
The roles and mechanisms of miR-26 derived from exosomes of adipose-derived stem cells in the formation of carotid atherosclerotic plaque
Introduction
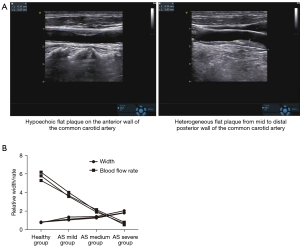
Atherosclerosis (AS) is a complex arterial disease characterized by inflammation and remodeling of the vascular wall that eventually forms atherosclerotic plaques (1). According to the site of plaque formation, clinical manifestations of AS can be coronary artery disease (CAD), carotid atherosclerosis (CAS), or peripheral artery disease (PAD) (2). The carotid artery is one of the specific sites for plaques to aggregate, especially at the opening and bifurcation of the common carotid artery, which may be related to the altered blood flow at these sites (3). CAS is a bellwether of systemic vascular AS, and its emergence indicates the greatly increased risk of AS in intracranial arteries, coronary arteries, and both lower limb arteries. CAS will lead to thickening of the vascular wall, narrowing of the vascular lumen, reduced blood flow, followed by intracranial ischemia, resulting in dizziness, blurred vision, tinnitus, nausea, and other symptoms. If the plaque is unstable and suddenly ruptures, it can be rushed off with blood flow, resulting in cerebral infarction after blocking the blood vessels of the brain, or blindness after blocking the blood vessels of the fundus oculi. In addition, AS can reflexively raise blood pressure after narrowing the carotid artery, resulting in hypertension or cause the blood pressure of patients with hypertension to be out of control (4-6). If CAS is not detected in a timely manner and effectively controlled and treated, there is a high risk of coronary heart disease and/or cerebral infarction, which can severely affect a patient’s morbidity and mortality. Figure 1 shows a Doppler ultrasound image of a carotid artery plaque.
Human studies on CAS have mainly focused on determining the expression of microRNAs (miRNAs) in the carotid atherosclerotic plaques obtained during carotid endarterectomy in patients with symptomatic stenosis (7,8). Indeed, the miRNAs expressed in the serum of patients with carotid plaques differ from that of patients with healthy carotid arteries (9).
Abnormal lipid metabolism and imbalances in cholesterol homeostasis can lead to AS, including CAS. There are many types of miRNAs related to lipid metabolism, including miR-27, miR-122, miR-370, miR-33, and miR-143. However, the role of the miR-26 family in AS has been less well-studied. miR-26 regulates lipid metabolism and cholesterol at the post-transcriptional level, primarily by affecting the expression of genes involved in lipid synthesis, transportation, and oxidation. And miR-26 affects progression and stability of carotid plaque, and participates in programmed cell death, which are closely related to carotid atherosclerotic plaque formation (10). Similar to most regulatory mechanisms in vivo, the regulatory role of miRNAs on lipid metabolism is a complex system (11). Target genes of multiple miRNAs not only play a regulatory role independently, but also overlap and interact with each other (12). Furthermore, miRNAs may represent potential diagnostic and therapeutic tools for the development of novel target drugs in clinical practice.
Proangiogenic factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and tumor necrosis factor α (TNF-α), have been shown to decrease miR-26a expression in endothelial cells (ECs). This in turn inhibits SMAD1 expression by binding the 3'-untranslated region (UTR), thereby decreasing inhibitor of DNA binding 1 (ID1) and increasing the expression of cell growth arrest proteins, p21 and p27, resulting in reduced EC growth and angiogenesis. Neutralizing miR-26a with locked nucleic acid anti-miR-26a (LNA-anti-miR-26a) can alleviate the miR-26a-mediated inhibition of bone morphogenetic (BMP)/SMAD1 signaling pathway and increase EC growth and angiogenesis.
miR-26 is known to be involved in programmed cell death, oxidative stress, transforming growth factor (TGF) signaling pathways, and importantly, in smooth muscle cell proliferation. Indeed, miR-26 is involved in regulating the phenotypic transformation of vascular smooth muscle cells through SMAD1 (Figure 2) and SMAD4 proteins (13). A study has shown that miRNA-26a participates in the feedback network to control cholesterol and lipid homeostasis, and targeting reverse cholesterol transcription may be a novel approach for the treatment of cardiovascular diseases (14). It has been suggested that miR-26 may affect the nuclear factor κB (NF-κB) inflammatory signaling pathway associated with the formation of atherosclerotic plaques. Whether miR-26 plays a positive role in carotid AS warrants further investigation. In addition, angiogenesis in atherosclerotic plaques and its role in plaque progression may cause serious complications, such as plaque rupture, hemorrhage, and apoptosis. miR-26 can reduce the production of inflammatory factors, as well as inhibit apoptosis and differentiation of cell (15).

Adipose-derived stem cells (ADSCs) are a class of stem cells with a variety of differentiation potential, and thus, have been widely used for the rehabilitation of various types of tissue damage. ADSCs can secrete many cytokines, proteins, growth factors, and extracellular vesicles that can be used in regenerative medicine with great potential. In addition, exosomes also carry and secrete miRNAs, such as miR-21, miR-24, and miR-26 (16). Recently, it was demonstrated that ADSCs can act through certain miRNAs secreted by the exosomes. Therefore, developing new therapeutic strategies involving ADSC secretions is of great interest globally.
In this study, ADSC lines expressing miR-26 were constructed to generate exosomes that overexpress miR-26 [exosomes of adipose-derived stem cells (ADSC-exos)]. The roles and mechanisms of miR-26 derived from ADSC-exos in the formation of carotid atherosclerotic plaques were examined using a mouse model of CAS. Furthermore, the therapeutic effects of exosomes expressing miR-26 on CAS were investigated. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4247/rc).
Methods
Study participants
A total of 60 patients who were newly diagnosed with CAS and 60 healthy subjects were enrolled in this study. Male or female patients aged 40–75 years, who underwent ultrasonic testing in the Electrophysiology Department of The Second Affiliated Hospital of Qiqihar Medical University from 2018 to 2019, and who did not present with transient ischemic attack, minor stroke, nor transient dizziness within 6 months were included. Ultrasonic testing showed that patients with CAS had intima-media thickness (IMT) ≥1.0 mm and healthy subjects had IMT <1.0 mm. The following exclusion criteria were applied: patients with non-atherosclerotic carotid stenosis; patients with a pacemaker, metal implants, or claustrophobia; patients with acute myocardial infarction (MI), acute coronary syndrome, or stroke within 4 weeks prior to presentation; patients with severe cerebral artery stenosis or atrial fibrillation; patients with systemic diseases, such as liver, kidney, blood system, or other malignant diseases; patients with a history of bilateral carotid endarterectomy or have immediate planning for carotid endarterectomy; and pregnant women and nursing mothers. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Second Affiliated Hospital of Qiqihar Medical University (No. 2021138) and informed consent was taken from all the patients.
Ultrasound diagnosis
All subjects underwent ultrasonography with a GE VIVID E80 color Doppler ultrasound diagnostic instrument to measure the carotid IMT. The examinations were conducted by an experienced operator. The patient was place in a position that fully exposed the neck, with the 2D probe at a frequency of 7.5 kHz and the blood flow beam <60 ℃. Plaques with an IMT ≥1.5 mm were defined as atherosclerotic plaques. The subjects were graded according to the severity of AS using the classification standards for CAS. Grade I was defined as lumen area stenosis ≤25%; grade II was defined as lumen area stenosis between 26% and 50%; grade III was defined as lumen area stenosis was between 51% and 75%; and grade IV was defined as lumen area stenosis between 76% and 100%. Grade I–II were considered mild disease, grade III was considered moderate and grade IV was considered severe disease.
Cell lines and cell culture
Human ADSC lines were purchased from the American Type Culture Collection (ATCC) Cell Bank (CAT#PCS-500-011). Cells were cultured in Minimum Essential Medium α (α-MEM; Gibco, CAT#12561056), supplemented with 10% fetal bovine serum (Gibco, CAT#10099141C) and antibacterial agent (100 U/mL penicillin, 100 µg/mL streptomycin, CAT#15140122), and maintained in an incubator containing 5% CO2 at 37 ℃.
RNA extraction and quantitative polymerase chain reaction (qPCR) amplification
Total RNA was extracted from ADSCs with TRIzol reagent (InvitrogenTM, CAT#15596018) and reverse-transcribed to cDNA using the TaqMan Advanced MicroRNA assay kit (Applied Biosystems, CAT#A28007). miR-26 was amplified with the Taqman MicroRNA amplification kit (Applied Biosystems, CAT#A25576) and GAPDH was employed as the internal control to calculate the relative expression of miR-26. All experiments were performed in triplicates. See Table 1 for primer sequences.
Table 1
| Name | Primer sequences |
|---|---|
| miR-26 | Reverse transcription primer: 5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCCTA-3' |
| Forward primer: 5'-ATGGCTTCAAGTAATCCAGGA-3' | |
| Reverse primer: 5'-GTGCAGGGTCCGAGGT-3' |
Establishment of the mouse model of CAS
ApoE knocked out mice (ApoE−/− C57BL/6; 6 weeks old, male, purchased from Cyagen Biosciences) were used as the model group and C57BL/6 mice (6 weeks old, male, purchased from Cyagen Biosciences) were used as the control group. There were 10 mice in each group. C57BL/6 mice in the control group were fed a general diet (Trophic Animal Feed High-tech Co., Ltd., China; CAT#LAD0011), and mice in the model group were fed a rich-fat diet containing 21% fat and 0.15% cholesterol (Trophic Animal Feed High-tech Co., Ltd., China; CAT#LAD0011). Carotid artery ligation was performed on mice after feeding for one week, and the lipid index levels of each group was tested at 12 weeks after feeding. Mice from the model group could be used only after satisfying the conditions of CAS. The inclusion criteria for AS in mice were carotid artery width IMT ≥1.5 mm and low-density lipoprotein cholesterol (LDL-C) ≥2.5 mmol/L. The exclusion criteria were carotid artery width IMT <1.5 mm and LDL-C <2.5 mmol/L. The animal experiments were approved by the Ethics board of Second Affiliated Hospital of Qiqihar Medical University (No. 2021138), in compliance with Second Affiliated Hospital of Qiqihar Medical University institutional guidelines for the care and use of animals.
Vector construct and retrovirus transfection
The 293T cell line (Chinese Academy of Sciences Cell Bank, CAT#GNHu17) was co-transfected with the lentivirus plasmids, pLVPTtTRKRAB-LNC-miR-26-NC or the pLVPTtTRKRAB-LNC-miR-26-promoterRNA (synthesized by RiBoBio), or the packaging plasmid. To prepare lentivirus particles, cells were cultured until 40–50% confluency. The medium was replaced with 10 mL of fresh DMEM medium (GIBCO, CAT#10566016) supplemented with 10% fetal bovine serum (Gibco, CAT#10099141C), without double antibodies. After 30 minutes, cells were transfected with the plasmids. After 8 h of transfection, the original medium was removed, and 7 mL of complete medium was added. After 24 h of transfection, about 3 mL of complete medium was supplemented. After 48 h of transfection, the supernatant containing the virus was carefully harvested and centrifuged at 1,000 g for 5 minutes. The supernatant was collected, filtered using a 0.2-pm filter, and frozen at −80 ℃ for later use.
Cell transfection
ADSCs were culture in 6-well plates for 12 h until cells reached 50% confluency. The original culture medium was then removed, and the cells washed with phosphate buffered saline (PBS). Approximately 1 mL of the virus solution was added to each well and the cells were incubated for 10 h. The virus medium was then aspirated and replaced with complete culture medium. The expression of the viral vectors reached a peak after 72 h of infection, and stable cell lines expressing miR-26 were obtained. The cells were harvested for further in vitro experiments.
Western blot analysis
Total protein was extracted from ADSCs and the protein concentration was measured using BCA solution (Enzyme-linked Biotechnology Co., Ltd., CAT#ml095490). After the protein concentration was determined, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed for each tissue/cell and transferred to polyvinylidene fluoride (PVDF) membranes (Enzyme-linked Biotechnology Co., Ltd., CAT#ml017798). The membranes were blocked with PBS Tween (PBST) containing 5% skim milk (Enzyme-linked Biotechnology Co., Ltd., CAT#ml095678) for 1 h at room temperature, followed by incubation with primary antibodies at 4 ℃ overnight, in accordance with the manufacturer’s instructions. The primary antibodies used were anti-human CD63 (LifeSpan, CAT#LS-C5463-100, RRID#AB_859461), anti-human CD9 (LifeSpan, CAT#LS-C46004-100, RRID#AB_1053357), anti-human CD81 (LifeSpan, CAT#LS-C5595-100, RRID#AB_859702), anti-human CD90 (Dianova, CAT#DIA-10, RRID#AB_2756409), anti-human TNF-α (Biolegend, CAT#502801), anti-human interleukin (IL)-6 (LifeSpan, CAT#LS-C7940-500, RRID#AB_864897), and anti-human IL-1β (Biolegend, CAT#508201). After 3 washes with PBST for 5 minutes each time, the membranes were incubated with the secondary horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Biolegend, CAT#405306) at room temperature for 1 h. Following another 3 washes in PBST, the protein bands were detected with enhanced chemiluminescence (ECL) chemiluminescence solution (Merck Millipore, CAT#WBKLS0010) according to the manufacturer’s instructions, and visualized with a gel chemiluminescence imager.
Exosomes extraction
ADSCs stably expressing miR-26 were harvested at 1×106 and centrifuged at 2,000 g for 10 minutes. The supernatant was collected and centrifuged at a high speed at 10,000 g for 30 minutes. The resultant supernatant was then centrifuged at 140,000 g for 90 minutes. The obtained precipitation containing the exosomes was washed with PBS buffer (Enzyme-linked Biotechnology Co., Ltd., CAT#WBKLS0010), resuspended, and centrifuged again at 140,000 g for 90 minutes. The pellet was then resuspended, precipitated with PBS buffer, and stored at 4 ℃ for later use.
Therapeutic effects of exosomes on the mouse model of CAS
ADSC-exos stably expressing miR-26 were injected into the tail vein (1×1010/mouse) of model mice at 200 µL/mouse. The plaque size and carotid width were measured with oil red O staining solution at 1 and 2 weeks post-injection. The levels of TNF-α, IL-6, and IL-1β in the plaques and serum of mice after exosome treatment were detected by Western blot, enzyme-linked immunosorbent assay (ELISA), and qPCR. The levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and LDL-C in the serum were detected by biochemical methods (17).
Statistical analysis
Statistical analyses were performed using the SPSS software. Pearson correlation was used for the pairwise correlation of the degree of AS sclerosis, carotid IMT, serum miR-26, and blood lipids (TC, TG, LDL-C, and HDL-C). An absolute value of correlation coefficient between 0.1 and 0.3 was considered as a weak correlation between variables, an absolute value of correlation coefficient between 0.3 and 0.5 was considered as a moderate correlation between variables, and an absolute value of correlation coefficient greater than 0.5 was considered as a strong correlation between variables. Differences between the two groups were analyzed with the t-test, and differences among multiple groups were analyzed by analysis of variance (ANOVA). A P value less than 0.05 was considered statistically significant and indicated as follows: *P<0.05, **P<0.01, and ***P<0.001. All experiments were replicated 3 times, and abnormal data outside the inclusion or exclusion criteria were excluded from data analysis.
Results
The degree of AS was positively correlated with the carotid IMT
The carotid IMT in subjects with different severity of AS was diagnosed by ultrasound. The results showed that the carotid IMT in patients with CAS was significantly higher than that in healthy subjects (Figure 3A). Pearson correlation analysis showed that carotid blood flow velocity was significantly negatively correlated with carotid IMT (r=−0.91; Figure 3B).
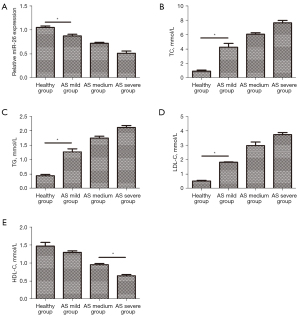
The degree of AS in patients was inversely associated with serum miR-26 levels
The levels of miR-26 in the serum of patients with and without AS were determined by qPCR. There was a negative correlation between the degree of sclerosis and serum miR-26 levels in AS patients (Figure 4A). Biochemical testing revealed that the degree of sclerosis in AS patients was positively associated with the levels of blood lipids, including TC, TG, and LDL-C (Figure 4B-4D), and negatively correlated with HDL-C levels (Figure 4E).
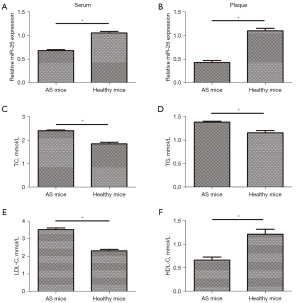
A mouse model of CAS showed decreased expression of miR-26 in tissues and serum, and abnormal blood lipid levels
ApoE−/− mice were used to establish a CAS model in which mice were fed a rich-fat diet. The miR-26 levels in plaques and serum of mice were examined by qPCR. The results showed that the miR-26 levels were significantly lower in the plaques and serum of model mice with AS compared to those in healthy mice (Figure 5A,5B). Biochemical testing revealed that the levels of blood lipids (TC, TG, and LDL-C) in model mice with AS were significantly higher than those in healthy mice (Figure 5C-5E). Furthermore, the HDL-C levels in model mice with AS were significantly lower than that in healthy mice (Figure 5F).
The levels of inflammatory factors significantly decreased in the supernatant of ADSCs overexpressing miR-26
ADSCs were stably transfected with the miR-26-promoter or the negative control, miR-NC. The miR-26 expression levels in the supernatant were significantly higher in cells overexpressing miR-26 compared to cells transfected with the vector control (Figure 6A). The levels of inflammatory factors, TNF-α, IL-6, and IL-1β, in the supernatant of ADSCs overexpressing miR-26 were significantly decreased compared to cells transfected with the negative control (Figure 6B-6D).

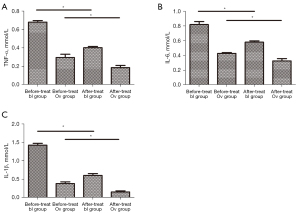
The NF-κB inhibitor synergistically enhanced the miR-26-mediated downregulation of inflammatory factors
ADSCs stably transfected with the miR-26-promoter or miR-NC were treated with an NF-κB inhibitor. Treatment with the NF-κB inhibitor significantly decreased the expression of TNF-α, IL-6, and IL-1β in both groups (Figure 7A-7C), suggesting that the NF-κB inhibitor enhanced the miR-26-mediated downregulation of inflammatory factors.
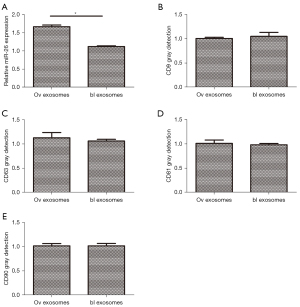
Identification of exosome markers
Exosomes were extracted from the stably transfected ADSCs and the expression of miR-26 was assessed. The miR-26 expression in the exosomes of ADSCs overexpressing miR-26 was significantly higher than that detected in the exosomes of the control ADSCs (Figure 8A). Western blot analyses demonstrated that there was no significant difference in the expression of CD63, CD9, CD89, nor CD90 between the 2 groups (Figure 8B-8E).
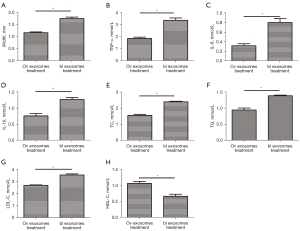
Treatment with exosomes improved the AS in mice
ADSC-exos overexpressing miR-26 were injected into the tail vein of the CAS mouse model. The plaque size, cross-sectional area, and area stenosis of the carotid intima were measured. The IMT in mice treated with miR-26-overexpressing exosomes was significantly decreased compared to that in control mice (Figure 9A). The levels of TNF-α, IL-6, and IL-1β in both the plaques and serum of exosome-treated mice were significantly decreased compared to that in control mice (Figure 9B-9D). Biochemical testing revealed that mice treated with the miR-26-overexpressing exosome had significantly lower levels of TC, TG, and LDL-C compared to the control group (Figure 9E-9G), and significantly elevated HDL-C levels (Figure 9H).
Discussion
The plaque in CAS is a common cause of carotid artery stenosis, that can lead to ischemic stroke. The etiology of CAS is multifactorial, with a complex pathogenesis, neither of which has been fully elucidated. The main risk factors include hypertension, hyperlipidemia, diabetes, obesity, smoking and drinking, genetic factors, high-salt diet, lack of exercise, nervous mental activity, irregular lifestyle, age, and gender. The formation of AS is complex, and is mainly caused by repeated and minor damages to the endarterium (endothelium) through various mechanisms (18-22). The current treatments for AS are mainly surgery and symptomatic drug treatment. Unfortunately, there is no rapid and effective specific drug for the treatment of AS. Drugs designed to regulate lipid metabolism at the molecular level may be a potential treatment modality for AS in the future (23).
A basic progression of AS is the aggregation of monocytes/macrophages on the vascular wall to produce proinflammatory cytokines. It has been reported that molecular-engineered exosomes derived from M2 macrophage can relieve inflammation by promoting the release of anti-inflammatory cytokines. Paeoniflorin can inhibit AS by significantly enhancing miR-223 expression in monocyte exosomes and inhibiting the STAT3 pathway. Exosomes loading heat shock protein 27 (HSP27) can significantly stimulate and activate NF-κB to release IL-10. The study suggests that exosomes may be used as a vector for anti-inflammatory therapy (24).
Mesenchymal stem cell-derived exosomes (MSC-exos) have gained much interest in the regeneration and rehabilitation of diseases such as ischemic stroke. Chen et al. showed in a rat model that ADSC-exos can enhance neural regeneration and significantly reduce infarcts after ischemic stroke (25). The administered dose of ASC-exos can reduce brain edema and contraction on the third day after the occurrence of acute ischemic stroke (26). It has been shown that ASC-exos overexpress miR-21, thereby promoting the vascularization of ECs. Interestingly, the increased platelet-derived growth factor (PDGF) levels alter the content of RNAs and proteins in the extracellular vesicles by changing the expression of anti-inflammatory and immunoregulatory factors. These changes can enhance the production of IL-10 and TGF-β1, and stimulate the formation of T cells, thereby protecting muscles from being affected by acute ischemia in vivo (27).
Exosomes have superior and irreplaceable biological functions, and therapeutic exosomes have a high potential for clinical applications. First, due to the protection of the phospholipid bilayer, exosomes can avoid phagocytosis and bypass lysosomes, thereby exhibiting a longer circulating half-life. Second, the phospholipid bilayer of exosomes also favors the fusion of recipient cells to the cell membrane. Third, exosomes from animals or patients have high homology and low immune response to avoid exosome degradation. Finally, exosomes with homing effect play a role in regulating the cell types produced by needle exosomes. The value of MSC-exo treatment in cardiac protection was highlighted by Piffoux et al., who showed that MSCs secrete the largest number of exosomes (24). Song et al. reported that local injection of exosomes can attenuate the apoptosis of cardiomyocytes and ECs in an animal model of preclinical MI (28). As additional substances in exosomes, miRNA can be selectively released from blood vessels or blood cells into the peripheral blood circulation and transferred to recipient cells. Unlike other extracellular vesicles, exosomes are thought to be released by living cells through an active process in response to environmental perturbations, thereby transferring miRNA to recipient cells, and playing a role in cell-to-cell communication. As natural drug delivery vectors, exosomes have good biocompatibility and targeting ability. Exosomes released from various cells contain potentially valuable biological information about the development and progression of CAS (29). There is increasing evidence that cell-derived exosomes modulate exosome-mediated intercellular crosstalk in AS (30). Based on their potential roles as biomarkers and pharmacological targets, cell-contained exosomes are considered for their use in novel drug delivery devices (31). EC-derived miR-92a exosomes regulate paracrine macrophage overexpression of KLF4 during AS. Furthermore, miR-92a exosomes play a key role in AS-susceptible endothelium by downregulating KLF2 and KLF4 (32).
CircRNAs have attracted attention as new diagnostic markers for diseases including cancer. CircRNAs constitute an abundant, stable, diverse and conserved family of RNA molecules. Emerging evidence suggests that circRNAs are involved in various biological processes, such as angiogenesis, proliferation, and differentiation (33). Recently, Zhang et al. demonstrated that crosstalk between circRNAs and their competing mRNAs may play a critical role in the development of AS by regulating cell adhesion, migration, and activation (34). Furthermore, exosomal circRNAs from peripheral circulating serum were associated with UA formation. Jiang et al. reported that peripheral circulating serum-exos could induce TNF-α and IL-6 production in macrophages via miRNA cargo (35). The miRNA-26 family, including miR-26a, miR-26b, miR-1297, and miR-4465, is a group of widely conserved miRNAs with identical seed region sequence (36). They play multiple key roles in regulating the growth, development, and activation of cells. Recent evidence supports the central role of the miR-26 family in cardiovascular diseases. Indeed miR-26 has been shown to controlled key signaling pathways, such as the BMP/SMAD1 signaling pathway (Figure 2), as well as key targets associated with EC growth, angiogenesis, and left ventricular (LV) function after MI. Recent study has suggested that miR-26 may play an important role in a range of cardiovascular rehabilitation mechanisms, as well as in antigen presenting cell (APC) differentiation and adipose tissue metabolism (37). Deletion of all miR-26 coding sites in mice resulted in rapid expansion of adipose tissues in adult animals fed with normal diet. In contrast, overexpression of miR-26a transgene protected mice from rich-fat diet-induced obesity, indicating that miR-26 plays an important role in lipid metabolism (38).
Furthermore, miR-26 can directly target ATP-binding cassette transporter A1 (ABCA1) and participate in feedforward or feedback networks to control cholesterol and lipid homeostasis. ABCA1 is a key regulator of cellular cholesterol efflux and HDL maturation. ABCA1 mRNA has an abnormally long 3'-UTR, which makes it very vulnerable to targeting and inhibition by miRNAs. Indeed, multiple miRNAs have been reported to directly target ABCA1, including miR-33a/b, miR-26, miR-106b, and miR-758 (39).
CAS is closely linked to blood lipid indicators, such as serum levels of TC, TG, LDL-C, and HDL-C. The accumulation of LDL-C in the endarterium is a key step in the initiation and development of AS. Recent study showed that the maximum LDL-C concentration was detected directly downstream of carotid stenosis, which supports the observation that the existing atherosclerotic lesions tend to develop downstream of the stenosis (40). A dependence between potential blood flow patterns and LDL-C concentration uptake on arterial wall was detected using mathematical and computational models (41).
Study has shown that antibodies targeting IL-1β combined with canakinumab can reduce the risk of CAS recurrence, suggesting that anti-inflammatory strategies may have a great promise (42). Since inflammation and metabolic changes are closely linked in immune cells and ECs, therapeutic targets that change the cellular metabolism of activated cells may have great potential in the treatment of patients with cardiovascular diseases (43).
In this current investigation, the levels of miR-26 in the serum of patients with CAS were explored. Decreased miR-26 levels had a negative effect on blood lipids, inflammatory factors, and other related indicators in patients with CAS. In vivo animal experimental data demonstrated that miR-26 derived from ASC-exos positively regulated the secretion of inflammatory factors and the levels of TC, TG, HDL-C, and LDL-C in the serum of mice in the carotid AS model. Herein, miR-26 levels were shown to be negatively correlated with blood lipids and inflammatory factors in the serum, and exosomes expressing miR-26 had a positive therapeutic effect on carotid atherosclerotic vascular rehabilitation, suggesting that miR-26 may be a novel target for the treatment of patients with CAS.
AS and CAS are risk factors for the development of stroke. Studies using endarterectomy and plasma/urine samples to reveal biomarkers of instability of plaques or severity of diseases clearly suggest that miRNA can serve not only as a potential diagnostic target, but also as a therapeutic target in CAS (44-46). Further studies on larger cohorts and patients involving various comorbidities and using animal models of instability of plaques are needed to reveal the true potential of miRNAs in CAS and provide evidence for future clinical practice. In conclusion, miRNAs derived from ADSC-exos may have potential therapeutic and diagnostic benefits in CAS.
The severity of CAS in patients was negatively correlated with serum miR-26 levels. The levels of inflammatory factors significantly decreased in the supernatant of ADSCs overexpressing miR-26. The NF-κB inhibitors collaboratively enhanced the miR-26-mediated downregulation of inflammatory factors. After treating the mice in the CAS model with ADSC-exos loaded with miR-26, the CAS was improved, as shown by the significantly decreased levels of inflammatory factors and the return of blood lipids to acceptable values, indicating that miR-26 may have an active role in the treatment of CAS.
Acknowledgments
Funding: This study is funded by the basic scientific research funds of Heilongjiang provincial colleges and universities (No. 2021-KYYWF-0366).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4247/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4247/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4247/coif). All authors report that this study is funded by the basic scientific research funds of Heilongjiang provincial colleges and universities (No. 2021-KYYWF-0366). The authors have no other conflicts of interest to declare.
(English Language: A. Kassem)
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Second Affiliated Hospital of Qiqihar Medical University (No. 2021138) and informed consent was taken from all the patients. The animal experiments were approved by the Ethics board of Second Affiliated Hospital of Qiqihar Medical University (No. 2021138), in compliance with Second Affiliated Hospital of Qiqihar Medical University institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peng Y, Ao M, Dong B, et al. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des Devel Ther 2021;15:4503-25. [Crossref] [PubMed]
- Topaz G, Yehezkel E, Benchetrit S, et al. Non-coronary cardiac calcifications and outcomes in patients with heart failure. J Cardiol 2021;77:83-7. [Crossref] [PubMed]
- Kumar S, Andueza A, Kim J, et al. Isolation of Endothelial Cells from the Lumen of Mouse Carotid Arteries for Single-cell Multi-omics Experiments. J Vis Exp 2021; [Crossref] [PubMed]
- Zhang J, Liu Y, Deng Y, et al. Non-invasive Global and Regional Myocardial Work Predicts High-Risk Stable Coronary Artery Disease Patients With Normal Segmental Wall Motion and Left Ventricular Function. Front Cardiovasc Med 2021;8:711547. [Crossref] [PubMed]
- Bays HE, Baum SJ, Brinton EA, et al. Effect of bempedoic acid plus ezetimibe fixed-dose combination vs ezetimibe or placebo on low-density lipoprotein cholesterol in patients with type 2 diabetes and hypercholesterolemia not treated with statins. Am J Prev Cardiol 2021;8:100278. [Crossref] [PubMed]
- Garner M, Yilmaz U, Behnke S. Spontaneous craniocervical dissection. Radiologe 2021;61:729-35. [Crossref] [PubMed]
- Sardu C, Modugno P, Castellano G, et al. Atherosclerotic Plaque Fissuration and Clinical Outcomes in Pre-Diabetics vs. Normoglycemics Patients Affected by Asymptomatic Significant Carotid Artery Stenosis at 2 Years of Follow-Up: Role of microRNAs Modulation: The ATIMIR Study. Biomedicines 2021;9:401. [Crossref] [PubMed]
- Telkoparan-Akillilar P, Cevik D. Identification of miR-17, miR-21, miR-27a, miR-106b and miR-222 as endoplasmic reticulum stress-related potential biomarkers in circulation of patients with atherosclerosis. Mol Biol Rep 2021;48:3503-13. [Crossref] [PubMed]
- Trusinskis K, Lapsovs M, Paeglite S, et al. Plasma circulating microRNAs in patients with stable coronary artery disease - Impact of different cardiovascular risk profiles and glomerular filtration rates. J Clin Transl Res 2021;7:270-6. [PubMed]
- Wu H, Feng K, Zhang C, et al. Metformin attenuates atherosclerosis and plaque vulnerability by upregulating KLF2-mediated autophagy in apoE-/- mice. Biochem Biophys Res Commun 2021;557:334-41. [Crossref] [PubMed]
- Volný O, Kašičková L, Coufalová D, et al. microRNAs in Cerebrovascular Disease. Adv Exp Med Biol 2015;888:155-95. [Crossref] [PubMed]
- Chen W, Li L, Wang J, et al. The ABCA1-efferocytosis axis: A new strategy to protect against atherosclerosis. Clin Chim Acta 2021;518:1-8. [Crossref] [PubMed]
- Zhang W, Wang Q, Xing X, et al. The antagonistic effects and mechanisms of microRNA-26a action in hypertensive vascular remodelling. Br J Pharmacol 2021;178:1037-54. [Crossref] [PubMed]
- Aryal B, Singh AK, Rotllan N, et al. MicroRNAs and lipid metabolism. Curr Opin Lipidol 2017;28:273-80. [Crossref] [PubMed]
- Gao W, Liu H, Yuan J, et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell Mol Med 2016;20:2318-27. [Crossref] [PubMed]
- Trzyna A, Banaś-Ząbczyk A. Adipose-Derived Stem Cells Secretome and Its Potential Application in "Stem Cell-Free Therapy". Biomolecules 2021;11:878. [Crossref] [PubMed]
- Ma X, Wang Y, Shi Y, et al. Effect of Xiaoji Recipe on stability of carotid artery vulnerable plaque in elderly patients with type 2 diabetes mellitus and type H hypertension. Zhongguo Zhong Yao Za Zhi 2020;45:4246-53. [PubMed]
- Katakami N, Mita T, Yoshii H, et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol 2020;19:110. [Crossref] [PubMed]
- Ramuš SM, Petrovič D. Genetic Variations and Subclinical Markers of Carotid Atherosclerosis in Patients with Type 2 Diabetes Mellitus. Curr Vasc Pharmacol 2019;17:16-24. [Crossref] [PubMed]
- Seo DH, Lee YH, Suh YJ, et al. Low muscle mass is associated with carotid atherosclerosis in patients with type 2 diabetes. Atherosclerosis 2020;305:19-25. [Crossref] [PubMed]
- Haberka M, Skilton M, Biedroń M, et al. Obesity, visceral adiposity and carotid atherosclerosis. J Diabetes Complications 2019;33:302-6. [Crossref] [PubMed]
- Amarenco P, Hobeanu C, Labreuche J, et al. Carotid Atherosclerosis Evolution When Targeting a Low-Density Lipoprotein Cholesterol Concentration <70 mg/dL After an Ischemic Stroke of Atherosclerotic Origin. Circulation 2020;142:748-57. [Crossref] [PubMed]
- Wu G, Zhang J, Zhao Q, et al. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew Chem Int Ed Engl 2020;59:4068-74. [Crossref] [PubMed]
- Piffoux M, Volatron J, Silva AKA, et al. Thinking Quantitatively of RNA-Based Information Transfer via Extracellular Vesicles: Lessons to Learn for the Design of RNA-Loaded EVs. Pharmaceutics 2021;13:1931. [Crossref] [PubMed]
- Chen KH, Chen CH, Wallace CG, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016;7:74537-56. [Crossref] [PubMed]
- An Y, Zhao J, Nie F, et al. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci Rep 2019;9:12861. [Crossref] [PubMed]
- Liu Y, Li C, Wu H, et al. Paeonol Attenuated Inflammatory Response of Endothelial Cells via Stimulating Monocytes-Derived Exosomal MicroRNA-223. Front Pharmacol 2018;9:1105. [Crossref] [PubMed]
- Song Y, Zhang C, Zhang J, et al. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 2019;9:2346-60. [Crossref] [PubMed]
- Boulanger CM, Loyer X, Rautou PE, et al. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017;14:259-72. [Crossref] [PubMed]
- Wang Y, Xie Y, Zhang A, et al. Exosomes: An emerging factor in atherosclerosis. Biomed Pharmacother 2019;115:108951. [Crossref] [PubMed]
- Yin M, Loyer X, Boulanger CM. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol 2015;763:90-103. [Crossref] [PubMed]
- Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol 2012;32:979-87. [Crossref] [PubMed]
- Pan RY, Zhao CH, Yuan JX, et al. Circular RNA profile in coronary artery disease. Am J Transl Res 2019;11:7115-25. [PubMed]
- Zhang F, Zhang R, Zhang X, et al. Comprehensive analysis of circRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of atherosclerosis in rabbits. Aging (Albany NY) 2018;10:2266-83. [Crossref] [PubMed]
- Jiang K, Yang J, Guo S, et al. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol Ther 2019;27:1758-71. [Crossref] [PubMed]
- Li C, Li Y, Lu Y, et al. miR-26 family and its target genes in tumorigenesis and development. Crit Rev Oncol Hematol 2021;157:103124. [Crossref] [PubMed]
- Zeitels LR, Acharya A, Shi G, et al. Tumor suppression by miR-26 overrides potential oncogenic activity in intestinal tumorigenesis. Genes Dev 2014;28:2585-90. [Crossref] [PubMed]
- Acharya A, Berry DC, Zhang H, et al. miR-26 suppresses adipocyte progenitor differentiation and fat production by targeting Fbxl19. Genes Dev 2019;33:1367-80. [Crossref] [PubMed]
- Wu X, Cheng B, Guo X, et al. PPARα/γ signaling pathways are involved in Chlamydia pneumoniae-induced foam cell formation via upregulation of SR-A1 and ACAT1 and downregulation of ABCA1/G1. Microb Pathog 2021;161:105284. [Crossref] [PubMed]
- Yi L, Tang J, Shi C, et al. Pentraxin 3, TNF-α, and LDL-C Are Associated With Carotid Artery Stenosis in Patients With Ischemic Stroke. Front Neurol 2019;10:1365. [Crossref] [PubMed]
- Spanos K, Petrocheilou G, Karathanos C, et al. Carotid Bifurcation Geometry and Atherosclerosis. Angiology 2017;68:757-64. [Crossref] [PubMed]
- Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol 2021;18:2114-27. [Crossref] [PubMed]
- Ali L, Schnitzler JG, Kroon J. Metabolism: The road to inflammation and atherosclerosis. Curr Opin Lipidol 2018;29:474-80. [Crossref] [PubMed]
- Huang P, He XY, Xu M. The Role of miRNA-146a and Proinflammatory Cytokines in Carotid Atherosclerosis. Biomed Res Int 2020;2020:6657734. [Crossref] [PubMed]
- Maitrias P, Metzinger-Le Meuth V, Nader J, et al. The Involvement of miRNA in Carotid-Related Stroke. Arterioscler Thromb Vasc Biol 2017;37:1608-17. [Crossref] [PubMed]
- Wu Y, Zhang F, Lu R, et al. Functional lncRNA-miRNA-mRNA networks in rabbit carotid atherosclerosis. Aging (Albany NY) 2020;12:2798-813. [Crossref] [PubMed]



