
Effects of hydrogen rich water and pure water on periodontal inflammatory factor level, oxidative stress level and oral flora: a systematic review and meta-analysis
Introduction
As the oral cavity is an important structure connecting the human body with the external environment, many microorganisms in the external environment are easy to enter the oral cavity and reproduce in it, thus forming a unique oral micro ecosystem(1). Among these microorganisms, >700 kinds of bacteria are related to the occurrence of diseases (2). If good oral hygiene is not maintained, bacteria adhere to the tooth surface through an acquired membrane, forming a plaque biofilm. The formed dental plaque secretes a variety of virulence factors that act on the teeth and periodontal tissue, which in turn can cause tooth defects and lead to periodontal inflammatory injury (3). With the widespread application of implant denture restoration in clinical settings, peri-implantitis has become an important factor affecting the prognosis of implants, and has an incidence rate of approximately 9% (4). The initiating factor of peri-implantitis is plaque biofilm, and its main pathogen is the same as that of periodontitis. Additionally, periodontitis is an important risk factor for peri-implantitis (5).
The production of reactive oxygen species (ROS), which is a normal immune reaction product that inhibits pathogens, such as bacteria in vivo, can effectively control the inflammatory response progress. However, the excessive production of ROS induces a serious oxidative stress reaction and further aggravates oxidative damage to periodontal and peri-implant tissues (6). Oxidative stress is also an important factor leading to inflammatory diseases. The continuous accumulation of ROS further promotes the release of related pro-inflammatory factors, such as interleukin (IL)-1β and IL-6, which leads to the continuous progression of oral diseases, such as periodontitis or peri-implantitis (7). These oral diseases cause tissue damage, tooth loss, and implant loss, which seriously affect patients’ quality of life. Thus, the question of how to inhibit the formation of dental plaque, and effectively control the inflammatory response in the oral cavity are popular areas of research in stomatology.
At present, brushing and flossing are widely used to remove oral biofilm. However, the use of these mechanical methods to remove dental plaque is not ideal. Thus, mouthwash gargling has become an auxiliary method to control the formation of dental plaque. Chlorhexidine is the most commonly used mouthwash, which can effectively inhibit plaque formation and prevent the occurrence of periodontitis (8). However, Chlorhexidine also has many adverse reactions related to use, such as gum swelling, bleeding, taste change, staining, and mucosal soreness. Additionally, as usage increases, drug resistance to relevant oral bacteria develops (9,10). Such reactions have adversely affected the use of Chlorhexidine and led to the development of safer and more effective oral hygiene products.
Relevant studies have shown that hydrogen (H2) is an effective therapeutic antioxidant that can reduce the degree of oxidative damage by selectively scavenging oxygen free radicals in tissues (11,12). Its antioxidant process is mild and does not interfere with the normal redox reaction of the body. Additionally, it does not have an adverse effect on ROS that transduce signals (13). Further, a previous study has shown that H2 can also inhibit the release of proinflammatory factors (14). The above research shows that the use of hydrogen rich water (HRW) for oral cleaning can restore the redox balance in the oral cavity and reduce the oxidative stress response.
However, in an experiment of beagle dog peri-implant inflammation model, after washing with HRW, the gingival index, improved bleeding index, and probe depth of the animal models were improved to some extent compared with those of pure water (PW) group, but not significantly. Besides, according to 16SRNA sequencing technology, HRW can inhibit the proliferation of oral pathogenic bacteria to a certain extent, but the change is not significant, which will not be conducive to inhibiting the formation of dental plaque (15). In addition, He et al. (16) found that long-term drinking of HRW may cause abnormal liver function and increase blood lipid levels in rat models. The reason maybe that the active hydrogen in HRW water is extremely unstable and prone to volatilization, resulting in a large reduction of active hydrogen molecules, which will greatly affect its clinical efficacy. Besides, the molecular mechanism of HRW’s biological effect has not been fully clarified, and long term drinking may affect the body function. Therefore, the clinical treatment of HRW for oral diseases remains controversial. This meta-analysis sought to summarize and analyze the data of previous studies to determine the effects of HRW on the inhibiting inflammatory reaction and oxidative stress levels to provide clinical guidance for controlling dental plaque formation and treating periodontitis and peri-implantitis. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4422/rc).
Methods
Data retrieval and collection
We searched the PubMed, Web of Science, Embase, Cochrane, China Knowledge Resource Integrated, Wanfang, and Weipu databases for relevant articles using the following key words: “Hydrogen-rich water” or “Hydrogen” and “Gingivitis” or “Periodontitis” or “Oral cavity” and “Oxidative stress” or “Inflammation” or “Bacteria.” The retrieval time was limited to March 2022. The literature retrieval was conducted by 2 individuals with >3 years of retrieval experience.
Inclusion criteria
To be eligible for inclusion in this meta-analysis, articles had to meet the following inclusion criteria: (I) comprise subjects who experienced various inflammatory reactions (e.g., gingivitis, periodontitis, and peri-implantitis.) (II) concern a randomized controlled trial; (III) include a HRW group, which received HRW only, and a pure water (PW) control group, which received PW; and (IV) have study indicators that included related inflammatory factors [e.g., IL-1β, IL-6, or tumor necrosis factor alpha (TNF-α)], oxidative stress indicators [e.g., 8-hydroxyguanosine (8-OHdG), reactive oxygen metabolites (ROM), or glutathione peroxidase (GPx)], or oral flora.
Exclusion criteria
Articles were excluded from the meta-analysis if they met any of the following exclusion criteria: (I) concerned case reports, reviews, comments, or guidelines; (II) concerned pseudo-randomized controlled trials; (III) had incomplete data or the complete data could not be obtained; (IV) included none of the relevant study indicators; and/or (V) were duplicate articles.
Data extraction
For all the articles included in the study, we extracted the author, year of publication, study subjects, number of samples, and IL-1β, IL-6, TNF-α, 8-OHdG, ROM, GPx, and bacteriostasis results. If necessary, we contacted the author of the article to obtain more detailed data.
Quality evaluation of the included articles
The Cochrane risk of bias assessment tool was used to assess the quality of included articles. The assessment includes the following 6 aspects: (I) random allocation method; (II) allocation concealment; (III) blinding; (IV) data integrity; (V) selective reporting; (VI) other bias. The evaluation degree of the above six items was divided into three grades: low bias, high bias, and uncertainty bias.
Statistical analysis
Revman 5.3 was used for the meta-analysis. The continuous variables of inflammation, oxidative stress, and colony formation unit (CFU) are expressed as the standardized mean differences (SMDs) and the 95% confidence intervals (CIs), and forest plots were generated using a random-effects or fixed-effects model. For each meta-analysis, Cochrane Q test and I2 index were used to evaluate the heterogeneity of the study, if P>0.1 or I2≤50% mean that no heterogeneity existed between studies, and fixed effect model was used for analysis; If P≤0.1 or I2>50% indicated that the research heterogeneity was obvious, and subgroup analysis or sensitivity analysis would be carried out. For studies that still could not eliminate heterogeneity but have clinical consistency, random effect model would be used for analysis. Meanwhile, funnel plot distribution was used to determine whether there was publication bias in the included literature. If the combined SMD and the 95% CI in the meta-analysis did not overlap with 0, the effect of the outcome index was considered statistically significant. P<0.05 indicated significant difference.
Results
Literature search
In total, 1,541 relevant articles were retrieved. After deleting duplicate articles, we screened the remaining 1,123 records according to the title and abstract, and 761 additional articles were excluded. The remaining 362 articles were further screened, and 345 records were excluded (41 case studies, 175 reviews, 58 pseudo-randomized controlled trials, and 71 studies with insufficient data). Ultimately, 17 studies, comprising 304 subjects, were included in the meta-analysis (11-14,17-29). In all the studies, the subjects in the HRW group received the HRW intervention, while those in the PW group received a placebo or PW. The retrieval process is shown in Figure 1, and the research characteristics are shown in Table 1.
Table 1
| Author, year | Research object | Total cases | IL-1β | TNF-α | IL-6 | GPx | 8-OHdG | ROM | CFU | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRW | PW | HRW | PW | HRW | PW | HRW | PW | HRW | PW | HRW | PW | HRW | PW | HRW | PW | |||||||||
| Ara (11), 2018 | Chronic forced swimming | 7 | 7 | 5.82±0.67 | 3.96±0.67 | 5.98±0.57 | 9.81±1.79 | 9.63±2.23 | 12.02±2.05 | 0.35±0.03 | 0.29±0.02 | |||||||||||||
| 4.93±0.3 | 5.07±0.75 | 8.65±0.45 | 9.06±0.67 | 14.45±1.34 | 6.79±0.59 | 0.36±0.02 | 0.27±0.04 | |||||||||||||||||
| Asada (12), 2020 | Diabetes | 9 | 9 | 6.35±0.98 | 7.78±1.13 | |||||||||||||||||||
| 3.73±0.54 | 7.78±1.13 | |||||||||||||||||||||||
| Azuma (13), 2015 | Periodontitis | 7 | 6 | 28.22±29.39 | 55.12±53.39 | |||||||||||||||||||
| −24.07±73.48 | 51.33±90.58 | |||||||||||||||||||||||
| Dobashi (14), 2020 | Severe exercise | 4 | 4 | 303.4±13.8 | 268.1±9.8 | |||||||||||||||||||
| 303.5±14.3 | 273.1±9.5 | |||||||||||||||||||||||
| Kasuyama (17), 2011 | Periodontitis | 7 | 7 | 8.12±0.87 | 5.88±1.25 | 0.097±0.036 | 0.32±0.072 | 324.68±16.34 | 382.48±20.79 | |||||||||||||||
| 378.65±32.67 | 479.53±41.58 | |||||||||||||||||||||||
| Kim (18), 2017 | Oral bacteria | 18 | 18 | 12.18±11.23 | 189.84±121.96 | |||||||||||||||||||
| Lee (19), 2006 | Oral bacteria | 16 | 16 | 231.09±10.51 | 2037.82±52.52 | |||||||||||||||||||
| 136.56±21.01 | 1313.03±42.01 | |||||||||||||||||||||||
| 199.58±10.5 | 1722.69±42.02 | |||||||||||||||||||||||
| 199.58±21.01 | 1817.23±84.03 | |||||||||||||||||||||||
| Lee (20), 2013 | Oral bacteria | 3 | 3 | 228.01±91.21 | 11902.3±638.4 | |||||||||||||||||||
| 137.85±47.13 | 4265.99±447.81 | |||||||||||||||||||||||
| 108.48±54.23 | 5532.2±515.25 | |||||||||||||||||||||||
| Nayak (21), 2021 | Chronic periodontitis | 20 | 20 | 59.55±47.35 | 81.72±55.63 | |||||||||||||||||||
| 45.72±30.51 | 81.72±55.63 | |||||||||||||||||||||||
| 53.55±38.71 | 81.72±55.63 | |||||||||||||||||||||||
| 75.2±44.92 | 115.2±74.11 | |||||||||||||||||||||||
| 70.3±37.53 | 115.2±74.11 | |||||||||||||||||||||||
| 61±38.29 | 115.2±74.11 | |||||||||||||||||||||||
| Saitoh (22), 2022 | Gingival cells | 6 | 6 | 19.19±0.58 | 19.77±0.07 | 8.64±0.38 | 10.27±0.67 | 7.26±1.81 | 13.12±2.09 | |||||||||||||||
| Si (23), 2021 | Kidney injury | 8 | 8 | 140.39±5.77 | 151.92±1.92 | 711.54±28.85 | 788.46±19.23 | 515.39±46.15 | 407.69±30.77 | 1.08±0.058 | 2.53±0.059 | |||||||||||||
| Sim (24), 2020 | Healthy adults | 20 | 18 | 0.73±0.6 | 1.04±0.59 | 384.4±96.4 | 349±56.1 | |||||||||||||||||
| Song (25), 2022 | Chronic ulcerative colitis | 5 | 4 | 3.88±0.92 | 9.55±1.27 | 5.66±2.25 | 10.95±2.3 | 39.74±11.6 | 13.91±2.31 | |||||||||||||||
| Tamaki (26), 2016 | Oral palatal wound | 6 | 6 | 82.11±8.58 | 198.53±36.76 | 28.82±3.43 | 52.5±5.49 | 8.04±0.97 | 15.29±2.84 | 260.59±7.72 | 372.79±28.98 | |||||||||||||
| 94.36±17.16 | 123.78±31.86 | 37.06±5.15 | 44.95±4.46 | 11.67±1.28 | 13.14±1.37 | 225.39±5.77 | 258.36±9.64 | |||||||||||||||||
| Tomofuji (27), 2014 | Aging periodontal tissues | 6 | 6 | 6.79±0.36 | 1.7±0.1 | 0.392±0.204 | 0.644±0.136 | |||||||||||||||||
| 0.18±0.04 | 0.2±0.05 | |||||||||||||||||||||||
| 0.17±0.03 | 0.25±0.04 | |||||||||||||||||||||||
| 0.19±0.04 | 0.27±0.05 | |||||||||||||||||||||||
| Yoneda (28), 2017 | High-fat diet | 6 | 6 | 0.12±0.03 | 0.17±0.05 | |||||||||||||||||||
| Yuan (29), 2018 | Traumatic brain injury | 6 | 6 | 22.87±2.73 | 9.24±3.08 | |||||||||||||||||||
Data are presented as No. and mean ± standard deviation. HRW, hydrogen rich water; PW, pure water; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor alpha; GPx, glutathione peroxidase; 8-OHdG, 8-hydroxyguanosine; ROM, reactive oxygen metabolites; CFU, colony formation unit.
Quality evaluation
The Cochrane risk of bias assessment tool was used to evaluate the quality of the articles and showed that the total risk of bias was medium to low. After the risk of bias graph and bias summary were generated for the included studies, we found that 2 studies mentioned unreasonable random assignment, 2 studies mentioned data shedding, 1 study mentioned “selective reporting”, and no studies mentioned “allocation concealment” or the “blinding of outcome assessment” (see Figure 2).
Effects of HRW on the release of inflammatory factors
After dental plaque is formed, it stimulates the body to secrete a large number of inflammatory factors. As an important immune mediator in the inflammatory response process, changes in inflammatory factor levels have a key effect on the degree of the inflammatory response and the development of infection.
Of the included studies, 5 reported changes in IL-1β after HRW therapy. The results showed that after treatment with HRW, the IL-1β level of the HRW group decreased significantly (SMD =–0.73; 95% CI: –1.29 to –0.18; P=0.009; see Figure 3A). Additionally, by summarizing the TNF-α data of 7 groups in 5 studies, we found that HRW effectively reduced the TNF-α level (SMD =–2.51; 95% CI: –3.56 to –1.46; P<0.00001; see Figure 3B). Finally, by summarizing the IL-6 data of 6 groups in 4 studies, we observed that the IL-6 level of the HRW group decreased significantly (SMD =–1.31; 95% CI: –1.96 to –0.67; P<0.0001; see Figure 3C).

Effects of HRW on the oxidative stress response
The formation of excessive ROS leads to deoxyribonucleic acid (DNA) and lipid damage, which in turn cause apoptosis and strengthen the degree of the inflammatory response. Thus, monitoring the levels of oxidative stress in vivo effectively reflects the effect of HRW on inhibiting the inflammatory response.
GPx is considered the first defense line of the antioxidant enzyme system in inhibiting the production of ROS. By summarizing and analyzing the data of 6 groups in 5 studies, we found that the GPx level of the HW group was higher than that of the PW group, and better inhibited the formation of ROS (SMD =2.5; 95% CI: 1.85 to 3.15; P<0.00001; see Figure 4A). Additionally, as the main product of endogenous DNA damage, 8-OHdG is widely used as a marker of the oxidative stress response. The data of 9 groups in 5 studies showed changes in 8-OHdG levels after HRW treatment. It was found that the level of 8-OHdG of the HW group decreased significantly (SMD =–1.61; 95% CI: –2.35 to –0.87; P<0.0001; see Figure 4B). Additionally, ROM effectively reflects the total oxidant capacity in vivo, including the level of ROS. Data from 9 groups in 5 studies reported ROM indicators, and we observed that HRW effectively reduced the ROM levels (SMD =–0.49; 95% CI: –0.91 to –0.06; P=0.02; see Figure 4C). Thus, HRW treatment effectively inhibits the degree of oxidative stress response in vivo.

Effects of HRW on CFU
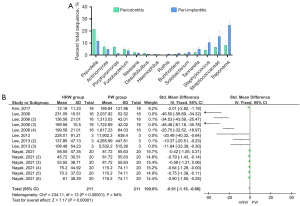
First, we summarized and analyzed the pathogenic bacteria of periodontitis and peri-implantitis, and found that they share a variety of the same pathogenic bacteria, of which Porphyromonas, Actinomyces, Streptococcaceae, and Treponema were the most common (5,30-36) (see Figure 5A). Staphylococcus aureus (S. mutans) and Porphyromonas gingivalis (P. gingivalis) are commonly used to study the bacteriostasis of HRW, and their CFUs effectively reflect the antibacterial effect of HRW. Through a comprehensive analysis of the CFU data from 14 groups in 4 studies, we found that HRW effectively inhibits the formation of bacterial colonies in the oral cavity (SMD =–0.91; 95% CI: –1.16 to –0.66; P<0.00001; see Figure 5B).
Publication bias analysis of included literature
When the number of studies included in the meta-analysis is less than 10, publication bias will generally be found in the funnel plot. In the evaluation of the effectiveness of HRW in improving oral inflammation, because less than 10 articles were included in each outcome index, funnel plot mapping and publication bias analysis were not conducted.
Discussion
As people’s quality of life has improved, they have begun to pay more and more attention to their oral health, including tooth whitening, controlling gum bleeding, and maintaining fresh breath. Periodontitis is the 2nd most common disease in the oral cavity (34). Additionally, dental implant is more and more commonly used because of its good appearance and functional restoration effect. Periodontitis and peri-implantitis are both infectious oral diseases, and their initiating factor is plaque biofilm. At present, guided-biofilm therapy emphasizes that good plaque control is key to the successful treatment of periodontitis and peri-implantitis (30). Plaque biofilm on the surface of the tooth and periodontal tissue continuously secretes virulence factors, which can stimulate periodontal tissue and cells to produce various inflammatory mediators. Subsequently, the persistent inflammatory reaction will result in irreversible tissue damage, and eventually cause the loss of teeth and failure of implant repair (21,27).
Periodontitis is also related to the occurrence of metabolic syndrome, atherosclerosis, Parkinson’s syndrome, and other systemic diseases. Thus, plaque control and the reduction of inflammatory injury of periodontal tissue and peri-implant supporting tissue are of great significance in the prevention of periodontitis, the improvement of the long-term dental implanting success rate, and the maintenance of oral and general health.
At present, many studies have examined whether HRW can be used to inhibit the inflammatory response, but such studies have used many different research indicators, and their conclusions have differed. Thus, we summarized and analyzed the intervention effects of HRW on the inflammatory response, oxidative stress response, and CFUs in various studies to analyze the bacteriostatic effects of HRW on oral pathogenic bacteria.
Inflammatory factors have a key effect on the development of periodontitis. The pathogenic bacteria attached to plaque biofilm not only produces a large number of toxic factors that directly destroy periodontal tissue, but also mediates the host immune and inflammatory response to promote the release of inflammatory factors, which in turn aggravate the destruction of periodontal tissue. For example, TNF-α can activate periodontal osteoclasts and collagenase to promote alveolar bone absorption and destruction (37). Meanwhile, IL-1β and IL-6 can further promote the inflammatory response and effectively reflect the severity of periodontitis (2,38).
After a comprehensive analysis of the levels of inflammatory factors in various studies, we found that only two groups of data suggested that the IL-1β level of the HRW group was higher than that of the PW group. Additionally, only 1 group of data showed that the IL-6 level of the HRW group was higher than that of the PW group, and the remaining data all showed that HRW greatly reduced the release of inflammatory factors and the degree of inflammatory reaction.
Oxidative stress promotes the occurrence of the inflammatory response and is a key factor in leading to periodontal disease development. Through the comprehensive analysis of 25 groups of research data, we found that HRW effectively improves the GPx level and reduces the 8-OHdG level. Thus, HRW effectively improves the antioxidant level in vivo to reduce DNA damage.
In terms of the ROM indicators, 3 groups of data showed that the ROM level of the HRW group was higher than that of the PW group, and all the other data showed that HRW effectively reduces the ROM level, which may be caused by H2 selectively removing hydroxyl radicals without affecting other ROS.
In addition, after a comprehensive analysis of 14 groups of data on the correlation between HRW and CFU, we found that HRW effectively inhibits the proliferation of a variety of oral pathogens, including S. mutans, P. gingivalis, and Actinobacillus actinomycetemcomitans (18-21), which effectively reduces the virulence of pathogens thus reduce inflammatory injury. And the reduction of the amount of bacteria can reduce the level of oxidative stress and inflammatory reaction in the body, further reducing inflammatory damage. Therefore, HRW can effectively control the formation of dental plaque, reduce the release of inflammatory factors in the mouth, and reduce the degree of oxidative stress.
However, the sample sizes of the included studies were small, and there were certain differences in the measurement methods and sample sources of various research indicators, which may have been the sources of the heterogeneity in this meta-analysis. In a follow-up study, we intend to collect more data and conduct a subgroup analysis of the sample sources and inflammation types to provide a more detailed summary.
Conclusions
In conclusion, HRW effectively inhibits the inflammatory reaction, oxidative stress level, and bacterial proliferation activity, which can be used to inhibit the formation of oral plaque biofilm, prevent and control inflammatory injury, and preserve periodontal and peri-implant supporting tissues to better maintain oral health. At the same time, prevention and effective treatment of periodontal disease is of great significance to the overall health.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4422/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4422/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Azuma MM, Samuel RO, Gomes-Filho JE, et al. The role of IL-6 on apical periodontitis: a systematic review. Int Endod J 2014;47:615-21. [Crossref] [PubMed]
- Albuquerque CM, Cortinhas AJ, Morinha FJ, et al. Association of the IL-10 polymorphisms and periodontitis: a meta-analysis. Mol Biol Rep 2012;39:9319-29. [Crossref] [PubMed]
- Toker H, Görgün EP, Korkmaz EM. Analysis of IL-6, IL-10 and NF-κB Gene Polymorphisms in Aggressive and Chronic Periodontitis. Cent Eur J Public Health 2017;25:157-62. [Crossref] [PubMed]
- Ephros H, Kim S, DeFalco R. Peri-implantitis: Evaluation and Management. Dent Clin North Am 2020;64:305-13. [Crossref] [PubMed]
- Sousa V, Nibali L, Spratt D, et al. Peri-implant and periodontal microbiome diversity in aggressive periodontitis patients: a pilot study. Clin Oral Implants Res 2017;28:558-70. [Crossref] [PubMed]
- Wang Y, Andrukhov O, Rausch-Fan X. Oxidative Stress and Antioxidant System in Periodontitis. Front Physiol 2017;8:910. [Crossref] [PubMed]
- Hernández-Ríos P, Pussinen PJ, Vernal R, et al. Oxidative Stress in the Local and Systemic Events of Apical Periodontitis. Front Physiol 2017;8:869. [Crossref] [PubMed]
- Faramarzi M, Shirmohammadi A, Chitsazi M, et al. The clinical and metabolic effects of subgingival application of xanthan-based chlorhexidine gel in Type 2 diabetic patients with chronic periodontitis. Dent Res J (Isfahan) 2017;14:299-305. [Crossref] [PubMed]
- Jagadish Pai BS, Rajan SA, Srinivas M, et al. Comparison of the efficacy of chlorhexidine varnish and chip in the treatment of chronic periodontitis. Contemp Clin Dent 2013;4:156-61. [Crossref] [PubMed]
- Chauhan AS, Bains VK, Gupta V, et al. Comparative analysis of hyaluronan gel and xanthan-based chlorhexidine gel, as adjunct to scaling and root planing with scaling and root planing alone in the treatment of chronic periodontitis: A preliminary study. Contemp Clin Dent 2013;4:54-61. [Crossref] [PubMed]
- Ara J, Fadriquela A, Ahmed MF, et al. Hydrogen Water Drinking Exerts Antifatigue Effects in Chronic Forced Swimming Mice via Antioxidative and Anti-Inflammatory Activities. Biomed Res Int 2018;2018:2571269. [Crossref] [PubMed]
- Asada R, Tazawa K, Sato S, et al. Effects of hydrogen-rich water prepared by alternating-current-electrolysis on antioxidant activity, DNA oxidative injuries, and diabetes-related markers. Med Gas Res 2020;10:114-21. [Crossref] [PubMed]
- Azuma T, Yamane M, Ekuni D, et al. Drinking Hydrogen-Rich Water Has Additive Effects on Non-Surgical Periodontal Treatment of Improving Periodontitis: A Pilot Study. Antioxidants (Basel) 2015;4:513-22. [Crossref] [PubMed]
- Dobashi S, Takeuchi K, Koyama K. Hydrogen-rich water suppresses the reduction in blood total antioxidant capacity induced by 3 consecutive days of severe exercise in physically active males. Med Gas Res 2020;10:21-6. [Crossref] [PubMed]
- Zhao YW. Preliminary study on the effect of hydrogen-rich water on peri-implant inflammation. Southern Medical University 2021.
- He J, Xun ZM, Xie F, et al. Effects of Long-term Drinking of Hydrogen-rich Water on the Physiological Function of Normal Rats. Curr Biotechno 2021;11:353-60.
- Kasuyama K, Tomofuji T, Ekuni D, et al. Hydrogen-rich water attenuates experimental periodontitis in a rat model. J Clin Periodontol 2011;38:1085-90. [Crossref] [PubMed]
- Kim J, Lee HJ, Hong SH. Inhibition of streptococcal biofilm by hydrogen water. J Dent 2017;58:34-9. [Crossref] [PubMed]
- Lee SH, Choi BK. Antibacterial effect of electrolyzed water on oral bacteria. J Microbiol 2006;44:417-22. [PubMed]
- Lee SH, Baek DH. Antibacterial activity of hydrogen-rich water against oral bacteria. International Journal of Oral Bioiogy 2013;38:81-5. [Crossref]
- Nayak A, Bhatt A, Bhat K, et al. Assessment of antibacterial effect of hydrogen water on plaque from patients with chronic periodontitis. J Indian Soc Periodontol 2021;25:193-6. [Crossref] [PubMed]
- Saitoh Y, Yonekura N, Matsuoka D, et al. Molecular hydrogen suppresses Porphyromonas gingivalis lipopolysaccharide-induced increases in interleukin-1 alpha and interleukin-6 secretion in human gingival cells. Mol Cell Biochem 2022;477:99-104. [Crossref] [PubMed]
- Si Y, Liu L, Cheng J, et al. Oral Hydrogen-Rich Water Alleviates Oxalate-Induced Kidney Injury by Suppressing Oxidative Stress, Inflammation, and Fibrosis. Front Med (Lausanne) 2021;8:713536. [Crossref] [PubMed]
- Sim M, Kim CS, Shon WJ, et al. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: a randomized, double-blind, controlled trial. Sci Rep 2020;10:12130. [Crossref] [PubMed]
- Song L, Zhang Y, Zhu C, et al. Hydrogen-rich water partially alleviate inflammation, oxidative stress and intestinal flora dysbiosis in DSS-induced chronic ulcerative colitis mice. Adv Med Sci 2022;67:29-38. [Crossref] [PubMed]
- Tamaki N, Orihuela-Campos RC, Fukui M, et al. Hydrogen-Rich Water Intake Accelerates Oral Palatal Wound Healing via Activation of the Nrf2/Antioxidant Defense Pathways in a Rat Model. Oxid Med Cell Longev 2016;2016:5679040. [Crossref] [PubMed]
- Tomofuji T, Kawabata Y, Kasuyama K, et al. Effects of hydrogen-rich water on aging periodontal tissues in rats. Sci Rep 2014;4:5534. [Crossref] [PubMed]
- Yoneda T, Tomofuji T, Kunitomo M, et al. Preventive Effects of Drinking Hydrogen-Rich Water on Gingival Oxidative Stress and Alveolar Bone Resorption in Rats Fed a High-Fat Diet. Nutrients 2017;9:64. [Crossref] [PubMed]
- Yuan J, Wang D, Liu Y, et al. Hydrogen-rich water attenuates oxidative stress in rats with traumatic brain injury via Nrf2 pathway. J Surg Res 2018;228:238-46. [Crossref] [PubMed]
- Al-Radha AS, Pal A, Pettemerides AP, et al. Molecular analysis of microbiota associated with peri-implant diseases. J Dent 2012;40:989-98. [Crossref] [PubMed]
- Schmalz G, Tsigaras S, Rinke S, et al. Detection of five potentially periodontal pathogenic bacteria in peri-implant disease: A comparison of PCR and real-time PCR. Diagn Microbiol Infect Dis 2016;85:289-94. [Crossref] [PubMed]
- da Silva ES, Feres M, Figueiredo LC, et al. Microbiological diversity of peri-implantitis biofilm by Sanger sequencing. Clin Oral Implants Res 2014;25:1192-9. [Crossref] [PubMed]
- Kumar PS, Mason MR, Brooker MR, et al. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol 2012;39:425-33. [Crossref] [PubMed]
- Benrachadi L, Bouziane A, Azziman Z, et al. Screening for periodontopathogenic bacteria in severe chronic periodontitis in a Moroccan population. Med Mal Infect 2012;42:599-602. [Crossref] [PubMed]
- Gajardo M, Silva N, Gómez L, et al. Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Chilean population. J Periodontol 2005;76:289-94. [Crossref] [PubMed]
- Takeuchi Y, Umeda M, Ishizuka M, et al. Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Japanese population. J Periodontol 2003;74:1460-9. [Crossref] [PubMed]
- Fredriksson M, Bergström K, Asman B. IL-8 and TNF-alpha from peripheral neutrophils and acute-phase proteins in periodontitis. J Clin Periodontol 2002;29:123-8. [Crossref] [PubMed]
- Noh MK, Jung M, Kim SH, et al. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med 2013;6:847-51. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)




