154). Patients with acute LVO-related AIS who underwent EVT within 24 hours were enrolled and followed up for 90 days (26). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the medical ethics committee of the Beijing Tiantan Hospital, Capital Medical University (No. KY2018-057-01). All participating hospitals/institutions were informed and agreed the study. All patients or their legally authorized representatives provided written informed consent before taking part in the study.
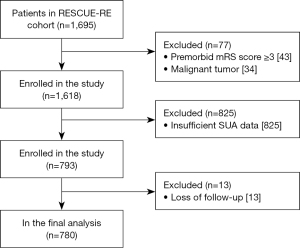
Patients of the current study were screened from the RESCUE-RE registry. Enrollment criteria were: (I) >18 years of age; (II) acute ischemic stroke with LVO; (III) EVT within 24 hours of stroke onset; and (IV) with SUA data recorded within 24 hours of EVT. The following patients were excluded: a premorbid modified Rankin Scale (mRS) (27) score ≥3, diagnosed with malignant tumor, or loss of follow-up at 90 days. Data related to this study are available from the corresponding author upon reasonable request.
Treatment procedures
Intravenous recombinant human tissue-type plasminogen activator (r-tPA) was administered within 4.5 hours of the onset of symptoms when indicated. Mechanical thrombectomy (MT) was conducted with stent retrievers and/or thrombus aspiration, intra-arterial thrombolysis (with r-tPA or urokinase), balloon angioplasty, stent implantation, or various combinations of these approaches, upon the decision of the treating neurologists/neurointerventionalists.
Clinical data and parameters
Baseline data, including demographic characteristics, history of symptomatic cardiovascular diseases and vascular risk factors, prior medications (especially those that may affect SUA level), characteristics of the index stroke and LVO, treatment procedures and laboratory results, were collected. Neurological deficits were assessed by stroke neurologists using the NIHSS. The location of LVO was identified on computed tomography or magnetic resonance angiography (CTA/MRA) or digital subtraction angiography (DSA). Stroke subtype was classified according to the Trial of ORG 10172 in Acute Stroke Treatment classification (TOAST). Successful reperfusion was defined as a modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b/3 (28). Postprocedural blood pressure parameters were measured and recorded every 2 hours for 24 hours after EVT. SUA and serum creatinine levels were measured within 24 hours of EVT using standard laboratory procedures. Estimated glomerular filtration (eGFR) was calculated using the formula adapted from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation on the basis of data from an Asian population (29). For any other relevant laboratory test of interest, the worst result within 48 hours of EVT, if there were multiple measures, was recorded.
Clinical outcomes
Primary outcome was defined as an excellent 90-day outcome by an mRS score of 0 to 1. Secondary outcomes included a favorable 90-day outcome defined by an mRS score of 0 to 2, symptomatic intracranial hemorrhage (sICH) within 24 hours of EVT according to the European Cooperative Acute Stroke Study II (ECASS II) classification (30), and 90-day mortality.
Statistical analysis
Baseline characteristics were described with means and standard deviation (SD) or medians and interquartile range (IQR) for quantitative variables and with numbers and percentage for qualitative variables. χ2 test or Fisher’s exact test was used for univariate analyses of categorical variables. Student t-test or Wilcoxon signed-rank test was used for continuous variables.
Significant variables associated with SUA or 90-day mRS according to univariate logistic regression (P value <0.05) were then entered into multivariable logistic regression analysis, respectively, and significant variables from the multivariable logistic regression were included in the adjusted model. In the analysis of factors affecting SUA level, SUA was used as a dependent variable for multiclassification multivariate logistic regression analysis. Multivariable logistic regression was performed to assess whether SUA level was associated with the primary outcome (mRS 0–1 at 90 days), adjusting for potential confounders, including: age, male sex, atrial fibrillation, diabetes mellitus, baseline NIHSS, prior stroke, smoking, drinking, onset to recanalization time, location of LVO, and stroke subtype (factors that have had an impact on 90-day functional outcomes in previous studies); diuretics before stroke onset, triglyceride level, blood urea nitrogen, and eGFR (factors affecting SUA level in the present study); and intravenous r-tPA, white blood cell (WBC), and mTICI 2b/3 (factors affecting 90-day functional outcomes in the current study). Sensitivity analysis was further performed to confirm that SUA level was an important predictor of 90-day functional outcome. All statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA), and two-sided P value <0.05 was considered statistically significant.
Results
Baseline characteristics of the population
Overall, 780 patients enrolled in RESCUE-RE between July 2018 and October 2020 were analyzed in the current study (Figure 1). The mean age was 64.40±12.14 years, and 66.28% were male (Table S1).
The mean SUA level within 24 hours of EVT was 310.02±96.22 µmol/L (Table S1). Compared with patients in the first quartile, those with SUA level in the fourth quartile were more likely to be male (78.46% vs. 51.28%; P<0.001), smokers (41.54% vs. 25.64%; P=0.002), drinkers (50.26% vs. 33.85%; P=0.007), and on diuretics (6.67% vs. 1.54%; P=0.003), and they were more likely to have a history of hypertension (66.67% vs. 52.82%; P=0.034), a higher level of triglycerides (1.56±0.96 vs. 1.33±1.17 mmol/L; P<0.001), a lower level of high density lipoprotein cholesterol (HDL-C) (1.07±0.29 vs. 1.21±0.36 mmol/L; P<0.001), a higher level of blood urea nitrogen (6.85±3.03 vs. 4.85±1.65 mmol/L; P<0.001), and a lower level of eGFR (80.78±27.18 vs. 101.39±17.09 mL/min/1.73 m2; P<0.001) (Table 1). Table 2 lists the factors that affected SUA level, including being male, a smoker, on diuretics before stroke onset, triglyceride level, blood urea nitrogen level, and eGFR.
Table 1
| Characteristics | Serum uric acid (μmol/L) | P | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Age (years), | 63.95±11.30 | 64.65±11.61 | 63.52±13.15 | 65.49±12.41 | 0.386 |
| Male | 100 (51.28) | 122 (62.56) | 142 (72.82) | 153 (78.46) | <0.001* |
| BMI (kg/m2) | 23.89±3.68 | 23.89±4.29 | 24.30±4.63 | 24.53±5.57 | 0.518 |
| Risk factors | |||||
| Diabetes mellitus | 52 (26.67) | 39 (20.00) | 39 (20.00) | 47 (24.10) | 0.310 |
| Hypertension | 103 (52.82) | 109 (55.90) | 117 (60.00) | 130 (66.67) | 0.034* |
| Coronary heart disease | 32 (16.41) | 39 (20.00) | 31 (15.90) | 38 (19.49) | 0.628 |
| Lipid metabolism disorders | 17 (8.72) | 17 (8.72) | 24 (12.31) | 12 (6.15) | 0.205 |
| Smoker | 50 (25.64) | 76 (38.97) | 82 (42.05) | 81 (41.54) | 0.002* |
| Using alcohol | 66 (33.85) | 81 (41.54) | 91 (46.67) | 98 (50.26) | 0.007* |
| Prior medications | |||||
| Aspirin before stroke onset | 39 (20.00) | 45 (23.08) | 39 (20.00) | 39 (20.00) | 0.840 |
| Diuretics before stroke onset | 3 (1.54) | 3 (1.54) | 3 (1.54) | 13 (6.67) | 0.003* |
| Prior statin treatment | 10 (5.13) | 10 (5.13) | 11 (5.64) | 7 (3.59) | 0.802 |
| Baseline parameters | |||||
| Baseline NIHSS | 15 [11–20] | 15 [12–20] | 15 [11–18] | 15 [9–21] | 0.476 |
| Location of occlusion | 0.534 | ||||
| ICA | 66 (33.85) | 71 (36.41) | 57 (29.23) | 63 (32.31) | |
| MCA (M1/M2) | 82 (42.05) | 83 (42.56) | 93 (47.69) | 89 (45.64) | |
| ACA | 0 (0) | 2 (1.03) | 3 (1.54) | 2 (1.03) | |
| VA | 30 (15.38) | 17 (8.72) | 19 (9.74) | 20 (10.26) | |
| BA | 17 (8.72) | 22 (11.28) | 23 (11.79) | 21 (10.77) | |
| Stroke subtype | 0.064 | ||||
| Large-artery atherosclerosis | 118 (60.51) | 124 (63.59) | 122 (62.56) | 113 (57.95) | |
| Cardioembolic | 61 (31.28) | 62 (31.79) | 57 (29.23) | 77 (39.49) | |
| Others | 16 (8.21) | 9 (4.62) | 16 (8.21) | 5 (2.56) | |
| Procedure process | |||||
| Onset to recanalization (min) | 432 [230–705] | 450 [225–649] | 410 [255–600] | 430 [305–590] | 0.694 |
| Laboratory data | |||||
| Fasting blood glucose (mmol/L) | 7.98±2.99 | 7.24±2.74 | 8.00±3.38 | 7.61±2.92 | 0.062 |
| Triglycerides (mmol/L) | 1.33±1.17 | 1.30±0.89 | 1.19±0.70 | 1.56±0.96 | <0.001* |
| Total cholesterol (mmol/L) | 4.16±1.15 | 4.23±0.96 | 4.13±1.05 | 4.27±1.17 | 0.727 |
| HDL-C (mmol/L) | 1.21±0.36 | 1.18±0.31 | 1.13±0.27 | 1.07±0.29 | <0.001* |
| LDL-C (mmol/L) | 2.50±0.96 | 2.59±0.82 | 2.55±0.89 | 2.73±0.98 | 0.124 |
| Serum albumin (g/L) | 37.86±5.67 | 38.41±5.20 | 38.80±5.84 | 38.08±6.27 | 0.283 |
| Blood urea nitrogen (mmol/L) | 4.85±1.65 | 5.11±1.56 | 5.53±2.17 | 6.85±3.03 | <0.001* |
| eGFR (mL/min/1.73 m2) | 101.39±17.09 | 97.92 ±18.54 | 94.78±21.29 | 80.78±27.18 | <0.001* |
The data are expressed as mean ± SD or n (%) or median [IQR]. Quartile 1: <245.85; Quartile 2: 245.85–297.45; Quartile 3: 297.45–364.95; Quartile 4: ≥364.95. *, P<0.05. SD, standard deviation; BMI, body mass index; NIHSS, National Institute of Health stroke scale; IQR, interquartile range; ICA, internal carotid artery; MCA (M1/M2), middle cerebral artery (the first/second segment of MCA); ACA, anterior cerebral artery; VA, vertebral artery; BA, basilar artery; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate.
Table 2
| Variable | SUA (μmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||
| aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | ||||
| Male | Reference | 0.671 (0.378–1.191) | 0.173 | 0.333 (0.185–0.600) | <0.001* | 0.151 (0.076–0.297) | <0.001* | ||
| Hypertension | Reference | 1.023 (0.669–1.564) | 0.916 | 1.102 (0.710–1.709) | 0.665 | 0.933 (0.571–1.523) | 0.780 | ||
| Smoker | Reference | 2.012 (1.158–3.494) | 0.013* | 1.831 (1.062–3.160) | 0.030* | 1.999 (1.117–3.579) | 0.020 | ||
| Using alcohol | Reference | 0.901 (0.511–1.588) | 0.718 | 0.921 (0.527–1.610) | 0.774 | 1.031 (0.570–1.865) | 0.919 | ||
| Diuretics before stroke onset | Reference | 0.749 (0.121–4.651) | 0.756 | 1.159 (0.217–6.203) | 0.863 | 4.780 (1.084–21.084) | 0.039* | ||
| Triglyceride | Reference | 0.912 (0.730–1.140) | 0.418 | 0.709 (0.539–0.932) | 0.014* | 1.126 (0.906–1.399) | 0.286 | ||
| HDL-C | Reference | 0.872 (0.455–1.669) | 0.679 | 0.561 (0.278–1.128) | 0.105 | 0.466 (0.207–1.046) | 0.064 | ||
| Blood urea nitrogen | Reference | 1.020 (0.887–1.172) | 0.783 | 1.098 (0.957–1.258) | 0.182 | 1.262 (1.095–1.455) | 0.001* | ||
| eGFR | Reference | 0.982 (0.969–0.996) | 0.010* | 0.975 (0.962–0.988) | <0.001* | 0.950 (0.937–0.964) | <0.001* | ||
Quartile 1: <245.85; Quartile 2: 245.85–297.45; Quartile 3: 297.45–364.95; Quartile 4: ≥364.95. *, based on multivariate regression analysis. SUA, serum uric acid; aOR, adjusted odds ratio; CI, confidence interval; HDL-C, high density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate.
Association between SUA level in quartiles and the primary outcome
Among the 780 patients, 230 (29.49%) had an excellent 90-day outcome, the primary outcome of our study (Table S1). Patients with an excellent outcome were younger (61.89±12.70 vs. 65.45±11.75 years; P<0.001), less likely to have a history of diabetes mellitus (13.91% vs. 26.36%; P<0.001), hypertension (52.61% vs. 61.45%; P=0.022), or prior stroke (13.91% vs. 22.36%; P=0.007), and had a lower baseline NIHSS {12 [8–17] vs. 16 [12–22]; P<0.001}. Patients with an excellent outcome more likely had intravenous r-tPA (27.83% vs. 17.82%; P=0.002), a higher rate of successful reperfusion (82.17% vs. 74.00%; P=0.014), lower maximum systolic blood pressure (SBP) within 24 hours of EVT (144.16±21.09 vs. 150.53±26.30 mmHg; P<0.001), lower WBC [(9.80±5.13 vs. 11.14±3.73)×109/L; P<0.001], lower fasting blood glucose (6.69±2.44 vs. 8.07±3.13 mmol/L; P<0.001), and a higher level of SUA (320.72±97.59 vs. 305.54±95.37 µmol/L; P=0.049) (Table S1).
Patients with an SUA level in the fourth quartile were less likely to achieve poor 90-day functional outcome compared with those in the first quartile in univariate logistic regression [66.67% vs. 76.92%; odds ratio (OR), 0.600; 95% confidence interval (CI), 0.384–0.938; P=0.025]. However, the rates of poor outcome were similar in patients with an SUA level in the first (76.92%), second (68.21%), and third (70.26%) quartiles (Table 3). In multivariate logistic regression, SUA level in the fourth quartile was independently associated with a lower risk of achieving poor outcome after adjusting for potential confounders in 3 models [adjusted odds ratio (aOR) in Model 3, 0.367; 95% CI, 0.154–0.876; P=0.024] (Table 3). This result was verified by sensitivity analysis (aOR, 0.354; 95% CI, 0.137–0.912; P=0.032) (Table S2).
Table 3
| SUA (μmol/L) |
mRS 2–6, n (%) |
Unadjusted model | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | |||||
| Quartile 1 | 150 (76.92) | Reference | Reference | Reference | Reference | |||||||
| Quartile 2 | 133 (68.21) | 0.644 (0.411–1.009) | 0.055 | 0.621 (0.382–1.008) | 0.054 | 0.675 (0.311–1.462) | 0.319 | 0.624 (0.285–1.365) | 0.238 | |||
| Quartile 3 | 137 (70.26) | 0.709 (0.451–1.115) | 0.136 | 0.754 (0.461–1.233) | 0.260 | 1.000 (0.449–2.231) | 0.999 | 0.905 (0.405–2.021) | 0.807 | |||
| Quartile 4 | 130 (66.67) | 0.600 (0.384–0.938) | 0.025 | 0.567 (0.345–0.933) | 0.026 | 0.364 (0.154–0.858) | 0.021 | 0.367 (0.154–0.876) | 0.024 | |||
Quartile 1: <245.85; Quartile 2: 245.85–297.45; Quartile 3: 297.45–364.95; Quartile 4: ≥364.95. Model 1, adjusted for age, male, atrial fibrillation, diabetes mellitus, baseline NIHSS, prior stroke, smoker, using alcohol, onset to recanalization, location of occlusion, and stroke subtype. Model 2, Model 1 + diuretics before stroke onset, triglyceride level, blood urea nitrogen, and eGFR (significant variables from Table 2). Model 3, Model 2 + intravenous r-tPA, mTICI 2b/3, and WBC (significant variables from Table S3). SUA, serum uric acid; mRS, modified Rankin Scale; OR, odds ratio; CI, confidence interval; aOR, adjusted odds ratio; NIHSS, National Institute of Health stroke scale; eGFR, estimated glomerular filtration rate; r-tPA, recombinant human tissue-type plasminogen activator; mTICI, modified thrombolysis in cerebral infarction score; WBC, white blood cell.
Association between SUA level as a continuous variable and the primary outcome
The SUA level, when analyzed as a continuous variable, was also associated with the primary outcome in univariate logistic regression (Tables S1,S3). In multivariate logistic regression, SUA level as a continuous variable (aOR, 0.998; 95% CI, 0.996–1.000; P=0.018), history of diabetes mellitus (aOR, 2.009; 95% CI, 1.257–3.209; P=0.004), baseline NIHSS (aOR, 1.090; 95% CI, 1.059–1.121; P<0.001), use of intravenous r-tPA (aOR, 0.631; 95% CI, 0.422–0.945; P=0.026), successful reperfusion (mTICI 2b/3; aOR, 0.565; 95% CI, 0.366–0.871; P=0.010), and WBC level (aOR, 1.071; 95% CI, 1.016–1.129; P=0.011) were independently associated with the primary outcome (Table S3).
Association between SUA level and secondary outcomes
For the secondary outcomes, 328 (42.05%), 47 (6.03%), and 144 (18.46%) patients had a favorable 90-day outcome, sICH, and 90-day death, respectively. There was no significant association for SUA level as a continuous variable with a favorable 90-day outcome, sICH, or 90-day mortality (P>0.05) (Table S4). The same results were obtained when further analyzed in quartiles (P>0.05) (Table 4 and Table S5).
Table 4
| Characteristics | Serum uric acid (μmol/L) | P value | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Primary outcome, n (%) | |||||
| 90-day mRS 0–1 | 45 (23.08) | 62 (31.79) | 58 (29.74) | 65 (33.33) | 0.125 |
| Secondary outcomes, n (%) | |||||
| 90-day mRS 0–2 | 75 (38.46) | 88 (45.13) | 81 (41.54) | 84 (43.08) | 0.595 |
| sICH | 14 (7.18) | 9 (4.62) | 15 (7.69) | 9 (4.62) | 0.426 |
| 90-day mortality | 29 (14.87) | 34 (17.44) | 40 (20.51) | 41 (21.03) | 0.362 |
Quartile 1: <245.85; Quartile 2: 245.85–297.45; Quartile 3: 297.45–364.95; Quartile 4: ≥364.95. mRS, modified Rankin Scale; sICH, symptomatic intracranial hemorrhage.
Discussion
In this prospective, nationwide registry, a higher SUA level within 24 hours of EVT was associated with a higher chance of achieving an excellent 90-day functional outcome in patients with AIS from an LVO, independent of other confounders, and was not significantly associated with favorable 90-day outcome, risk of sICH, and 90-day mortality.
The central nervous system is highly vulnerable to oxidative damage because of its high oxygen consumption, high concentrations of peroxidizable lipids, low levels of protective antioxidants, and high levels of iron acting as pro-oxidants under pathological conditions (31). Oxidative stress begins early after stroke onset and increases sharply and rapidly after recanalization (32). Reactive oxygen/nitrogen species (ROS/RNS) generated by pericytes are highly expressed after ischemia and reperfusion, leading to structural and functional alterations in the microvasculature. This process may partially offset or outweigh the benefit of recanalization/reperfusion (33), which may be a major mechanism in futile reperfusion (34). Uric acid could ameliorate secondary hypoperfusion after reperfusion by blocking the generation of free radicals in the vessel wall in the brain and inducing passive lumen expansion (10). In the current study, LVO-related AIS patients with higher SUA level within 24 hours of EVT were less likely to have a poor 90-day outcome (OR, 0.600; 95% CI, 0.384–0.938; P=0.025). This relationship was independent of confounding factors associated with 90-day mRS and SUA (aOR in Model 3, 0.367; 95% CI, 0.154–0.876; P=0.024) (Table 3).
Two previous studies on the relationship between SUA and functional prognosis after reperfusion therapy in stroke had smaller sample sizes. Moreover, these 2 studies only enrolled patients with LVO-related AIS in the anterior circulation (24), or those who received intravenous thrombolysis (25). The time of onset to recanalization or mTICI were not adjusted in multivariate analyses (25). Discrepancies between the findings of previous studies and the current study may have been due to differences in patients’ age, sex, race, time when SUA was measured, heterogeneity of stroke subtypes, means of intervention, and outcome measures. Although SUA level is easily affected by diet (9), medications (35), renal function and metabolic syndrome (36), and dynamic after stroke (37), the current study indicated that SUA level in the acute stage predicted the 90-day outcome, which was confirmed by sensitivity analysis. This was likely due to the neuronal protection from reducing the exacerbated oxidative stress after recanalization in LVO-related AIS patients with EVT. The current study minimized possible interference through rigorous screening of SUA, a relatively large sample size, and correction of factors affecting SUA level.
In the phase 2b/3 URICO-ICTUS trial, the safety of adding uric acid to alteplase treatment was confirmed (38). In post-hoc analysis of the URICO-ICTUS data, uric acid was associated with reduced infarct growth in patients with early recanalization (39) and a reduced rate of early ischemic progression in patients with good pretreatment collaterals (40) but not in those with delayed or no recanalization (38). These observations suggested that the benefit of uric acid as a neuroprotective agent might be relatively more prominent in circumstances of higher oxidative stress, such as in those with early reperfusion (41). A previous study has considered hyperuricemia as a risk factor for stroke through several potential pathophysiological mechanisms, such as enhancing lipid peroxidation and causing vascular inflammation (42). The balance between the antioxidant and pro-oxidant effects of SUA may shift in favor of tissue protection in conditions of extraordinary oxidative stress (43). A recent study indicated that the hyperactive microglia in ischemic penumbra might play a key role in post-stroke inflammation, while uric acid could alleviate the ischemia-reperfusion injury by suppressing the excessive activation of microglia (44). All patients enrolled in this study were treated with EVT, which could supply oxygen to the ischemic area rapidly when blood flow was re-established through early and rapid recanalization, accelerating the oxidative damage. In our study, the potential protective effect for the 90-day functional outcome was only found for SUA in the highest quartile (Table 3), indicating that only a certain level of SUA could offset the damage caused by oxidative stress. The same result was derived by sensitivity analysis (Table S2), providing evidence for treatment with exogenous uric acid as an antioxidant agent after EVT and thereby, a potentially beneficial neuroprotective strategy.
The current study had a number of limitations. First, although most factors affecting level of SUA were analyzed, information about the history of gout and dietary structure was absent. Second, the proportion of favorable 90-day outcome was modestly higher in the fourth quartile than in the first, but there was no statistical significance. This may have been related to the relatively weak effect of SUA and relatively small sample size. Third, as there was only one measure of SUA, we failed to observe the effect of changes in SUA level on 90-day functional outcomes.
Conclusions
In summary, higher SUA level within 24 hours of EVT in AIS was associated with a higher chance of an excellent 90-day functional outcome. The possible beneficial effect of SUA in such patients warrants further investigation in prospective studies or therapeutic trials.
Acknowledgments
We thank all the participants and investigators who took part in the RESCUE-RE study (Registration Study for Critical Care of Acute Ischemic Stroke After Recanalization).
Funding: This research was supported by the National Key R&D Program of China (grant Nos. 2016YFC1307301, 2018YFC1312402), the National Natural Science Foundation of China (grant No. 81820108012), Medical Science Research Project of Hebei Province (grant No. 20211242) and partially supported by Boehringer-Ingelheim China.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4494/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4494/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4494/coif). All authors report that this research was supported by the National Key R&D Program of China (grant Nos. 2016YFC1307301, 2018YFC1312402), the National Natural Science Foundation of China (grant No. 81820108012), Medical Science Research Project of Hebei Province (grant No. 20211242) and partially supported by Boehringer-Ingelheim China. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the Beijing Tiantan Hospital, Capital Medical University (No. KY2018-057-01). All participating hospitals/institutions were informed and agreed the study. Informed consent was obtained from all subjects involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019;50:e344-418. [Crossref] [PubMed]
- Writing Group for the BASILAR Group. Assessment of Endovascular Treatment for Acute Basilar Artery Occlusion via a Nationwide Prospective Registry. JAMA Neurol 2020;77:561-73. [Crossref] [PubMed]
- Solla DJF, Argolo FC, Budohoski KP, et al. Is more evidence needed for thrombectomy in basilar artery occlusion? The BASICS and BEST meta-analytical approaches. Stroke Vasc Neurol 2021;6:671-2. [Crossref] [PubMed]
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-31. [Crossref] [PubMed]
- Cho BR, Jang DK, Jang KS, et al. Predictors for intracerebral hemorrhage after intravenous or intraarterial recanalization in acute major cerebral artery occlusion in Korean patients. Int J Neurosci 2022; Epub ahead of print. [Crossref] [PubMed]
- Ghandehari K. Challenging comparison of stroke scales. J Res Med Sci 2013;18:906-10. [PubMed]
- Xu H, Jia B, Huo X, et al. Predictors of Futile Recanalization After Endovascular Treatment in Patients with Acute Ischemic Stroke in a Multicenter Registry Study. J Stroke Cerebrovasc Dis 2020;29:105067. [Crossref] [PubMed]
- Sun MS, Jin H, Sun X, et al. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid Med Cell Longev 2018;2018:3804979. [Crossref] [PubMed]
- El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: A review. J Adv Res 2017;8:487-93. [Crossref] [PubMed]
- Onetti Y, Dantas AP, Pérez B, et al. Middle cerebral artery remodeling following transient brain ischemia is linked to early postischemic hyperemia: a target of uric acid treatment. Am J Physiol Heart Circ Physiol 2015;308:H862-74. [Crossref] [PubMed]
- Netea MG, Kullberg BJ, Blok WL, et al. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood 1997;89:577-82. [Crossref] [PubMed]
- Irfan M, Jawaid W, Hashmat O, et al. Association Between Hyperuricemia and Acute Ischemic Stroke in Patients at a Tertiary Care Hospital. Cureus 2020;12:e10899. [Crossref] [PubMed]
- Hu G, Li J, Wang Q, et al. J-shaped relationship between serum uric acid levels and the risk of ischemic stroke in high-risk individuals: A hospital-based observational study. Clin Neurol Neurosurg 2020;195:105906. [Crossref] [PubMed]
- Yang Y, Zhang X, Jin Z, et al. Association of serum uric acid with mortality and cardiovascular outcomes in patients with hypertension: a meta-analysis. J Thromb Thrombolysis 2021;52:1084-93. [Crossref] [PubMed]
- Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum 2009;61:885-92. [Crossref] [PubMed]
- Li M, Hou W, Zhang X, et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis 2014;232:265-70. [Crossref] [PubMed]
- Shi X, Yang J, Wang L, et al. Prospective study of serum uric acid levels and stroke in a Chinese hypertensive cohort. Clin Exp Hypertens 2017;39:527-31. [Crossref] [PubMed]
- Cheng Z, Zheng T, Zhang D, et al. High-level uric acid in asymptomatic hyperuricemia could be an isolated risk factor of cardio-cerebrovascular diseases: A prospective cohort study. Nutr Metab Cardiovasc Dis 2021;31:3415-25. [Crossref] [PubMed]
- Amaro S, Jiménez-Altayó F, Chamorro Á. Uric acid therapy for vasculoprotection in acute ischemic stroke. Brain Circ 2019;5:55-61. [Crossref] [PubMed]
- Jiménez-Xarrié E, Pérez B, Dantas AP, et al. Uric Acid Treatment After Stroke Prevents Long-Term Middle Cerebral Artery Remodelling and Attenuates Brain Damage in Spontaneously Hypertensive Rats. Transl Stroke Res 2020;11:1332-47. [Crossref] [PubMed]
- Aliena-Valero A, López-Morales MA, Burguete MC, et al. Emergent Uric Acid Treatment is Synergistic with Mechanical Recanalization in Improving Stroke Outcomes in Male and Female Rats. Neuroscience 2018;388:263-73. [Crossref] [PubMed]
- Yang Y, Zhang Y, Li Y, et al. U-Shaped Relationship Between Functional Outcome and Serum Uric Acid in Ischemic Stroke. Cell Physiol Biochem 2018;47:2369-79. [Crossref] [PubMed]
- Wang Z, Lin Y, Liu Y, et al. Serum Uric Acid Levels and Outcomes After Acute Ischemic Stroke. Mol Neurobiol 2016;53:1753-9. [Crossref] [PubMed]
- Chen Z, Chen H, Zhang Y, et al. Lower uric acid level may be associated with hemorrhagic transformation but not functional outcomes in patients with anterior circulation acute ischemic stroke undergoing endovascular thrombectomy. Metab Brain Dis 2020;35:1157-64. [Crossref] [PubMed]
- Wang C, Cui T, Wang L, et al. Prognostic significance of uric acid change in acute ischemic stroke patients with reperfusion therapy. Eur J Neurol 2021;28:1218-24. [Crossref] [PubMed]
- Wei Y, Pu Y, Pan Y, et al. Cortical Microinfarcts Associated With Worse Outcomes in Patients With Acute Ischemic Stroke Receiving Endovascular Treatment. Stroke 2020;51:2742-51. [Crossref] [PubMed]
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. [Crossref] [PubMed]
- Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650-63. [Crossref] [PubMed]
- Teo BW, Xu H, Wang D, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis 2011;58:56-63. [Crossref] [PubMed]
- Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245-51. [Crossref] [PubMed]
- Jelinek M, Jurajda M, Duris K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants (Basel) 2021;10:1886. [Crossref] [PubMed]
- Li C, Sun T, Jiang C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta Pharm Sin B 2021;11:1767-88. [Crossref] [PubMed]
- Jung JE, Kim GS, Chen H, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol 2010;41:172-9. [Crossref] [PubMed]
- Fisher M, Savitz SI. Pharmacological brain cytoprotection in acute ischaemic stroke - renewed hope in the reperfusion era. Nat Rev Neurol 2022;18:193-202. [Crossref] [PubMed]
- Ben Salem C, Slim R, Fathallah N, et al. Drug-induced hyperuricaemia and gout. Rheumatology (Oxford) 2017;56:679-88. [PubMed]
- Yanai H, Adachi H, Hakoshima M, et al. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int J Mol Sci 2021;22:9221. [Crossref] [PubMed]
- Brouns R, Wauters A, Van De Vijver G, et al. Decrease in uric acid in acute ischemic stroke correlates with stroke severity, evolution and outcome. Clin Chem Lab Med 2010;48:383-90. [Crossref] [PubMed]
- Chamorro A, Amaro S, Castellanos M, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol 2014;13:453-60. [Crossref] [PubMed]
- Chamorro Á, Amaro S, Castellanos M, et al. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int J Stroke 2017;12:377-82. [Crossref] [PubMed]
- Amaro S, Laredo C, Renú A, et al. Uric Acid Therapy Prevents Early Ischemic Stroke Progression: A Tertiary Analysis of the URICO-ICTUS Trial (Efficacy Study of Combined Treatment With Uric Acid and r-tPA in Acute Ischemic Stroke). Stroke 2016;47:2874-6. [Crossref] [PubMed]
- Amaro S, Chamorro Á. Should uric acid be administered alongside thrombolysis for stroke patients? Expert Rev Cardiovasc Ther 2016;14:407-9. [Crossref] [PubMed]
- Zhong C, Zhong X, Xu T, et al. Sex-Specific Relationship Between Serum Uric Acid and Risk of Stroke: A Dose-Response Meta-Analysis of Prospective Studies. J Am Heart Assoc 2017;6:e005042. [Crossref] [PubMed]
- Proctor PH. Uric acid: neuroprotective or neurotoxic?. Stroke 2008;39:e88-author reply e89. [PubMed]
- Wang Q, Zhao H, Gao Y, et al. Uric acid inhibits HMGB1-TLR4-NF-κB signaling to alleviate oxygen-glucose deprivation/reoxygenation injury of microglia. Biochem Biophys Res Commun 2021;540:22-8. [Crossref] [PubMed]


