
Role of ROR2 in promoting gastric cancer metastasis by enhancing c-JUN-mediated MMP3 transcription
Introduction
Gastric cancer is the fifth most frequently diagnosed cancer and the fourth leading cause of cancer-related death worldwide (1). This disease exhibits considerable geographic variation, and more than half of the total incidence and deaths occur in the Chinese population (2). Most patients present with advanced disease at diagnosis, and consequently miss the chance for radical surgery, and are likely to have poor outcomes. Therefore, there is an increasing need to identify the key molecular mechanisms driving metastasis that can be targeted to prolong the survival of patients with advanced and metastatic gastric cancer.
Receptor tyrosine kinases (RTKs) are a superfamily of glycoproteins that regulate important cell functions, including migration, metabolism, and survival. RTK-like orphan receptor 2 (ROR2) is a transmembrane receptor, which was identified as a member of the orphan RTK family in 1992 (3). The extracellular region contains immunoglobin (Ig)-like, cysteine rich, and Kringle domains, while the cytoplasmic region contains tyrosine kinase, proline-rich, and serine/threoninerich domains. ROR2 expression has been detected in many developing tissues but is rarely found in adult tissues. ROR2 plays especially critical roles in skeletal and neural systems, germline mutations of which have been reported as being associated with brachydactyly type B (4,5) and autosomal recessive Robinow syndrome (6).
Expression of ROR2 varies across different malignancies. ROR2 expression has been reported to be higher in cervical, ovarian, breast, osteosarcoma, and non-small cell lung cancer cells than in non-tumor adjacent tissues, and reduced in liver and oral squamous cell cancers (7-13). ROR2 plays different roles in the malignant behavior of tumors. For instance, ROR2 accelerates tumor proliferation and migration in renal and esophageal cancers, promote migration rather than proliferation in some breast and ovarian cancer cells, and inhibit both proliferation and migration in certain colorectal cancer cells related to promoter hypermethylation (8,9,14-16). However, the role of ROR2 in gastric cancers has not been extensively studied. Takiguchi et al. (17) reported that Wnt5a interacted with ROR2 and promoted gastric cancer proliferation. In contrast, Yan et al. (18) demonstrated that ROR2 knockdown negatively affects tumor growth. The effect of ROR2 on the mechanism of gastric cancer metastasis and the association between ROR2 and gastric cancer prognosis has not yet been explored.
In this study, our aim was to determine the role of ROR2 in gastric cancer, regarding expression, metastasis promotion and its possible mechanism. Furthermore, we investigated the correlation between ROR2 expression and the prognosis of gastric cancer patients. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4583/rc).
Methods
Cell lines and cell culture
AGS, GTL16, SNU216, SNU601, SNU16, MKN45, MKN28, KATOIII, SNU5, NCIN87, HGC27, SGC7901, and MGC803 human gastric cancer cell lines; immortalized normal gastric epithelial cell line GES-1; and HEK293T cells were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All cell lines were verified by short tandem repeat DNA profiling analysis. AGS cells were cultured in F12K (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), GTL16 cells in high glucose Dulbecco’s modified Eagle’s medium (Gibco), and other cells in Roswell Park Memorial Institute-1640 medium (Gibco) containing 10% fetal bovine serum (FBS; Gibco) at 37 ℃ in a humidified atmosphere with 5% carbon dioxide. All cells except HEK293T cells were cultured with 5% penicillin-streptomycin (Invitrogen, Thermo Fisher Scientific).
Clinical tissue samples
All gastric tumor samples were collected from routine surgical operations at Fudan University Shanghai Cancer Center from January 1, 2010, to December 31, 2011. Characterization of the clinicopathological features of all the patients was followed up to December 31, 2015. No patients had received chemotherapy or radiotherapy before surgery. Adjacent normal gastric tissues were histologically confirmed as cancer free. All sample collections were obtained with informed consent and approved by the institutional review board of Fudan University Shanghai Cancer Center (No. ZRB1612167-18). The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki (as revised in 2013).
Lentivirus production and transduction
The ROR2-overexpressing (ROR2 WT) and the empty vector plasmids (Con) as well as shROR2#1, shROR2#2, and the empty vector plasmids (NC) were purchased from Hanyin Biotechnology (Shanghai, China). The plasmids were transfected into HEK293T cells by Lipofectamine 2000 (Invitrogen) to produce the lentivirus. Viruses were harvested 48 hours (hr) later. AGS, GTL16, SNU216, and SNU601 were infected with the ROR2-overexpressing lentivirus, SGC7901 and MGC803 were infected with the shROR2#1 and shROR2#2 lentivirus in the presence of 4–7 µg/mL of polybrene (Sigma-Aldrich, St Louis, MO, USA). Puromycin was added to select cells 72 hours after transfection. All the plasmid sequences are listed in Tables S1,S2.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from cell lines with RNAiso plus (Takara Bio, Shiga, Japan) and transcribed into complementary DNA (cDNA) using PrimeScript RT reagent Kit (Takara). cDNAs were quantified by SYBR Premix Ex Taq (Takara) using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific) for quantitative real-time polymerase chain reaction (qRT-PCR). All primers are listed in Tables S1,S2.
Western blot analysis and enzyme-linked immunosorbent assay
Cells were lysed in radioimmunoprecipitation assay buffer (Shanghai Beyotime Biotechnology Co. Ltd., Shanghai, China) supplemented with complete protease inhibitor cocktail (Roche, Basel, Switzerland). A nucleus protein extraction kit (Beyotime) was used to obtain proteins from the nucleus and cytoplasm according to the manufacturer’s instructions. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Beyotime). Western blot was carried out with 30 µg total proteins, which were probed with primary antibodies, incubated with the horseradish peroxidase-conjugated secondary antibody, and then detected by an enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). Antibodies against ROR2, β-actin, vinculin, AXIN1, GSK-3β, phospho-GSK-3β (Ser21), E-cadherin, β-catenin, p-β-catenin, c-JUN, JNK1/2, and p-JNK1/2 (Thr183/Tyr185) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against GAPDH and MMP3 were purchased from Proteintech (Rosemont, IL, USA). MMP3 concentrations of sample medium were determined using a human MMP3 enzyme-linked immunosorbent assay (ELISA) kit (Multi Sciences Biotech Co., Ltd., Hangzhou, China) according to the manufacturer’s instructions.
Signaling explorer antibody microarray
ROR2 overexpression and control cells were seeded into a culture dish at 3.0×106 cells/dish (10 cm) overnight to adhere. After 24 hours, total protein was extracted and sent to Super Biotech Corporation (Shanghai, China) for signaling explorer antibody microarray.
Wound-healing, transwell, and 3-dimensional culture assays
Cells were seeded into 96-well plates (Essen ImageLock; Essen Instruments, Ann Arbor, MI, USA) in triplicate for incubation. After 24 hours, when the cells reached 90% confluence, a single wound was created with a wound scratcher (Essen Instruments). Wound confluence was monitored with a Live-Cell Imaging System and software (Essen Instruments). Wound closure was observed every 2 hours for 24 hours by comparing the mean relative wound density.
We used a 24-well 2-chamber plate (BD Biosciences, San Jose, CA, USA), with an 8-µm (pore size) polycarbonate filter between chambers for the transwell migration assay. Matrigel (Becton Dickinson; Franklin Lakes, NJ, USA) was additionally used to precoat the membrane to simulate a matrix barrier for the transwell invasion assay. We added 1,000–4,000 cells into each upper chamber and allowed them to migrate at 37 ℃ for 16–48 hours toward the down chamber containing medium with 20% FBS. The cells were then fixed in 4% paraformaldehyde for 30 min and stained with crystal violet for 20 min. The migrated cells were counted as those that passed through the membrane separating the chamber.
To examine 3-dimensional cell growth, cells were seeded on 24-well ultra-low attachment surface plates as described previously (19). After 2 weeks of culture, cells were observed and counted under microscopy.
Luciferase reporter assay
The promoter regions were synthesized by polymerase chain reaction (PCR; PrimerSTAR, Takara) and cloned into the pGL3 vector between SacI and NheI. HEK293T cells were cotransfected with cloned pGL3 vector and Renilla luciferase vector with or without the c-JUN-overexpression plasmid. After 48-hour transfection, relative luciferase activity was calculated by a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using the SimpleChIP Enzymatic Chromatin IP Kit (magnetic beads) with c-JUN ChIP antibody (Cell Signaling Technology) according to the manufacturer’s instructions. Quantitative results were expressed as the corresponding fold change, and anti-rabbit IgG was used as a negative control.
Xenograft experiments
A protocol was prepared before the study without registration. Male BALB/c nude mice (4–6 weeks old) were purchased from the Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). They were housed in climate-controlled quarters with a 12-hour day-night cycle and had free access to food and water. All animal experiments were approved by the institutional review board of Shanghai East Hospital (No. 2022-060) and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Tongji University for the care and use of animals. For the experimental pulmonary metastasis assay, a total of 12 nude mice were acclimated 3 days before the study and were randomly divided into two groups using the standard = RAND function in Microsoft Excel. SNU601-ROR2 WT or SNU601-ROR2 Con cells (1×106) in 200 µL of phosphate-buffered saline (PBS) were respectively injected into tail veins. After injection, the mice were marked and sent back to their original cages. Mice were killed 8 weeks after injection, and the lungs were harvested for photographing. Metastatic nodules in the lungs were counted microscopically. In addition, paraffin-embedded lung tissue sections were stained by hematoxylin and eosin (HE) to examine for the presence of micrometastases.
Immunohistochemical staining
Tumor tissues were deparaffinized in xylene and rehydrated in a graded series of ethanol, and sections were subjected to antigen retrieval by boiling in 0.01 mol/L of sodium citrate buffer (pH 6.0) in a microwave oven for 10 minutes. After endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide and nonspecific protein binding was blocked with 1.5% normal goat serum, the sections were incubated overnight with an antibody at 4 ℃ in a humid chamber. Then, antibody of ROR2 (Abgent, AP7672, 1:100) was localized by incubating sections with biotinylated goat anti-rabbit IgG for 30 minutes and detected with the labeled streptavidin biotin system (Dako, Glostrup, Denmark). Rabbit anti-human ROR2 polyclonal antibody was purchased from Abgent Company (San Diego, USA). The positive controls were the positive breast cancer sections provided by Abgent Company. PBS was used in place of primary antibody as negative controls. For each sample, semiquantitative H-scoring assessment was performed by multiplying staining intensity (0, negative; 1+, weak; 2+, moderate; and 3+, strong) with the percentage of positive cells (0–100%). Two independent pathologists calculated the strength of positivity by considering the percentage of positive cells and the staining intensity.
Statistical analysis
Results are reported as mean ± standard deviation. One-way analyses of variance and Student’s t-tests were used to evaluate the between-group differences. The Spearman correlation was used for correlation analysis. Disease-free survival (DFS) and overall survival (OS) were estimated using the Kaplan-Meier method. All the relative analyses were performed using the SPSS 16.0 software (IBM Corp., Armonk, NY, USA) for Windows and GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Values of P<0.05 were considered statistically significant.
Results
ROR2 was upregulated in gastric cancer and associated with poor prognosis
To investigate the role of ROR2 in gastric cancer progression, we first analyzed the messenger RNA (mRNA) expression of ROR2 in 124 pairs of human gastric tumor specimens and matched normal gastric tissues by qRT-PCR. Results showed that the mRNA expression of ROR2 was upregulated in 77 of 124 (62.1%) gastric cancer samples as compared with their normal counterparts (P<0.05) (Figure 1A). We then analyzed ROR2 protein expression by immunohistochemistry (IHC) in 81 surgical specimens from gastric cancer patients with survival information followed-up by December 2015. The clinical characteristics are listed in Table 1. Representative images for ROR2 staining are shown in Figure 1B. Upregulation of ROR2 was associated with nerve infiltration (P=0.026). However, there was no statistically significant difference between ROR2 expression level and age, sex, tumor differentiation, T stage, N stage, TNM stage, or vascular infiltration (Table 1). Univariate analyses showed that tumor stage and ROR2 expression level were associated with both reduced DFS and OS (Table 2). Kaplan-Meier analysis (Figure 1C) revealed that patients with high ROR2 expression had worse OS (P=0.037) and DFS (P=0.030). Median OS and DFS in ROR2-high expression group was 34.2 and 23.9 months, respectively, and neither was reached in ROR2-low expression group by the end of follow-up period. Collectively, analyses of the clinical data suggested that ROR2 was upregulated in gastric cancer and associated with poor prognosis in patients with gastric cancer.
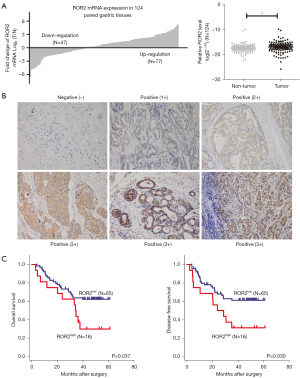
Table 1
| Variables | Expression of ROR2 | P value | |
|---|---|---|---|
| Low (N=65) (%) | High (N=16) (%) | ||
| Age (years) | 0.159 | ||
| ≤60 | 41 (63.08) | 7 (43.75) | |
| >60 | 24 (36.92) | 9 (56.25) | |
| Gender | 0.497 | ||
| Male | 43 (66.15) | 12 (75.00) | |
| Female | 22 (33.85) | 4 (25.00) | |
| Tumor differentiation | 0.387 | ||
| Undifferentiated | 3 (4.62) | 2 (12.50) | |
| Poor | 44 (67.69) | 8 (50.00) | |
| Moderate | 13 (20.00) | 3 (18.75) | |
| Missing data | 5 (7.69) | 3 (18.75) | |
| T stage | 0.185 | ||
| T2 + T3 | 28 (43.08) | 4 (25.00) | |
| T4 | 37 (56.92) | 12 (75.00) | |
| N stage | 0.95 | ||
| N0 + N1 | 29 (44.62) | 7 (43.75) | |
| N2 + N3 | 36 (55.38) | 9 (56.25) | |
| TNM stage | 0.314 | ||
| II | 25 (38.46) | 4 (25.00) | |
| III | 40 (61.54) | 12 (75.00) | |
| Vascular invasion | 0.873 | ||
| Yes | 38 (58.46) | 10 (62.50) | |
| No | 25 (38.46) | 6 (37.50) | |
| Missing data | 2 (3.08) | – | |
| Nerve invasion | 0.026 | ||
| Yes | 22 (33.85) | 15 (93.75) | |
| No | 42 (64.62) | 1 (6.25) | |
| Missing data | 1 (1.53) | – | |
Table 2
| Variables | Disease-free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recurrence | N (%) | P value | Death | N# (%) | P value | ||||
| Yes | No | Yes | No | ||||||
| Age (years) | 0.264 | ||||||||
| ≤60 | 18 | 30 | 48 (59.3) | 0.276 | 18 | 29 | 47 (50.6) | ||
| >60 | 17 | 16 | 33 (40.7) | 17 | 16 | 33 (49.4) | |||
| Gender | 0.983 | ||||||||
| Male | 24 | 31 | 55 (67.9) | 0.990 | 24 | 31 | 55 (81.2) | ||
| Female | 11 | 15 | 26 (32.1) | 11 | 14 | 25 (18.8) | |||
| Tumor differentiation | 0.179 | ||||||||
| Undifferentiated/poor | 27 | 30 | 57 (70.4) | 0.179 | 27 | 29 | 56 (64.7) | ||
| Moderate/well | 5 | 11 | 16 (29.6) | 5 | 11 | 16 (35.3) | |||
| Vascular invasion | 0.521 | ||||||||
| Yes | 23 | 25 | 48 (60.8) | 0.481 | 23 | 24 | 47 (62.4) | ||
| No | 12 | 19 | 31 (39.2) | 12 | 19 | 31 (37.6) | |||
| Nerve invasion | 0.377 | ||||||||
| Yes | 27 | 30 | 57 (28.7) | 0.286 | 27 | 29 | 56 (70.9) | ||
| No | 8 | 15 | 23 (71.3) | 8 | 15 | 23 (29.1) | |||
| T stage | 0.007 | ||||||||
| T2 + T3 | 8 | 24 | 32 (39.5) | 0.008 | 8 | 24 | 32 (40.0) | ||
| T4 | 27 | 22 | 49 (60.5) | 27 | 21 | 48 (60.0) | |||
| N stage | 0.002 | ||||||||
| N0 + N1 | 8 | 28 | 36 (44.4) | 0.001 | 8 | 27 | 35 (43.8) | ||
| N2 + N3 | 27 | 18 | 45 (55.6) | 27 | 18 | 45 (56.2) | |||
| TNM stage | 0.025 | ||||||||
| II | 7 | 22 | 29 (23.5) | 0.015 | 7 | 21 | 28 (35.4) | ||
| III | 28 | 24 | 52 (76.5) | 28 | 24 | 51 (64.6) | |||
| Adjuvant chemotherapy | <0.001 | ||||||||
| Yes | 23 | 42 | 65 (80.2) | <0.001 | 23 | 41 | 64 (80.0) | ||
| No | 12 | 4 | 16 (19.8) | 12 | 4 | 16 (20.0) | |||
| ROR2 | 0.037 | ||||||||
| High | 11 | 5 | 16 (19.8) | 0.030 | 11 | 5 | 16 (20.0) | ||
| Low | 24 | 41 | 65 (80.2) | 24 | 40 | 64 (80.0) | |||
P value: statistical analysis was conducted by Kaplan-Meier method (log-rank test). #, data available from 80 cases with complete survival records. Cases with incomplete records were processed as missing data.
ROR2 promoted gastric cancer cell migration, invasion, and metastasis
To explore the potential roles of ROR2 in gastric cancer, we first examined the expression of ROR2 in gastric cancer cell lines as well as the immortalized normal gastric mucosal epithelial cell line GES1 by qRT-PCR and western blot. As shown in Figure 2A, MGC803 and SGC7901 expressed relatively high levels of ROR2 when compared with immortalized normal gastric mucosal epithelial cell line GES1 as well as AGS, GTL16, SNU216, and SNU601. To investigate the influence of ROR2 on malignant phenotypes of gastric cancer cells, we stably expressed ROR2 in AGS, GTL16, SNU216, and SNU601 cells, and depleted ROR2 in SGC7901 and MGC803 cells by infection with lentiviral vectors encoding ROR2 and short hairpin (shRNA) targeting ROR2 (shROR2#1 and shROR2#2), respectively. The expression status of ROR2 in the resultant cell lines was verified (Figure 2B). ROR2 expression level transfected with shROR2#1 was lower than that transfected with shROR2#2 in both MGC803 and SGC7901 cells, so cells transfected with shROR2#1 were chosen in the subsequent experiments.

We assessed whether ROR2 could affect the migratory and invasive properties of gastric cancer cells. Wound-healing assays, transwell migration and invasion assays showed that knockdown of ROR2 in MGC803 and SGC7901 cells suppressed cell migration and invasion compared to their control cells. In contrast, ectopic expression of ROR2 in AGS, SNU601, and GTL16 cells promoted migratory and invasive capacity (Figure 3A-3C and Figure S1). Furthermore, the 3-dimensional culture assay showed that the spheres formed by SNU601-ROR2 were looser and that the contact between adjacent cells was reduced, which facilitated the invasion of cells further outward (Figure 3D).

To examine whether ROR2 affects gastric cancer metastasis in vivo, SNU601 cells stably expressing ROR2 and SNU601 cells with the control vector were respectively injected into BALB/c nude mice through the tail vein. As shown in Figure 3E, SNU601-ROR2 WT cells increased the number of metastatic tumors in the lungs of nude mice. These results were supported by HE staining of the lung sections of these mice. The average lung weight in the SNU601-ROR2 WT mice was significantly higher than that in the control group (P<0.05) (20). Together, these results indicated that ROR2 could promote gastric cancer cell migratory and invasive behaviors in vitro and lung metastatic potential in vivo.
Screening for ROR2-modulating genes
To clarify the molecular mechanisms underlying the role of ROR2 in gastric cancer cells, we conducted a signaling explorer antibody microarray to identify proteins with an altered expression induced by ROR2. The heat map is shown in Figure 4A. To compare the protein expression of SNU216-Con and SNU216-ROR2 WT cells, we made a list of candidates that exhibited a more than 15% difference in protein expression amounts. By searching the literature, a total of 23 protein-encoding genes closely related to tumor proliferation and migration were identified and chosen to validate the array result. The mRNA expression of 23 genes was quantified by qRT-PCR in AGS-ROR2 WT, GTL16-ROR2 WT, SNU216-ROR2 WT, SNU601-ROR2 WT, SGC7901-shROR2, MGC803-shROR2, and their respective control cells. Representative qRT-PCR images of 8 genes are illustrated in Figure 4B, and those of the other genes in Figure S2.

As demonstrated, MMP3 was upregulated in ROR2-overexpressed cells and downregulated in shROR2 cell lines compared with those in their parental cells. MMP3 protein expression detected by ELISA (Figure 5A) and western blot confirmed the qRT-PCR results (Figure 5B). To further explore the relationship between MMP3 and ROR2, we detected the mRNA expression of ROR2 and MMP3 in 32 cases of gastric cancer by qRT-PCR. The MMP3 mRNA level consistently and positively correlated with ROR2 (R2=0.738; P<0.001; Figure 5C). These results demonstrated that MMP3 may be the main downstream factor of ROR2.

ROR2 activated the non-canonical Wnt pathway but not the canonical Wnt pathway
To gain mechanistic insights into how ROR2 regulates MMP3, we examined changes in the downstream pathway signaling along with ROR2 expression alteration. As an important receptor of the Wnt pathway, ROR2 has been reported to have different effects in many tumors through the canonical Wnt pathway or non-canonical Wnt pathway. We first detected key proteins of the canonical Wnt pathway in ectopic ROR2 expression cells and control cells. Western blot showed that overexpression or depletion of ROR2 did not affect the expression of AXIN1, GSK-3β, p-GSK-3β, E-cadherin, β-catenin, or p-β-catenin, suggesting that ROR2 does not activate the canonical Wnt pathway (Figure 6A,6B).

It has been reported that ROR2 can activate JNK1/2 in the non-canonical Wnt pathway (21). We detected the expression of JNK1/2 and phosphorylated JNK1/2 in ectopic ROR2 expression cells and control cells by western blot. As in Figure 6A,6B, p-JNK1/2 was upregulated in ROR2-overexpression cells, and downregulated in shROR2 cells. Moreover, we found that p-JNK1/2 expression in the nucleus was increased when ROR2 was overexpressed (Figure 6C). The above results showed that ROR2 could activate downstream of JNK1/2 by translocating p-JNK1/2 into the nucleus through the non-canonical Wnt pathway.
c-JUN transcriptionally activated MMP3
c-JUN has been identified as a transcription factor (22-24), so we conducted bioinformatics prediction analysis, which showed that there were 3 potential binding sites of c-JUN in the promoter region of MMP3. Information for the 3 predicted sites is listed in Table S3.
To determine whether c-JUN regulates MMP3 transcription, we transfected c-JUN-overexpression plasmid and control plasmid into HEK293T cells. We found that MMP3 mRNA expression was greatly enhanced by c-JUN (Figure 7A). We then cloned the MMP3 promoter region and constructed the pGL3-MMP3 plasmid, and dual luciferase reporter assays were conducted to determine whether c-JUN was able to bind to the MMP3 promoter. As shown in Figure 7B, the activity of luciferase was significantly higher when transfected with the c-JUN-overexpression plasmid than with the control group in both HEK293T and AGS cells (P<0.01), which demonstrated that c-JUN could promote the transcriptional activity of the MMP3 promoter.
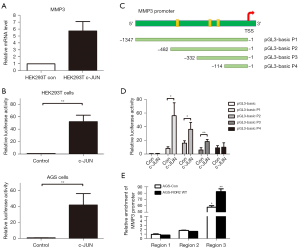
In order to more precisely examine the main region for c-JUN binding, truncated fragments of the MMP3 promoter of different lengths were constructed according to the predicted binding sites. We named the full-length-pGL3 plasmid P1 (–1 to –1,347 bp) and 3 truncated plasmids P2 (–1 to –482 bp), P3 (–1 to –332 bp), and P4 (–1 to –114 bp; Figure 7C). A dual luciferase reporter assay was employed to identify the exact promoter region for c-JUN binding. We found that the –332 to –114 bp locus was the main binding region of c-JUN, as the luciferase activities of P1, P2, and P3 were significantly increased, while that of P4 did not change significantly (Figure 7D). Moreover, a ChIP assay was conducted to verify the results. Three pairs of ChIP qRT-PCR primers corresponding to the MMP3 promoter binding sites were designed. We found that a fragment spanning the positions –332 to –114 bp (region 3) of the MMP3 promoter was enriched in c-JUN–precipitated chromosomal DNA in AGS-ROR2 WT cells compared with AGS control cells (Figure 7E). These results showed that ROR2 could activate c-JUN to upregulate MMP3 expression by translocating p-JNK1/2 into the nucleus and then activate c-JUN-mediated transcription by binding to the –332 to –114 bp locus of the MMP3 promoter.
Discussion
We present several novel findings concerning the role for ROR2 in the malignant progression of gastric cancer.
First, ROR2 is upregulated in tumor tissues of gastric cancer patients. The expression of ROR2 in different tumors is inconsistent. Previous studies showed an increase in ROR2 expression in laryngeal squamous cell carcinoma, tongue squamous cell carcinoma, oral squamous cell carcinoma, breast cancer, ovarian cancer, non-small cell lung cancer, colorectal cancer, and pancreatic ductal adenocarcinoma (8,9,11,25-27). In contrast, Geng et al. (12) found that the expression of ROR2 in 63% of liver cancer samples was significantly lower than that in paracancerous tissue. Lara and Ma’s research confirmed the presence of ROR2 promoter hypermethylation in colorectal cancer cells and tissues (14,28), while another study reported ROR2 expression in oral squamous cell carcinoma to be downregulated (13). Despite there being abundant research in other areas, studies of ROR2 expression in gastric cancer are rare. Yan et al. (18) found that ROR2 expression in 4 gastric cancer cell lines was lower than that in normal gastric epithelial cells but that this decrease was not significant. Collectively, these findings suggest that ROR2 functions differently depending on the tumor type. Our results showed that ROR2 was overexpressed in cell lines MGC803 and SGC7901 derived from metastatic foci. In addition, we found that the mRNA expression of ROR2 was upregulated in 77 of 124 (62.1%) gastric cancer samples. Moreover, the positive rate of ROR2 expression in another group of clinical gastric cancer samples detected by IHC was as high as 70.4% (57/81). Thus, this study is the first to demonstrate that the ROR2 expression level is upregulated in gastric cancer.
Second, high expression of ROR2 in gastric cancer is associated with poor prognosis. The expression of ROR2 in different tumors has an inconsistent relationship with prognosis. High expression of ROR2 has been found to be positively correlated with TNM stage and lymph node metastasis, which could be used as an independent prognostic factor in cervical cancer (29), non-small cell lung cancer (30), pancreatic ductal adenocarcinoma (26), breast cancer (8), ovarian cancer (9), chondrosarcoma (11), colorectal cancer (27), and clear cell renal cell carcinoma (31). However, in primary thyroid lymphoma, high expression of ROR2 was also shown to be related to local invasion and clinical stage, but not to OS time (P=0.256) (32). Conversely, high expression of ROR2 in neuroblastoma was found to be associated with better prognosis (33). In liver cancer, low expression of ROR2 was closely related to poor prognosis (P=0.007) (12), while Nema et al. (34) found the expression of ROR2 to be associated with a worse prognosis for patients with gastrointestinal cancer, particularly for those with the intestinal type. In this study, we assessed 81 gastric cancer specimens by IHC and found that a high expression of ROR2 was associated with poor DFS and OS, suggesting that ROR2 could be used as a prognostic marker in patients with gastric cancer. In this study, we confirmed both mRNA and protein elevated expression of ROR2 in gastric cancer clinical samples for the first time, on the basis of which we found high expression of ROR2 to be associated with poor DFS and OS.
Third, ROR2 exerts a premetastatic effect in gastric cancer progression. ROR2 has been reported to have different effects on cell proliferation, growth, migration, and invasion across different tumors. Many studies have shown that ROR2 promotes cell proliferation and migration in renal cell carcinoma (15), esophageal cancer cells WT33 (16), leiomyosarcoma, and gastrointestinal stromal tumor (35). Lara et al. (14) found that, along with overexpression of ROR2, proliferation and growth of colon cancer cells in vivo and in vitro were weakened. However, ROR2 in gastric cancer has rarely been explored, and the conclusions are inconsistent. Takiguchi et al. (17) found that ROR2 promoted proliferation of gastric cancer cells. On the other hand, Yan et al. (18) found that overexpression of ROR2 in AGS gastric cancer cells decreased cell proliferation, with the cells being blocked in the G0–G1 phase. In this study, the transwell assay was used to demonstrate that ROR2 promotes gastric cancer migration and invasion in vitro, and a BALB/c nude mice pulmonary metastasis model was used to ascertain the role of ROR2 in promoting metastasis in vivo. We confirm for the first time that ROR2 promotes gastric cancer metastasis, which further clarifies the function of ROR2 in gastric cancer progression.
Fourth, we revealed a novel mechanism by which ROR2 enhances metastasis by upregulating MMP3 expression via activating c-JUN interaction directly with the MMP3 promoter. ROR2 was reported to have an opposite role in the canonical Wnt pathway, inhibiting (36,37) and activating the canonical Wnt/β-Catenin pathway (38,39). Our data showed that ROR2 did not activate the canonical Wnt pathway in gastric cancer. Previous studies have shown that ROR2 stimulates the expression of PKC and JNK1/2 by activating the non-canonical Wnt pathway, thereby promoting synaptic transmission (21). Yamagata et al. (40) confirmed that ROR2 recruits transcription factors c-JUN and Atf2 to bind to and promote the activity of the MMP13 promoter by activating the JNK1/2 pathway, which eventually leads to an increase of MMP13 expression. Another study reported that Wnt5a could activate the JNK1/2 pathway, which is dependent on PKC, thus promoting cell migration (41). In this study, the activation of the JNK1/2 pathway in the non-canonical Wnt pathway by ROR2 was confirmed. Through a signaling explorer antibody microarray analysis, we identified MMP3 as a key downstream target of ROR2. Reducing MMP3 expression in gastric cancer cells reversed the promotion of cell progression induced by ROR2 expression. The JNK1/2-c-JUN pathway is frequently activated to promote tumor progression, proliferation, and metastasis (42-45). The transcription factor c-JUN phosphorylation was identified as an essential prerequisite for activation of the JNK1/2 pathway, which can promote transcriptional activity of oncogenes by binding to the promoters (22-24). Previous studies found MMP3 transcription to be enhanced by the JNK1/2-c-JUN axis (46-49). Here, we demonstrated that ROR2 could activate p-JNK1/2 in the nucleus and that the downstream transcription factor c-JUN of JNK1/2 binds to the MMP3 promoter at –332 to –114 bp to promote its expression at the transcriptional level. This study is the first of its kind to define the role of the ROR2/JNK1/2-c-JUN/MMP3 pathway in gastric cancer metastasis and supports the theory of ROR2 regulation of the non-canonical Wnt pathway.
Conclusions
Our findings demonstrate that ROR2 is upregulated in gastric cancer, promotes tumor metastasis, and correlates with the poor prognosis of patients with gastric cancer. The underlying mechanism involves the ROR2/JNK1/2-c-JUN-MMP3 axis in the progression of gastric cancer. Therefore, ROR2 may be a promising biomarker to predict prognosis in gastric cancer. Furthermore, silencing the JNK1/2-c-JUN pathway and thereby inhibiting MMP3 expression may serve as a promising strategy to inhibit gastric cancer progression.
Acknowledgments
The authors are grateful to pathologists Cong Tan and Jiaojie Lv (both from Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China; Institute of Pathology, Fudan University, Shanghai, China) for their contribution to the IHC staining score assessment. The authors appreciate the academic support from the AME Gastric Cancer Collaborative Group.
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81702360 and 81772561) and the Fundamental Research Funds for Central Universities (No. 22120180596).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4583/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4583/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4583/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were approved by the institutional review board of Shanghai East Hospital (No. 2022-060) and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Tongji University for the care and use of animals. All sample collections were obtained with informed consent and approved by the institutional review board of Fudan University Shanghai Cancer Center (No. ZRB1612167-18). The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. NCCN Clinical Practice Guidelines in Oncology Gastric Cancer, Version 2.2022. 2022.
- Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem 1992;267:26181-90. [Crossref] [PubMed]
- Afzal AR, Rajab A, Fenske CD, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 2000;25:419-22. [Crossref] [PubMed]
- Akbarzadeh S, Wheldon LM, Sweet SM, et al. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS One 2008;3:e1873. [Crossref] [PubMed]
- van Bokhoven H, Celli J, Kayserili H, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet 2000;25:423-6. [Crossref] [PubMed]
- Sun B, Ye X, Lin L, et al. Up-regulation of ROR2 is associated with unfavorable prognosis and tumor progression in cervical cancer. Int J Clin Exp Pathol 2015;8:856-61. [PubMed]
- Henry C, Quadir A, Hawkins NJ, et al. Expression of the novel Wnt receptor ROR2 is increased in breast cancer and may regulate both β-catenin dependent and independent Wnt signalling. J Cancer Res Clin Oncol 2015;141:243-54. [Crossref] [PubMed]
- Henry C, Llamosas E, Knipprath-Meszaros A, et al. Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion. Oncotarget 2015;6:40310-26. [Crossref] [PubMed]
- Enomoto M, Hayakawa S, Itsukushima S, et al. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene 2009;28:3197-208. [Crossref] [PubMed]
- He L, Yang Z, Zhou J, et al. The clinical pathological significance of FRAT1 and ROR2 expression in cartilage tumors. Clin Transl Oncol 2015;17:438-45. [Crossref] [PubMed]
- Geng M, Cao YC, Chen YJ, et al. Loss of Wnt5a and Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol 2012;18:1328-38. [Crossref] [PubMed]
- Liu G, Kukuruzinska MA, Xu X. Ror2 may be downregulated in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:120. [Crossref] [PubMed]
- Lara E, Calvanese V, Huidobro C, et al. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer 2010;9:170. [Crossref] [PubMed]
- Yang CM, Ji S, Li Y, et al. Ror2, a Developmentally Regulated Kinase, Is Associated With Tumor Growth, Apoptosis, Migration, and Invasion in Renal Cell Carcinoma. Oncol Res 2017;25:195-205. [Crossref] [PubMed]
- Lyros O, Nie L, Moore T, et al. Dysregulation of WNT5A/ROR2 Signaling Characterizes the Progression of Barrett-Associated Esophageal Adenocarcinoma. Mol Cancer Res 2016;14:647-59. [Crossref] [PubMed]
- Takiguchi G, Nishita M, Kurita K, et al. Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci 2016;107:290-7. [Crossref] [PubMed]
- Yan L, Du Q, Yao J, et al. ROR2 inhibits the proliferation of gastric carcinoma cells via activation of non-canonical Wnt signaling. Exp Ther Med 2016;12:4128-34. [Crossref] [PubMed]
- Lee GY, Kenny PA, Lee EH, et al. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 2007;4:359-65. [Crossref] [PubMed]
- Elkin M, Vlodavsky I. Tail vein assay of cancer metastasis. Curr Protoc Cell Biol 2001;Chapter 19:19.2.1-7.
- Cerpa W, Latorre-Esteves E, Barria A. RoR2 functions as a noncanonical Wnt receptor that regulates NMDAR-mediated synaptic transmission. Proc Natl Acad Sci U S A 2015;112:4797-802. [Crossref] [PubMed]
- Wu Q, Wu W, Fu B, et al. JNK signaling in cancer cell survival. Med Res Rev 2019;39:2082-104. [Crossref] [PubMed]
- Zhang D, Jiang Q, Ge X, et al. RHOV promotes lung adenocarcinoma cell growth and metastasis through JNK/c-Jun pathway. Int J Biol Sci 2021;17:2622-32. [Crossref] [PubMed]
- Shen S, Liang J, Liang X, et al. SNHG17, as an EMT-related lncRNA, promotes the expression of c-Myc by binding to c-Jun in esophageal squamous cell carcinoma. Cancer Sci 2022;113:319-33. [Crossref] [PubMed]
- Castro MV, Lopez-Bergami P. Cellular and molecular mechanisms implicated in the dual role of ROR2 in cancer. Crit Rev Oncol Hematol 2022;170:103595. [Crossref] [PubMed]
- Huang J, Fan X, Wang X, et al. High ROR2 expression in tumor cells and stroma is correlated with poor prognosis in pancreatic ductal adenocarcinoma. Sci Rep 2015;5:12991. [Crossref] [PubMed]
- Mei H, Lian S, Zhang S, et al. High expression of ROR2 in cancer cell correlates with unfavorable prognosis in colorectal cancer. Biochem Biophys Res Commun 2014;453:703-9. [Crossref] [PubMed]
- Ma SS, Srivastava S, Llamosas E, et al. ROR2 is epigenetically inactivated in the early stages of colorectal neoplasia and is associated with proliferation and migration. BMC Cancer 2016;16:508. [Crossref] [PubMed]
- Martinez S, Scerbo P, Giordano M, et al. The PTK7 and ROR2 Protein Receptors Interact in the Vertebrate WNT/Planar Cell Polarity (PCP) Pathway. J Biol Chem 2015;290:30562-72. [Crossref] [PubMed]
- Lu C, Wang X, Zhu H, et al. Over-expression of ROR2 and Wnt5a cooperatively correlates with unfavorable prognosis in patients with non-small cell lung cancer. Oncotarget 2015;6:24912-21. [Crossref] [PubMed]
- Rasmussen NR, Debebe Z, Wright TM, et al. Expression of Ror2 mediates invasive phenotypes in renal cell carcinoma. PLoS One 2014;9:e116101. [Crossref] [PubMed]
- Wang L, Yang D, Wang YH, et al. Wnt5a and Ror2 expression associate with the disease progress of primary thyroid lymphoma. Tumour Biol 2016;37:6085-90. [Crossref] [PubMed]
- Lee SE, Lim SD, Kang SY, et al. Prognostic significance of Ror2 and Wnt5a expression in medulloblastoma. Brain Pathol 2013;23:445-53. [Crossref] [PubMed]
- Nema R, Patel P, Kumar A. Prognostic Role of Receptor Tyrosine Kinase-Like Orphan Receptors in Intestinal-Type Gastric Cancer. Asian Pac J Cancer Prev 2021;22:2125-34. [Crossref] [PubMed]
- Edris B, Espinosa I, Mühlenberg T, et al. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol 2012;227:223-33. [Crossref] [PubMed]
- Bainbridge TW, DeAlmeida VI, Izrael-Tomasevic A, et al. Evolutionary divergence in the catalytic activity of the CAM-1, ROR1 and ROR2 kinase domains. PLoS One 2014;9:e102695. [Crossref] [PubMed]
- Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem 2009;284:30167-76. [Crossref] [PubMed]
- Li C, Chen H, Hu L, et al. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol 2008;9:11. [Crossref] [PubMed]
- Liu Y, Ross JF, Bodine PV, et al. Homodimerization of Ror2 tyrosine kinase receptor induces 14-3-3(beta) phosphorylation and promotes osteoblast differentiation and bone formation. Mol Endocrinol 2007;21:3050-61. [Crossref] [PubMed]
- Yamagata K, Li X, Ikegaki S, et al. Dissection of Wnt5a-Ror2 signaling leading to matrix metalloproteinase (MMP-13) expression. J Biol Chem 2012;287:1588-99. [Crossref] [PubMed]
- Nomachi A, Nishita M, Inaba D, et al. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem 2008;283:27973-81. [Crossref] [PubMed]
- Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res 2012;72:379-86. [Crossref] [PubMed]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol 2007;19:142-9. [Crossref] [PubMed]
- Dong C, Li X, Yang J, et al. PPFIBP1 induces glioma cell migration and invasion through FAK/Src/JNK signaling pathway. Cell Death Dis 2021;12:827. [Crossref] [PubMed]
- Kalli M, Li R, Mills GB, et al. Mechanical Stress Signaling in Pancreatic Cancer Cells Triggers p38 MAPK- and JNK-Dependent Cytoskeleton Remodeling and Promotes Cell Migration via Rac1/cdc42/Myosin II. Mol Cancer Res 2022;20:485-97. [Crossref] [PubMed]
- Lee MG, Lee KS, Nam KS. Anti-metastatic effects of arctigenin are regulated by MAPK/AP-1 signaling in 4T-1 mouse breast cancer cells. Mol Med Rep 2020;21:1374-82. [Crossref] [PubMed]
- Liu P, Liu Y, Zheng H, et al. Sulforaphane suppresses polyinosinic-polycytidylic acid-stimulated release of cytokines, chemokines and MMPs by human corneal fibroblasts. Mol Med Rep 2020;22:5463-71. [Crossref] [PubMed]
- Hu G, Zhao X, Wang C, et al. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis 2017;8:e3140. [Crossref] [PubMed]
- Chen T, Hou H, Fan Y, et al. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J Photochem Photobiol B 2016;165:34-41. [Crossref] [PubMed]
(English Language Editor: J. Gray)


