
Treatment of progressive nodular histiocytosis: a case report
Introduction
Progressive nodular histiocytosis (PNH) is a proliferative disorder of dermal dendrocytes and is further classified within as dendritic cell disorders, which include generalized eruptive histiocytosis, benign cephalic histiocytosis, PNH, and spindle cell xanthogranuloma (1). The disorder is an extremely rare variant of non-Langerhans cell histiocytosis (NLCH) and was first reported by Taunton et al. in 1978 (2). At present, about 20 reported cases can be found through a PubMed search. PNH is clinically characterized by the appearance of cutaneous lesions, mostly on the face, neck, and trunk, with leonine facies being a prominent characteristic according to some reports (3). Patients with PNH usually have numerous disseminated lesions, and the number and size of the of the lesions increase with the duration of the disease (4). The lesions are xanthoma-like yellowish-brown skin papules, including deeper subcutaneous nodules with sizes ranging from 5.0 mm to 5.0 cm (3), which are generally benign, usually without systemic involvement, and exhibit rare associations with the laryngeal, oral, and conjunctival mucosa (3).
PNH is generally chronic, progressive, with no spontaneous regression and is often treatment resistant thereby incurring significant disfigurement, disabling with consequent loss of quality of life (5). Most PNH cases have been described in the Western population, and reports in the Asian population have been rare. Adequate surgical resection of papules and nodules seems to be the main and most effective option for PNH, but recurrences were also common (5). Therefore, it is urgent to develop new and more effective treatment methods. In this article, we present a unique case of PNH in a 21-year-old Asian patient, who was diagnosed and exceptionally treated with a small molecule multi-target anti-vascular drug—regorafenib—which had an unexpected curative effect. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4987/rc).
Case presentation
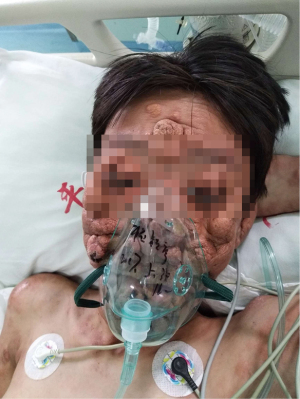
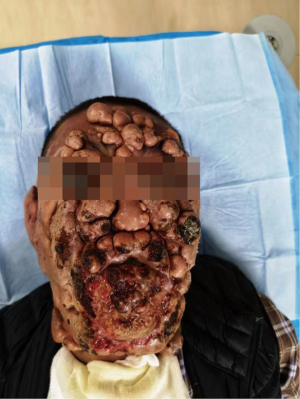
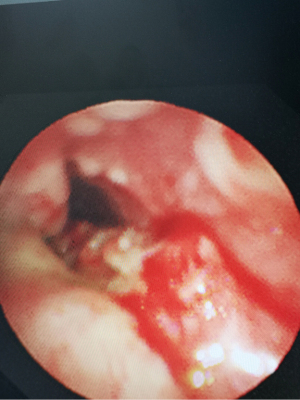
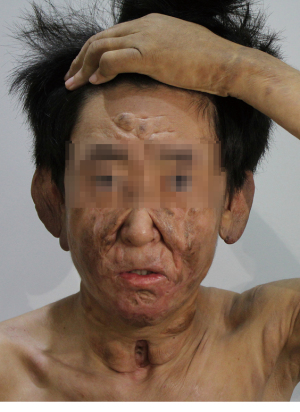
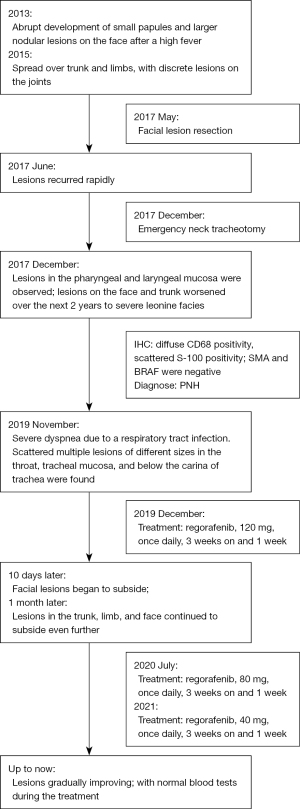
A 21-year-old male developed recurrent high fever at the age of 12 years in 2013, followed by the abrupt development of small papules and larger nodular lesions on the face. The lesions started from the eyelids, and then gradually expanded and worsened. In 2015, the nodular lesions spread over the facial region, trunk, and limbs, with discrete lesions on the joints (see Figure 1). In May 2017, the patient underwent facial lesion resection at a hospital, but the lesions recurred rapidly 1 month after the operation (see Figures 2,3). In December 2017, the patient had difficulty breathing due to respiratory tract infection. After an emergency neck tracheotomy, lesions in the pharyngeal and laryngeal mucosa were observed, a tracheostomy was indicated. The lesions on his face and trunk continued to worsen over the next 2 years, becoming confluent with severe leonine facies. In November 2019, the patient presented to our hospital for severe dyspnea due to a respiratory tract infection (see Figure 4). A fiberoptic bronchoscopy revealed scattered multiple lesions of different sizes in the throat, tracheal mucosa, and below the carina of trachea. Some of them were bleeding (see Figure 5). The patient had no family history of skin diseases or lipid storage disorders.

Histopathological and immunochemical examinations
Microscopic examinations of all the lesions demonstrated that the histiocytes proliferated and formed expansive cell nodules in the dermis and subcutaneous tissues. The lesions were composed of epithelioid and spindle cells with roughly similar vesicular nuclei, small nucleoli, abundant eosinophils, and had consistent vacuolar or xanthoma-like cytoplasm. Further, scattered Touton cells and a few small round lymphocytes were observed in the lesions. The cellular components were present in the sclerotic stroma characterized by thick collagen bundles. Immunohistochemical staining showed diffuse CD68 positivity and scattered S-100 positivity, while CD1α, smooth muscle actin (SMA) and B-Raf proto-oncogene, serine/threonine kinase (BRAF) were negative. Based on the presence and the nature of the skin lesions, along with the histopathological and microscopic findings, the patient was diagnosed with PNH (see Figure 6).
Treatment
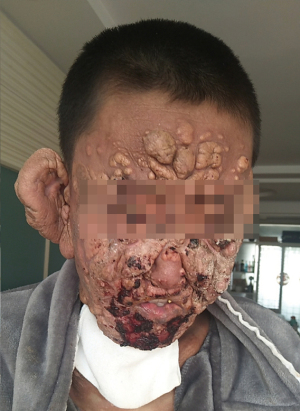
Recently, mitogen-activated protein kinase kinase 1 (MAP2K1) and mitogen-activated protein kinase 1 (MAPK1) mutations have been reported in histiocytosis, which strongly suggests that the disease may be driven by MAP kinase pathway activation (6). Given the severity of the patient’s condition and the ineffectiveness of other therapies, treatment with a small multi-kinase inhibitor that targets MAP kinase pathway, regorafenib, was initiated in December 2019, after obtaining informed consent from the patient and his parents. Following the standard dose for colon cancer, 120 mg of regorafenib was prescribed once daily for 3 weeks on and 1 week off. Results were seen as early as 10 days after treatment initiation (see Figure 7) and continued to improve thereafter. Hence, on July 2020, the dose of regorafenib was adjusted to 80 mg QD for 3 weeks on and 1 week off. In 2021, the regorafenib dose was subsequently decreased to 40 mg QD, with intermittent administration. Blood tests were within normal parameters, including liver and kidney function, during the treatment. At the moment, the patient’s overall condition is good, the lesions are gradually improving (see Figure 8), and he has returned to normal life and work, as outlined in Figure 9.
All the procedures performed in the study were conducted in accordance with the Declaration of Helsinki (as revised in 2013), and this study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYWZLL 26097). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In the present manuscript, we described a unique case of a patient who was diagnosed with severe disfiguring and life-threatening PNH and showed remarkably improvement with regorafenib. Systemic disease association with PNH is rare, however, Gonzalez et al. reported a case associated with hypercholesterolemia and chronic myeloid leukemia (1) and Taibo et al. described a patient with PNH that developed an extracutaneous peripheral T-cell lymphoma (7). Another unique PNH case reported by Beswick et al. was associated with hypothalamic involvement and growth hormone deficiency, and 1 case reported an association of PNH with Eale’s disease (8,9). However, these associations remain anecdotal (3). The present case was particularly challenging and unique due to the substantial involvement of pharyngeal and laryngeal mucosal lesions, which led to airway obstruction and urgent tracheotomy.
Since Karimi et al. (10) described the first PNH case involving pharyngeal and upper laryngeal mucosa, three cases including ours were reported (3). In previously reported cases, mucosal involvement was seen late in the disease course and possibly correlated with severe and extensive skin disease (3). Hence, we believe that these patients need regular monitoring life-long to ensure the timely detection of life‑threatening airway involvement.
As expected with PNH, our clinical observation and histopathology findings demonstrated 2 types of lesions (1,3,4); small superficial dermal papules with lymphocytic infiltrates, and larger and deeper nodules with storiform spindle cell infiltrate and scattered Touton giant cells. The immunohistochemistry results were positive for CD68 and S-100 protein as reported by Glavin et al. (3). Conversely, a study has reported that these infiltrating macrophages are negative for S-100 and CD1α (4). This unusual pattern of reactivity may be due to an unexplored phenomenon relating to different progenitor cells or different levels of maturation (4).
Current treatment for PNH is difficult and mostly unsuccessful (4,11). Previous attempts to treat the disease have included surgical/physical resection and chemotherapy (prednisolone, vincristine, and cyclophosphamide), but have proven to be unsuccessful (12,13). While histiocytic neoplasms are clinically and genetically heterogenous, in most cases, activating MAPK pathway mutations can be demonstrated (14). Hence, in 2019, we chose regorafenib to inhibit the MAPK kinase pathway, as it targets multiple kinases along MAPK signaling cascade such as RAF, RET and KIT. Indeed, the response to regorafenib was rapid (within 10 days) and dramatic. The involvement of MAPK was further highlighted by the recent hallmark phase II trial published in Nature. Eighteen histiocytosis patients (no PNH patients were included) were treated with a MEK inhibitor (cobimetinib) and 89% responded to the treatment (14). Since, 2 successful case reports using cobimetinib in PNH were reported (15,16). In our manuscript, the remarkable curative effect of regorafenib in this case also suggests that the pathogenesis of the disease may be associated with genetic site mutations. However, a further genetic analysis is required to confirm the hypothesis.
For our patient, regorafenib proved to have a curative effect, and thus the use of the drug should be further explored in the treatment of PNH, after excluding relevant contraindications. The patient in our case did not exhibit any adverse reactions; however, the occurrence of drug-related toxic side effects needs to be monitored closely.
Questions to be further discussed and considered
Question 1: The patient is currently taking an oral treatment of regorafenib (40 mg daily) intermittently and has shown a good response and no side effects but has not yet fully recovered. How long should the current dose be continued?
Ana Taibo: I guess that if the patient had a good response, I would try to low the dose.
Elena Netchiporouk: I don’t think anyone has the clear response to this yet. My gut feeling would be to continue on therapy, once he is in complete response ON therapy for 2 years, I would opt to STOP and keep following closely in case of recurrence. Of note, I think the nature study has a good 1 year drug free response rate.
Question 2: PNH is a rare disease, what are the possible targets of regorafenib?
Ana Taibo: Histiocytosis pathophysiology is still not well known so it is difficult to know the target that did work. I guess that regorafenib worked due to its anti-proliferative properties.
Elena Netchiporouk: See the suggestions made in the text. I think this case would benefit from targeted next generation sequencing of the tumor biopsy (FFPE from 2019?).
Question 3: In subsequent clinical practice, if we encounter a rare and life-threatening disease that significantly affects the quality of life, could this drug be used if no other effective therapy is available?
Ana Taibo: In case of proliferative diseases /neoplastic, it may help.
Elena Netchiporouk: Only if it makes sense biologically. But even for PNH I would be more inclined to try a more targeted MAPK inhibitor like cobi. I don’t think it would work in lipoid proteinosis or brooke spiegler by example. So it has to make sense biologically.
Acknowledgments
The authors appreciate the academic support from the AME Progressive Nodular Histiocytosis Collaborative Group.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4987/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4987/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures performed in the study were conducted in accordance with the Declaration of Helsinki (as revised in 2013), and this study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYWZLL 26097). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gonzalez Ruíz A, Bernal Ruíz AI, Aragoneses Fraile H, et al. Progressive nodular histiocytosis accompanied by systemic disorders. Br J Dermatol 2000;143:628-31. [Crossref] [PubMed]
- Taunton OD, Yeshurun D, Jarratt M. Progressive nodular histiocytoma. Arch Dermatol 1978;114:1505-8. [Crossref] [PubMed]
- Glavin FL, Chhatwall H, Karimi K. Progressive nodular histiocytosis: a case report with literature review, and discussion of differential diagnosis and classification. J Cutan Pathol 2009;36:1286-92. [Crossref] [PubMed]
- Nofal A, Assaf M, Tawfik A, et al. Progressive nodular histiocytosis: a case report and literature review. Int J Dermatol 2011;50:1546-51. [Crossref] [PubMed]
- Zelger BW, Sidoroff A, Orchard G, et al. Non-Langerhans cell histiocytoses. A new unifying concept. Am J Dermatopathol 1996;18:490-504. [Crossref] [PubMed]
- Diamond EL, Durham BH, Haroche J, et al. Diverse and Targetable Kinase Alterations Drive Histiocytic Neoplasms. Cancer Discov 2016;6:154-65. [Crossref] [PubMed]
- Taibo A, Martin I, Almagro M, et al. PUVA therapy in persistent cutaneous histiocytosis: Case report and literature review. Photodermatol Photoimmunol Photomed 2022; Epub ahead of print. [Crossref] [PubMed]
- Beswick SJ, Kirk JM, Bradshaw K, et al. Progressive nodular histiocytosis in a child with a hypothalamic tumor. Br J Dermatol 2002;146:138-40. [Crossref] [PubMed]
- Williams A, Thomas AG, Kwatra KS, et al. Progressive Nodular Histiocytosis Associated with Eale's Disease. Indian J Dermatol 2015;60:388-90. [Crossref] [PubMed]
- Karimi K, Jadidian A, Glavin FL, et al. Laryngeal involvement in progressive nodular histiocytosis: a case report. Arch Otolaryngol Head Neck Surg 2010;136:513-6. [Crossref] [PubMed]
- Nakayashiki N, Akita H, Mori W, et al. Effective surgical treatment of progressive nodular histiocytosis. J Plast Surg Hand Surg 2014;48:80-3. [Crossref] [PubMed]
- Watanabe T, Watanabe D, Tamada Y, et al. Progressive nodular histiocytosis - A five-year follow up. Eur J Dermatol 2008;18:200-2. [PubMed]
- Huet F, Brenaut E, Costa S, et al. Progressive nodular histiocytosis improved by methotrexate. Eur J Dermatol 2017;27:661-3. [Crossref] [PubMed]
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature 2019;567:521-4. [Crossref] [PubMed]
- Buján MM, Sánchez La Rosa C, Galluzzo Mutti ML, et al. Progressive nodular histiocytosis in a 9-year-old boy treated with cobimetinib. Pediatr Dermatol 2022;39:115-8. [Crossref] [PubMed]
- Berce PC, Cardwell L, Essenmacher AN, et al. Progressive nodular histiocytosis with dramatic response to cobimetinib. JAAD Case Rep 2022;22:110-3. [Crossref] [PubMed]




