
Low absolute CD4+ T cell counts in peripheral blood: an independent predictor of inferior survival in natural killer/T-cell lymphoma—a retrospective cohort study
Introduction
Extra-nodal natural killer (NK)/T-cell lymphoma (ENKTL), nasal type, is a heterogeneous histopathologic and characteristic non-Hodgkin’s lymphoma (NHL) subtype. It is associated with vascular destruction and damage, prominent necrosis, cytotoxic phenotype, and the Epstein-Barr virus (1). The incidence of ENKTL is lower in western populations than in Latin American and East Asian (especially Chinese) populations (2,3). ENKTL has aggressive clinical features and a poor prognosis with a 5-year survival rate ranging from 32–49% (4,5). The International Prognostic Index (IPI) is widely applied to predict the prognosis of patients with aggressive NHL, including ENKTL (6).
A new prognostic model, named the Korean Prognostic Index (KPI), is considered to have better prognostic value for nasal disease, especially in distinguishing between low- and high-risk ENKTL (4). However, these two models are based on clinical factors and do not take the tumor microenvironment and host immune response into account.
Research shows that Ann Arbor stage, B symptom, EBV-DNA level and cytokines such as IFN-γ, IL-6 and IL-10 were prognostic factors of ENKTL (4,7). However, whether the immune factors in the blood affect ENKTL is still lack of research. Profiling studies of gene expression have shown that systemic immune response of lymphoma may be reflected by peripheral blood lymphocytes (8-10). The absolute lymphocyte count (ALC) and absolute monocyte count, which could reflect systemic immunity, were independent prognostic indicators of survival in ENKTL (8,11). CD4+ T cells play a crucial role in inducing immune responses against tumors and influencing lymphoma outcomes. Clinical studies have reported that enhanced levels of blood CD4+ T cells are related to a better prognosis for various types of NHL, including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL) (12-15).
To date, only one study has reported the prognostic value of CD4+ T cell counts in patients with ENKTL (16). Zhang et al. reported that low ACD4Cs were associated with poorer survival in ENKTL patients, but there are only 70 cases in this study, which deserves further research (16). Thus, we retrospectively analyzed the peripheral blood samples of 176 newly diagnosed ENKTL patients, who had not yet received any treatment and examined the samples by flow cytometry (FCM) in order to study the prognostic value of peripheral blood lymphocytes on ENKTL outcomes. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4846/rc).
Methods
Patient population
A total of 193 patients who had been newly diagnosed with ENKTL were evaluated for inclusion in the study from 2000 to 2018. All the patients had typical ENKTL pathology and immunohistochemistry results as previously described (17). Of the 193 patients, 17 patients were excluded due to incomplete clinical data, and the remaining 176 patients were included in this study. Review charts to collect clinical baseline, treatment, and survival data.
The patients were staged according to the Ann-Arbor system (18). IPI scores of the patients were documented. IPI score includes age, performance status, stage, serum lactate dehydrogenase (LDH) levels, and extra-nodal sites. We also used KPI score, which includes B symptoms, stage, LDH levels, and regional lymph nodes (4,6). According to the location of the lesion, ENKTL is further classified into 2 subtypes; that is, upper aerodigestive tract (UNKTL) and extra-upper aerodigestive tract (EUNKTL), as previously described (2,19).
The patients’ clinical features, including their complete history and B symptoms, Eastern Cooperative Oncology Group performance status (ECOG PS), physical examination results, primary site, location of invasion, LDH levels, peripheral blood cell count, bone marrow aspiration, magnetic resonance imagining scans of the nasopharynx, neck, and computed tomography (CT) of chest, abdomen, and pelvis, or whole-body positron emission tomography/CT scan (PET/CT), date of diagnosis, therapeutic method, last follow-up and survival status were systematically reviewed. The Ethics Committee of the Integrated Hospital of Traditional Chinese Medicine, Southern Medical University authorized the experimental and research protocols of this study (ID: ZXY201709138). The study was conducted according with the ethical standards of the institutional research committee and with the Declaration of Helsinki (as revised in 2013). Written informed consent was provided and signed by all patients prior to sample collection.
Treatment
All enrolled patients received anthracycline-based or asparaginase-based chemotherapy regiments. Early patients received chemotherapy, followed by three-dimensional conformal radiotherapy or intensity modulated conformal radiotherapy. For patients with extensive IE/IE disease, after at least two chemotherapy cycles, they will receive IFRT for primary tumors, with a total dose of 36–68 Gy, 1.8–2.0 Gy each time, five times a week.
Flow cytometric analysis
The percentage of CD3+ T cells, CD3+CD8+ T cells, and CD3−CD16+CD56+ lymphocytes were detected by peripheral blood FCM (Becton Dickinson, Franklin Lakes, NJ, USA). Murine anti-human polyclonal antibodies were used, including anti-CD3-FITC, anti-CD4-phycoerythrin, anti-CD8-allophycocyanin, anti-CD16-phycoerythrin, and anti-CD56-allophycocyanin (BD Biosciences, San Diego, CA, USA). The lymphocytes were delineated by adequate forward and sidelight scatters. CellQuest software (BD Biosciences) was used to analyze the data. The peripheral blood ALC was obtained from the hematology laboratory records, and the percentages of the subtypes were determined using FCM; ACD4C refers to the CD3+CD4+ lymphocyte count, ACD8C refers to the CD3+CD8+ lymphocyte count, and ANKC refers to the CD16+CD56+ lymphocyte count in peripheral blood (15).
Response criteria and statistical analysis
The evaluation of treatment responses was carried out in accordance with the International Working Group Recommendations for Response Criteria for NHL (20). Overall survival (OS) was defined as the time from diagnosis to death or to the last follow-up visit. Progression-free survival (PFS) was defined the time from diagnosis to first progression, death, or to the last follow-up visit.
The optimal cut-off values for separating the lymphocyte subsets were selected by receiver operator characteristic (ROC) curve analysis with OS as the outcome (21). The categorical characteristics among patient groups stratified by lymphocyte subsets were compared using a chi-square test. The Kaplan-Meier method was used to estimate OS and PFS, and the survival curves among patient groups stratified by lymphocyte subsets were compared using the log-rank test. All the significant variables with a P value <0.05 in the univariate analysis were further included in the multivariate analysis. Univariate and multivariate Cox regression analysis were conducted to examine OS and PFS. Variables associated with a P value <0.2 were included in the multivariate analysis. A 2-tailed P value <0.05 was considered statistically significant. All the statistical calculations were made using the statistical software package SPSS 19.0 (SPSS, Chicago, USA).
Results
Patient characteristics
The clinical characteristics of the 176 patients are listed in Table 1. The study included 119 males and 57 females (male to female ratio: 2.1:1), with a median age of 45 years (range, 18–88 years). Most patients (139, 79.0%) displayed good performance status (ECOG PS 0–1). A total of 80 (45.5%) patients presented with B symptoms. Elevated LDH levels were detected in 45 (25.6%) patients. A total of 48 patients (27.3%) had ≥2 extra-nodal involvement sites. According to the Ann-Arbor staging system, 140 (79.5%) patients had stage I/II disease and 36 (20.5%) patients had stage III/IV disease. In total, 127 (72.2%) patients were diagnosed with UNKTL, and 49 patients (27.8%) were diagnosed with EUNKTL. The majority of patients belonged to the low-risk groups according to their IPI and KPI scores [112 patients (63.6%) and 64 patients (36.4%), respectively].
Table 1
| Clinical variable | Overall (N=176) |
|---|---|
| Gender, n (%) | |
| Female | 57 (32.4) |
| Male | 119 (67.6) |
| Age at diagnosis (years), n (%) | |
| ≤60 | 151 (85.8) |
| >60 | 25 (14.2) |
| ECOG PS, n (%) | |
| 0–1 | 139 (79.0) |
| ≥2 | 37 (21.0) |
| B symptom, n (%) | |
| Yes | 80 (45.5) |
| No | 96 (54.5) |
| Serum LDH levels, n (%) | |
| Normal | 131 (74.4) |
| Elevated | 45 (25.6) |
| Number of extra-nodal sites, n (%) | |
| 0–1 | 128 (72.7) |
| ≥2 | 48 (27.3) |
| Ann-Arbor stage, n (%) | |
| I and II | 140 (79.5) |
| III and IV | 36 (20.5) |
| Disease subtypes, n (%) | |
| U-NKTCL | 127 (72.2) |
| EU-NKTCL | 49 (27.8) |
| IPI score, n (%) | |
| 0–1 | 123 (69.9) |
| 2–5 | 53 (30.1) |
| KPI score, n (%) | |
| 0–1 | 112 (63.6) |
| 2–4 | 64 (36.4) |
| Lymphocyte subset, median (range) | |
| Leukocytes (×109 cells/L) | 1.50 (0.28–3.60) |
| CD3+ T cells (×109 cells/L) | 1.03 (0.12–3.48) |
| ACD4C (×109 cells/L) | 0.55 (0.11–1.38) |
| ACD8C (×109 cells/L) | 0.41 (0.08–2.15) |
| CD4/CD8 ratio | 1.33 (0.31–6.17) |
| ANKC (×109 cells/L) | 0.21 (0.01–1.02) |
NKTCL, natural killer/T-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; U-NKTCL, Upper aerodigestive tract natural killer/T-cell lymphoma; EU-NKTCL, Extra-upper aerodigestive tract natural killer/T-cell lymphoma; IPI, International Prognostic Index; KPI, Korean Prognostic Index; ACD4C, absolute CD4+ T cell count; ACD8C, absolute CD8+ T cell count; ANKC, absolute CD16+ CD56+ lymphocyte counts.
Cut-off values for lymphocytes, CD3+ T cells, ACD4C, ACD8C, CD4+/CD8+ T cell ratios and ANKC by ROC curves
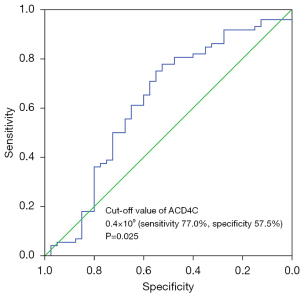
We defined the cut-off values by the maximal sensitivity and specificity of the ROC curves with OS as the outcome, and found that lymphocytes <1.43×109/L had an AUC of 0.620 (sensitivity 65.0%, specificity 64.9%, P=0.035), and an ACD4C <0.4×109 cells /L had an AUC of 0.628 (sensitivity 77.0%, specificity 57.5%, P=0.025) (see Figure 1), which were the most discriminative cut-off values. The cut-off values for CD3+ T cells, the ACD8C, CD4+/CD8+ T cell ratios and ANKC were also defined using the ROC curve analysis, but failed to reach statistical significance in our study cohort (P>0.05).
Association between ACD4C and clinical features
In our study cohort, the median value for the ACD4C in all patients was 0.50×109/L [range, (0.11 to 1.38)×109/L]. The low-ACD4C group was defined as patients with an ACD4C <0.4×109/L according to the previously defined cut-off value, and the high-ACD4C group was defined as patients with an ACD4C ≥0.4×109/L. Based on this classification, 51 patients (29.0%) had a low ACD4C, and 125 patients (71.0%) had a high ACD4C. The baseline clinical features of the patients in the low-ACD4C group were compared to those in the high-ACD4C group (see Table 2). The low-ACD4C group had significantly higher LDH levels, ≥2 extra-nodal sites, advanced stage (III/IV), extra-nasal ENKTL, and elevated KPI scores. There was no significant association between the ACD4C and any other clinical features.
Table 2
| Variable | ACD4C | P value | |
|---|---|---|---|
| <0.4×109/L (%) | ≥0.4×109/L (%) | ||
| No. of patients | 51 (29.0) | 125 (71.0) | – |
| Gender | 0.371 | ||
| Female | 14 (8.0) | 43 (24.4) | – |
| Male | 37 (21.0) | 82 (46.6) | – |
| Age at diagnosis (years) | 0.719 | ||
| ≤60 | 43 (24.4) | 108 (61.4) | – |
| >60 | 8 (4.6) | 17 (9.6) | – |
| ECOG PS | 0.031 | ||
| 0–1 | 35 (19.9) | 104 (59.1) | – |
| ≥2 | 16 (9.1) | 21 (11.9) | – |
| B symptom | 0.003 | ||
| Yes | 32 (18.2) | 48 (27.3) | – |
| No | 19 (10.8) | 77 (43.7) | – |
| Elevated serum LDH levels | <0.001 | ||
| Yes | 28 (15.9) | 103 (58.5) | – |
| No | 23 (13.1) | 22 (12.5) | – |
| Number of extra-nodal sites | 0.023 | ||
| 0–1 | 31 (17.6) | 97 (55.1) | – |
| ≥2 | 20 (11.4) | 28 (15.9) | – |
| Ann-Arbor stage | 0.007 | ||
| I–II | 34 (19.3) | 106 (60.2) | – |
| III–IV | 17 (9.7) | 19 (10.8) | – |
| Disease subtypes | 0.004 | ||
| U-NKTCL | 29 (16.5) | 98 (55.7) | – |
| EU-NKTCL | 22 (12.5) | 27 (15.3) | – |
| IPI score | 0.016 | ||
| 0–1 | 29 (16.5) | 94 (53.4) | – |
| 2–5 | 22 (12.5) | 31 (17.6) | – |
| KPI score | <0.001 | ||
| 0–1 | 18 (10.2) | 94 (53.4) | – |
| 2–4 | 33 (18.8) | 31 (17.6) | – |
NKTCL, natural killer/T-cell lymphoma; ACD4C, absolute CD4+ T cell count; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; U-NKTCL, Upper aerodigestive tract natural killer/T-cell lymphoma; EU-NKTCL, Extra-upper aerodigestive tract natural killer/T-cell lymphoma; IPI, International Prognostic Index; KPI, Korean Prognostic Index.
Treatment modalities and response
The primary treatment strategies were as follows: (I) 127 cases (72.2%) received chemotherapy followed by radiotherapy; and (II) 49 cases (27.8%) received chemotherapy alone. The treatment details and responses are set out in Table 3. A significantly greater proportion of the high ACD4C patients were treated with chemotherapy and radiotherapy over chemotherapy alone.
Table 3
| Treatment strategy | Overall (n=176) (%) | ACD4C | P value | |
|---|---|---|---|---|
| <0.4×109 cells/L (n=51) (%) | ≥0.4×109 cells/L (n=125) (%) | |||
| Chemotherapy alonea | 49 (27.8) | 22 (12.5) | 27 (15.3) | 0.004 |
| Chemotherapy followed by radiotherapyb | 127 (72.2) | 29 (16.5) | 98 (55.7) | – |
| Chemotherapy regimens | 0.141 | |||
| Anthracycline-based | 78 (44.3) | 27 (15.3) | 51 (29.0) | – |
| Asparaginase-based | 98 (55.7) | 24 (13.7) | 74 (42.0) | – |
| Response status at the end of treatment | ||||
| Complete remission | 128 (72.7) | 26 (15.1) | 102 (57.6) | <0.001 |
| Partial remission | 34 (19.3) | 19 (10.8) | 15 (8.5) | |
| Overall response | 162 (92.0) | 45 (25.6) | 117 (66.4) | 0.233 |
| Disease progression or relapse | 0.032 | |||
| Yes | 68 (38.6) | 26 (14.7) | 42 (23.9) | – |
| No | 108 (61.4) | 25 (14.2) | 83 (47.2) | – |
| Survival status | 0.002 | |||
| Dead | 50 (28.4) | 23 (13.1) | 27 (15.3) | – |
| Alive | 126 (71.6) | 28 (15.9) | 98 (55.7) | – |
| Overall survival rate (%) | 0.001 | |||
| 1-year | 85.0 | 69.1 | 91.5 | – |
| 3-year | 70.1 | 56.2 | 75.8 | – |
| 5-year | 65.3 | 48.8 | 72.5 | – |
| Progression-free survival rate (%) | 0.034 | |||
| 1-year | 74.5 | 64.9 | 78.4 | – |
| 3-year | 59.7 | 54.8 | 61.7 | – |
| 5-year | 50.2 | 37.1 | 56.8 | – |
a, 5 patients with stage IV disease received autologous hematopoietic stem cell transplantation after complete remission by chemotherapy; b, 2 patients with stage II disease received autologous hematopoietic stem cell transplantation after partial remission by chemotherapy, followed by radiotherapy. NKTCL, natural killer/T-cell lymphoma; ACD4C, absolute CD4+ T cell count.
The treatment response was determined in each patient. 128 patients (72.7%) achieved complete response (CR), 34 patients (19.3%) achieved partial response, and the remaining 14 patients (8.0%) exhibited stable disease or progressive disease. The CR rate was significantly higher in the high-ACD4C group than the low-ACD4C group (57.6% vs. 15.1%, respectively, P<0.001).
Survival analysis
Within a median follow-up time of 58.2 months (range, 5.5–179.6 months), the 1-, 3-, and 5-year OS rates were 85.0%, 70.1%, and 65.3%, respectively (see Figure 2A), and the 1-, 3-, and 5-year PFS rates were 74.5%, 59.7%, and 50.2%, respectively (see Figure 2B). As Figure 2C,2D show, patients in the high-ACD4C group had significantly better OS and PFS than those in the low-ACD4C group (P=0.001 and P=0.034, respectively).

The correlation between clinical features and survival outcomes was determined by univariate and multivariate analyses (see Table 4). In the univariate analysis, gender, ECOG PS, extra-nodal sites, Ann-Arbor stage, IPI score, KPI score, and ACD4C level were significantly correlated with both PFS and OS. All factors with a significant P value <0.05 in the univariate analysis were included in the multivariate analysis. The results of the multivariate Cox regression model revealed that ECOG PS, and ACD4C level were independent prognostic factors for OS, and ECOG PS was an independent prognostic factor for PFS (P=0.029). However, the ACD4C level trended towards significant correlation with PFS (P=0.085).
Table 4
| Parameter | OS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| P value | RR (95% CI) | P value | P value | RR (95% CI) | P value | ||||
| Gender, male | 0.393 | 5.231 (2.436–6.988) | 0.867 | 0.476 | 6.159 (3.367–8.991) | 0.691 | |||
| Age >60 years | 0.030 | 3.653 (1.217–4.883) | 0.289 | 0.081 | 2.363 (1.187–3.993) | 0.165 | |||
| ECOG PS ≥2 | <0.001 | 2.288 (1.209–4.328) | 0.011 | <0.001 | 1.858 (1.064–3.244) | 0.029 | |||
| Subtype, EU-NKTCL | 0.004 | 2.936 (1.117–3.852) | 0.197 | 0.001 | 1.751 (1.038–3.643) | 0.093 | |||
| B symptoms | 0.097 | 3.995 (2.015–4.943) | 0.313 | 0.067 | 2.287 (1.294–3.596) | 0.114 | |||
| Elevated serum LDH | 0.004 | 3.322 (1.518–4.295) | 0.241 | 0.033 | 2.954 (1.576–3.942) | 0.157 | |||
| Extra-nodal sites ≥2 | <0.001 | 2.395 (1.258–3.282) | 0.067 | <0.001 | 2.142 (1.156–3.488) | 0.124 | |||
| Ann-Arbor stage III–IV | <0.001 | 1.984 (1.002–4.086) | 0.096 | <0.001 | 2.077 (1.001–3.386) | 0.097 | |||
| IPI score ≥2 | <0.001 | 2.687 (1.341–3.779) | 0.081 | <0.001 | 1.900 (1.017–3.246) | 0.152 | |||
| KPI score ≥2 | 0.005 | 3.143 (1.622–4.785) | 0.193 | 0.002 | 1.786 (1.103–3.085) | 0.106 | |||
| ACD4C <0.4×109/L | 0.001 | 2.058 (1.070–3.968) | 0.031 | 0.034 | 2.006 (1.124–3.658) | 0.085 | |||
OS, overall survival; PFS, progression-free survival; ENKTL, extra-nodal natural killer (NK)/T-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; EU-NKTCL, extra-upper aerodigestive tract natural killer/T-cell lymphoma; LDH, lactate dehydrogenase; IPI, International Prognostic Index; KPI, Korean Prognostic Index; ACD4C, absolute CD4+ T cell count.
Discussion
The immune system often plays an important role in the development and progression of lymphoma. In epidemiologic studies, immune suppression is regarded as a risk factor for NHL (22,23). It is not yet known whether there is a correlation between lymphocytopenia and poor survival and an association between the specific subset of lymphocytes and inferior prognosis. Previous studies have reported that lymphopenia is an adverse prognostic factor in various solid tumors, Hodgkin’s lymphoma (HL), and NHL (24-27).
In this study, we investigated the prognostic value of lymphocyte counts in the peripheral blood of ENKTL patients. We found that the ACD4C (using a cut-off value of 0.4×109/L cells) was significantly correlated with OS according to the univariate analysis and multivariate analysis results. However, the ACD8C, or ANKC had no significant correlation with PFS or OS in our study.
In clinical studies, lymphocyte count is usually used as an indicator of immune status, and the decrease of ACD4C often indicates immunosuppression (8,11). The ACD4C has also been considered a key factor in predicting responses to chemotherapy and survival in patients with DLBCL, FL, and MCL. A low level of blood CD4+ T cells was shown to be correlated with inferior survival in DLBCL and FL, but had a negative prognostic effect in MCL, due to the heterogeneity of MCL (13-15). A study has reported the prognostic role of ACD4C in ENKTL in the past (16), but this study deserves further exploration due to the small number of cases (only 70 patients).
The present study appears to show that the ACD4C at diagnosis could be a prognostic factor for survival in ENKTL. Several different cut-off values for the ACD4C, including 0.4×109/L, 0.16×109/L, and 0.45×109/L, have been reported in patients with NHL (11-14). In our study cohort, the most discriminative threshold value of the ACD4C was <0.4×109/L. We also observed an obvious difference in clinical behaviors between the high- and low-ACD4C groups. Patients with a low ACD4C at diagnosis appeared to exhibit more adverse clinical features, such as bulky disease, B symptoms, poor ECOG PS, and an advanced stage. Additionally, patients with a high ACD4C were more likely to achieve a higher rate of CR. The multivariate analysis results demonstrated that an ACD4C ≤0.4×109/L at diagnosis had a significant adverse effect on the 5-year OS of ENKTL patients and was independent of the conventional prognostic index.
The remarkable difference in prognosis between patients with a high and low ACD4C may be the result of the different systemic immune mechanisms in these patients. EBV infection is specifically important for the prognosis and development of ENKTL (28-31). Therefore, the low ACD4C might be associated with higher EBV viral load in patients with ENKTL, which needs to be shown by further studies. On the other hand, a higher ACD4C is frequently associated with more available anti-tumor function and prolonged survival. For example, Protti et al. found that activated CD4+ T cells play an essential role in tumor immunity and inhibit tumor growth by lysing tumors cells or by releasing cytokines (e.g., IFN-γ and TNF-α), which could further activate other tumoricidal immune cells (32). Rakhra et al. discovered that CD4+ T cells were essential to block angiogenesis and induce cell senescence for tumor regression oncogene inactivation (33). We speculated that ACD4C was an important parameter, which could improve the efficacy of therapeutic agents, such as more intense chemotherapy regimens. Further, the combination of targeted therapy and immunotherapy might be a promising anti-tumor therapy for ENKTL patients (34).
The major limitation of this study was that it was a retrospective single-center study. Clearly, using OS as the outcome variable and determining ACD4C cutoff groupings based on this dataset will influence the predictive power of the ACD4C level in this cohort. This may explain why our multivariate analysis did not find a statistically significant effect of ACD4C on PFS, although there was a trend to significance. Additionally, a higher portion of patients in the high ACD4C received chemotherapy plus radiation compared to chemotherapy alone. The better prognosis in the high ACD4C group may be related to the different treatment methods. Thus, it is necessary for us to conduct further prospective investigations to evaluate whether the pretreatment ACD4C affects the survival of ENKTL patients. If confirmed, our findings would suggest the testable hypothesis that patients with a low ACD4C might be good candidates for immune modulatory therapy, such as therapies that use anti-PD-1 and anti-PD-L1 antibodies.
In summary, our study proposed that the ACD4C at diagnosis was a prognostic factor for survival in ENKTL patients. Further investigation with a larger cohort and multicenter registries need to be conducted to validate the prognostic value of the ACD4C in ENKTL and gain a better understanding of the mechanisms underlying the association between the lymphocyte count and different clinical outcomes.
Acknowledgments
The authors appreciate the academic support from the AME Lymphoma Collaborative Group.
Funding: This research was supported by the National Natural Scientific Research Fund of China (project No. 81970176) and by the Guangdong Province Traditional Chinese Medicine Research Project of China (project No. 20232077).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4846/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4846/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4846/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee of the Integrated Hospital of Traditional Chinese Medicine, Southern Medical University authorized the experimental and research protocols of this study (ID: ZXY201709138). The study was conducted according with the ethical standards of the institutional research committee and with the Declaration of Helsinki (as revised in 2013). Written informed consent was provided and signed by all patients prior to sample collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Polyatskin IL, Artemyeva AS, Krivolapov YA. Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition):lymphoid tumors. Arkh Patol 2019;81:59-65.
- He L, Zou Y, Tang X, et al. Survival trends for extranodal NK/T-cell lymphoma, nasal type from different anatomical sites: a population-based study. Ann Transl Med 2021;9:849. [Crossref] [PubMed]
- V S. A Diagnostic Dilemma of Sinonasal T Cell Lymphoma: Report of A Unique Case and Literature Review. Gulf J Oncolog 2019;1:83-9. [PubMed]
- Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 2006;24:612-8. [Crossref] [PubMed]
- Chen X, Liu X, Liu W, et al. Clinicopathological characteristics and treatment outcomes of cutaneous extranodal natural killer/T-cell lymphoma: a retrospective study in China. Transl Cancer Res 2020;9:6096-106. [Crossref] [PubMed]
- Zhang MC, Xu PP, Zhong HJ, et al. Prognostic significance of NCCN-International Prognostic Index (NCCN-IPI) for patients with peripheral T-cell lymphoma treated with CHOP-based chemotherapy. Zhonghua Xue Ye Xue Za Zhi 2017;38:772-7. [PubMed]
- He X, Gao Y, Li Z, et al. Review on natural killer/T-cell lymphoma. Hematol Oncol 2021; Epub ahead of print. [Crossref] [PubMed]
- Li N, Zhang L, Song HL, et al. Prognostic impact of absolute lymphocyte count/absolute monocyte count ratio and prognostic score in patients with nasal-type, extranodal natural killer/T-cell lymphoma. Tumour Biol 2017;39:1010428317705503. [Crossref] [PubMed]
- Gluzman DF, Zavelevich MP, Philchenkov AA, et al. Immunodeficiency-associated lymphoproliferative disorders and lymphoid neoplasms in post-COVID-19 pandemic era. Exp Oncol 2021;43:87-91. [PubMed]
- Pollari M, Leivonen SK, Leppä S. Testicular Diffuse Large B-Cell Lymphoma-Clinical, Molecular, and Immunological Features. Cancers (Basel) 2021;13:4049. [Crossref] [PubMed]
- de Pádua Covas Lage LA, Hamasaki DT, Moreira FR, et al. Absolute monocyte count is a predictor of overall survival and progression-free survival in nodal peripheral T cell lymphoma. Ann Hematol 2019;98:2097-102. [Crossref] [PubMed]
- Herrera A, Cheng A, Mimitou EP, et al. Multimodal single-cell analysis of cutaneous T-cell lymphoma reveals distinct subclonal tissue-dependent signatures. Blood 2021;138:1456-64. [Crossref] [PubMed]
- Sidorov S, Fux L, Steiner K, et al. CD4 + T cells are found within endemic Burkitt lymphoma and modulate Burkitt lymphoma precursor cell viability and expression of pathogenically relevant Epstein-Barr virus genes. Cancer Immunol Immunother 2022;71:1371-92. [Crossref] [PubMed]
- Zhang X, Ge R, Chen H, et al. Follicular Helper CD4+ T Cells, Follicular Regulatory CD4+ T Cells, and Inducible Costimulator and Their Roles in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Mediators Inflamm 2021;2021:2058964. [Crossref] [PubMed]
- Zhang XY, Xu J, Zhu HY, et al. Negative prognostic impact of low absolute CD4+ T cell counts in peripheral blood in mantle cell lymphoma. Cancer Sci 2016;107:1471-6. [Crossref] [PubMed]
- Zhang YP, Zhang R, Zhu HY, et al. Circulating Low Absolute CD4+ T Cell Counts May Predict Poor Prognosis in Extranodal NK/T-Cell Lymphoma Patients Treating with Pegaspargase-Based Chemotherapy. Cancer Res Treat 2019;51:368-77. [Crossref] [PubMed]
- Jiang L, Li P, Wang H, et al. Prognostic significance of Ki-67 antigen expression in extranodal natural killer/T-cell lymphoma, nasal type. Med Oncol 2014;31:218. [Crossref] [PubMed]
- Kim TM, Heo DS, Extranodal NK. T-cell lymphoma, nasal type: new staging system and treatment strategies. Cancer Sci 2009;100:2242-8. [Crossref] [PubMed]
- Lee J, Park YH, Kim WS, et al. Extranodal nasal type NK/T-cell lymphoma: elucidating clinical prognostic factors for risk-based stratification of therapy. Eur J Cancer 2005;41:1402-8. [Crossref] [PubMed]
- Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244. [Crossref] [PubMed]
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561-77. [Crossref] [PubMed]
- Cao X, Wang Y, Zhang W, et al. Targeting macrophages for enhancing CD47 blockade-elicited lymphoma clearance and overcoming tumor-induced immunosuppression. Blood 2022;139:3290-302. [Crossref] [PubMed]
- Ding Y, Ru Y, Song T, et al. Epstein-Barr virus and cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation in patients with non-Hodgkin lymphoma: the prevalence and impacts on outcomes: EBV and CMV reactivation post allo-HCT in NHL. Ann Hematol 2021;100:2773-85. [Crossref] [PubMed]
- Emile G, Penager S, Levy C, et al. Baseline lymphopenia as prognostic factor in patients with metastatic breast cancer treated with palbociclib. Oncol Lett 2022;23:25. [Crossref] [PubMed]
- Brice P, de Kerviler E, Friedberg JW. Classical Hodgkin lymphoma. Lancet 2021;398:1518-27. [Crossref] [PubMed]
- Pai SY, Lurain K, Yarchoan R. How immunodeficiency can lead to malignancy. Hematology Am Soc Hematol Educ Program 2021;2021:287-95. [Crossref] [PubMed]
- Mozas P, Sorigué M, López-Guillermo A. Follicular lymphoma: an update on diagnosis, prognosis, and management. Med Clin (Barc) 2021;157:440-8. [Crossref] [PubMed]
- Wang L, Wang H, Wang JH, et al. Post-treatment plasma EBV-DNA positivity predicts early relapse and poor prognosis for patients with extranodal NK/T cell lymphoma in the era of asparaginase. Oncotarget 2015;6:30317-26. [Crossref] [PubMed]
- Au WY, Pang A, Choy C, et al. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood 2004;104:243-9. [Crossref] [PubMed]
- Kim HS, Kim KH, Kim KH, et al. Whole blood Epstein-Barr virus DNA load as a diagnostic and prognostic surrogate: extranodal natural killer/T-cell lymphoma. Leuk Lymphoma 2009;50:757-63. [Crossref] [PubMed]
- Suzuki R, Yamaguchi M, Izutsu K, et al. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood 2011;118:6018-22. [Crossref] [PubMed]
- Protti MP, De Monte L, Di Lullo G. Tumor antigen-specific CD4+ T cells in cancer immunity: from antigen identification to tumor prognosis and development of therapeutic strategies. Tissue Antigens 2014;83:237-46. Erratum in: Tissue Antigens 2015;85:516. [Crossref] [PubMed]
- Rakhra K, Bachireddy P, Zabuawala T, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 2010;18:485-98. [Crossref] [PubMed]
- Li PF, Mao YZ, Bai B, et al. Persistent peripheral blood EBV-DNA positive with high expression of PD-L1 and upregulation of CD4 + CD25 + T cell ratio in early stage NK/T cell lymphoma patients may predict worse outcome. Ann Hematol 2018;97:2381-9. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


