
Megestrol acetate dispersible tablets with a 5-HT3 receptor antagonist and dexamethasone vs. 5-HT3 receptor antagonist plus dexamethasone, can better control chemotherapy-induced nausea and vomiting: a randomized controlled study
Introduction
Despite the ongoing advancements in treatment approaches and the discovery of novel drugs for cancer, chemotherapy continues to be the cornerstone of cancer treatment. Chemotherapy-induced nausea and vomiting (CINV) is a common side effect during chemotherapy protocols, which not only causes discomfort to patients and affects their quality of life (QOL) but also reduces the compliance of patients to the treatment, leading to delays or early termination of the anticancer treatment (1,2). Previous reports have revealed that 70% of patients experience CINV in cases where no preventive antiemetic drugs were given during the chemotherapy protocols (3). Importantly, CINV can also lead to adverse events, such as electrolyte imbalance, dehydration, anxiety, decreased physical condition score, and malnutrition (4-9). In addition to these clinical consequences, the need for extra rescue drugs for CINV and the subsequently extended length of hospital stay represent an increase in total medical costs. Thus, CINV represents a considerable challenge for both doctors and patients (10,11).
Cisplatin-containing chemotherapy is the standard treatment for lung, esophageal, breast, and cervical cancers, as well as other malignant tumors, and is classified as a highly emetogenic chemotherapy (HEC) (12). Although the triple regimen, which is recommended by the international antiemetic guidelines, reduces the incidence of HEC-induced acute CINV by more than 80%, there are still at least 30% of patients who do not achieve complete remission and experience delayed CINV (13-15). In clinical practice, since neurokinin-1 (NK-1) receptor antagonists are expensive and have not been included in the scope of medical insurance, they cannot be reasonably used in hospitals in middle- and low-income areas. Therefore, exploring cost-effective drugs to prevent HEC-induced delayed CINV is crucial.
Megestrol belongs to the 17α-hydroxyprogesterone derivative and is a highly effective synthetic progesterone. Recorded in the instructions may improve appetite and cachexia in patients with advanced tumors. In recent years, several studies have reported that megestrol acetate dispersible tablets combined with chemotherapy can improve CINV (16,17). In order to observe the efficacy of olanzapine combined with megestrol acetate in the treatment of advanced cancer anorexia, Zhao et al. (18) randomly divided 85 patients with cancer anorexia into the treatment group (olanzapine combined with megestrol acetate group + nutritional support), the control group (megestrol acetate group + nutritional support) and the simple nutritional support group, and observed the changes of appetite, weight, Karst score and immune function before and after treatment in each group, and evaluated adverse reactions. Li et al. (19) shows that megestrol acetate can significantly improve the appetite, body mass and Karnofsky score of patients with malignant tumor after radiotherapy, and there is no obvious adverse reaction. At present, it is considered to be an effective and safe drug for the treatment of anorexia of advanced cancer. As an oral contraceptive, megestrol can be used as a long-term oral drug for women of childbearing age, and its safety has been widely recognized. However, to date, the clinical potential of megestrol has only been assessed by retrospective studies with small sample sizes; randomized controlled prospective studies are needed. Therefore, a prospective, randomized controlled phase II clinical trial was designed to determine whether the combination of megestrol and a 5-HT3 receptor antagonist plus dexamethasone based on the standard regimen can effectively control HEC-induced CINV. We present the following article in accordance with the CONSORT reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4809/rc).
Methods
Study design and participants
This study was a prospective, randomized, controlled phase II clinical study. Patients with malignant tumors who were treated with cisplatin-containing chemotherapy in our hospital from September 2018 to December 2019 were enrolled. We used PASS operation to calculate the sample size. This study was approved by the Ethics Committee of Henan Cancer Hospital (ethics batch No. 2017098). Written informed consent was obtained from each participant. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Treatment plan
A total of 120 patients were randomly divided into megestrol (n=60) and control (n=60) groups. This is a two-parallel study and the allocation ratio is 1:1. In the megestrol group, an antiemetic regimen of megestrol acetate dispersible tablets with a 5-HT3 receptor antagonist and dexamethasone was administered, whereas participants in the control group received a 5-HT3 receptor antagonist plus dexamethasone. The specific drug dosages were as follows: (I) megestrol acetate dispersible tablets: 160 mg orally every morning from the day of chemotherapy; (II) palonosetron (5-HT3 receptor antagonist): 2.5 mg intravenously 30 min before chemotherapy on the first day; and (III) dexamethasone: 12 mg intravenously 30 min before chemotherapy on the first day, 8 mg intravenously 30 min before chemotherapy on the second to the fourth day, once a day. Megestrol will be taken orally daily during the treatment of the first chemotherapy until it lasts for ten days. If the patients experienced nausea and vomiting, additional rescue drugs (such as dopamine receptor antagonists, sedatives, or psychotropic drugs) were allowed after assessment and at the discretion of the attending clinician. Subject diary cards were issued on the first day of chemotherapy, where the frequency, time, and degree of nausea and vomiting were recorded every 24 h. The research physician collected statistics based on the diary cards filled in by the patients and reviewed the relevant data and information of each patient. All participants were followed up without data loss.
Evaluation of the antiemetic effect
The therapeutic effect was evaluated according to the World Health Organization standards and the recommended standards of the Fifth European Conference on Clinical Oncology and the Common Terminology Criteria for Adverse Events (CTCAE) 4.0 standards. Complete response (CR) was described as no incidence of vomiting and no requirement for antiemetic medicine, and the complete response rate was calculated by the number of complete response cases/total number of cases ratio. Complete protection (CP) was defined as the absence of nausea and vomiting, and the complete prevention rate was calculated by the number of complete prevention cases/total number of cases ratio.
The primary end point was the proportion of nausea and vomiting controlled by the two antiemetic regimens in the delayed period (24–120 hours after the start of chemotherapy), that is, the proportion of complete remission (no vomiting, no rescue treatment) and complete prevention (no nausea and vomiting). The secondary endpoint of the trial was the control ratio of nausea and vomiting in the two groups of antiemetic regimens in the acute (0–24 h) and overall (0–120 h) phases. The proportion of patients with grade 3–4 vomiting in the two groups during chemotherapy was also evaluated. The related adverse reactions of the antiemetic drugs were assessed according to the impact of the two antiemetic protocols on the QOL of the patients before and after treatment.
QOL assessment
The QOL of the patients was evaluated one day before chemotherapy and seven days after chemotherapy according to the QOL scale for Chinese cancer patients established in 1990. According to the QOL score, the appetite, spirit, sleep, fatigue, and pain symptoms of the patient were evaluated, along with information on social understanding and cooperation, the patient’s understanding of cancer, treatment attitude, daily life, adverse reactions, and facial expression. Each item was scored 1–5 points, up to a total score of 60 points, with the overall QOL being classified as good (51–60 points), satisfactory (41–50 points), general (31–40 points), poor (21–30 points), and very bad (<20 points).
Assessment of adverse reactions
All adverse reactions during chemotherapy were assessed according to the CTCAE 4.03 evaluation standard issued by the National Cancer Institute and divided into five grades according to severity. The common adverse reactions of megestrol include vaginal bleeding, peripheral edema, thrombosis, and dyspnea, but according to the drug instructions, they are occasional or rare. In order to ensure the safety of the subjects, the subjects have been informed of the corresponding risks and precautions, and provided with oxygen, hemostatic drugs, and other rescue measures. If necessary, they can be transferred to the intensive care unit for follow-up treatment immediately.
Statistical analysis
Patients were assigned to the megestrol or control groups by computer-generated random sequence. SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The measurement data were expressed by the mean ± standard error of the mean. The t-test was used for comparison between groups and the χ2 test was used to compare the numerical data between groups. Statistical significance was set at P<0.05.
Results
Case data and clinical characteristics of the two groups
A total of 133 patients with a malignant tumor, diagnosed by histopathology with at least one measurable lesion, who received cisplatin-based chemotherapy in the Department of Oncology of our hospital from September 2018 to December 2019, were recruited. Among them, 13 patients were excluded during the screening period, and a total of 120 patients were finally enrolled (Figure 1). The patients were randomly divided into megestrol and control groups, with 60 patients in each group, based on whether or not their chemotherapy drugs were combined with megestrol acetate dispersible tablets, and whether their compliance and tolerance were good. The clinical and demographic data of the two groups were similar (P>0.05) (Table 1).
Table 1
| Features | Megestrol group (n=60) | Control group (n=60) | Total number (n=120) | P | χ2 |
|---|---|---|---|---|---|
| Chemotherapy regimen | |||||
| Paclitaxel + cisplatin | 21 (35.0) | 24 (40.0) | 46 (38.3) | 0.527 | 0.320 |
| Docetaxel + cisplatin | 15 (25.0) | 15 (25.0) | 29 (24.1) | 1.000 | 0.000 |
| Etoposide + cisplatin | 5 (8.3) | 10 (16.7) | 15 (12.5) | 0.168 | 1.905 |
| Pemetrexed + cisplatin | 3 (5.0) | 1 (1.7) | 4 (3.3) | 0.309 | 1.034 |
| Fluorouracil + cisplatin | 4 (6.7) | 1 (1.7) | 5 (4.1) | 0.171 | 1.878 |
| Vinorelbine + cisplatin | 1 (1.7) | 0 (0) | 1 (0.8) | 0.315 | 1.008 |
| Irinotecan + cisplatin | 2 (3.3) | 0 (0) | 2 (1.7) | 0.154 | 2.034 |
| Gemcitabine + cisplatin | 8 (13.3) | 8 (13.3) | 16 (13.3) | 1.000 | 0.000 |
| Cisplatin | 1 (1.7) | 1 (1.7) | 2 (1.7) | 1.000 | 0.000 |
| Median age (range) | 54 [26–70] | 61 [25–70] | 56 [25–70] | 0.628 | 0.234 |
| Sex | |||||
| Male | 35 (58.0) | 45 (75.0) | 80 (66.7) | ||
| Female | 25 (42.0) | 12 (25.0) | 40 (33.3) | ||
| ECOG PS* | |||||
| 0 | 14 (23.3) | 13 (21.7) | 27 (22.5) | ||
| 1 | 46 (76.7) | 47 (78.3) | 93 (77.5) | ||
| TNM stage | |||||
| I-III | 30 (50.0) | 33 (55.0) | 63 (52.5) | ||
| IV | 30 (50.0) | 27 (45.0) | 57 (47.5) | ||
| Tumor site | |||||
| Gastric cancer | 11 (18.3) | 19 (31.7) | 30 (25.0) | 0.092 | 2.844 |
| Esophageal cancer | 18 (30.0) | 17 (28.3) | 25 (20.8) | 0.841 | 0.040 |
| Lung cancer | 6 (10.0) | 9 (15.0) | 15 (12.5) | 0.408 | 0.686 |
| Cervical cancer | 2 (3.3) | 3 (5.0) | 5 (4.2) | 0.648 | 0.209 |
| Breast cancer | 5 (8.3) | 2 (3.3) | 7 (5.8) | 0.243 | 1.365 |
| Ovarian cancer | 4 (6.7) | 1 (1.7) | 5 (4.2) | 0.171 | 1.878 |
| Other | 14 (23.3) | 9 (15.0) | 23 (19.2) | 0.246 | 1.345 |
| Risk factors | 8 (13.3) | 11 (18.3) | 19 (15.8) | 0.453 | 0.563 |
| History of previous surgery | 26 (43.3) | 22 (36.7) | 48 (40.0) | 0.456 | 0.556 |
| Previous use history of cisplatin | 32 (53.3) | 28 (46.7) | 60 (50.0) | 0.465 | 0.533 |
| Previous radiotherapy history | 3 (5.0) | 4 (6.7) | 7 (5.8) | 0.697 | 0.152 |
| History of previous chemotherapy | 19 (31.7) | 20 (33.3) | 39 (32.5) | 0.845 | 0.038 |
*, ECOG PS was measured on a 5-point scale, with 0 representing asymptomatic cases. The higher the score, the less suitable for chemotherapy. ECOG PS, European cooperative cancer team physical condition score; TNM, tumor stage.
Comparison of the control of nausea between the two antiemetic protocols in each period
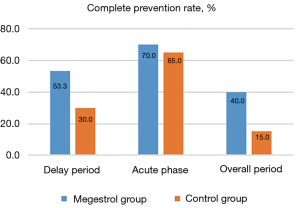
Both groups of patients successfully completed one cycle of treatment. In the delayed phase, 31 patients in the megestrol group and 18 patients in the control group reported complete prevention of nausea. The complete prevention rates in the delayed period of the megestrol and control groups were of 53.3% vs. 30.0%, respectively [risk ratio (RR): 1.751, 95% confidence interval (CI): 1.110–2.764; P=0.012]. Hence, the difference in the primary endpoint indicator between the two groups was statistically significant.
In the overall period, 24 patients in the megestrol group and nine patients in the control group achieved complete prevention. The complete prevention rates of the megestrol and control groups were of 40.0% vs. 15.0%, respectively (RR: 2.667; 95% CI: 1.355–5.250; P=0.002). Thus, a statistically significant difference in the nausea control efficacy in the overall period was noted between the groups, with the megestrol protocol showing enhanced efficacy.
In the acute phase, nausea was completely prevented in 47 and 39 patients in the megestrol and control groups, respectively, achieving complete prevention rates of 70.0% vs. 65.0% (RR: 1.077; 95% CI: 0.840–1.381; P=0.559), respectively. There was no marked difference in the nausea control effect between the two groups in the acute phase (Table 2 and Figure 2).
Table 2
| Study endpoint | Megestrol group (n=60), n (%) | Control group (n=60), n (%) | RR (95% CI) | χ2 | P |
|---|---|---|---|---|---|
| Delay period (24–120 h) | 31/60 (53.3) | 18/60 (30.0) | 1.751 (1.110–2.764) | 6.241 | 0.012 |
| Acute phase (0–24 h) | 42/60 (70.0) | 39/60 (65.0) | 1.077 (0.840–1.381) | 0.342 | 0.559 |
| Overall period | 24/60 (40.0) | 9/60 (15.0) | 2.667 (1.355–5.250) | 9.404 | 0.002 |
P value was calculated by using χ2 to test the nausea free ratio (CP). CP, complete prevention rate; RR, risk ratio.
Comparison of the control of vomiting between the two antiemetic protocols in each period
Comparing the control of vomiting, 46 patients in the megestrol group and 31 patients in the control group achieved complete remission, with complete remission rates of 76.7% vs. 51.7% (RR: 1.586; 95% CI: 1.179–2.134; P=0.001), respectively.
In the overall phase, 41 patients in the megestrol group and 28 patients in the control group achieved complete remission, with complete remission rates of 68.3% vs. 46.6% (RR: 1.464; 95% CI: 1.063–2.018; P=0.016), respectively. Statistically significant differences in the control of vomiting between the two groups in the overall phase were noted, with the megestrol protocol displaying enhanced efficacy.
In the acute phase, 49 patients in the megestrol group and 47 patients in the control group achieved the endpoint of complete remission. The complete remission rates of the two groups were 81.7% vs. 78.3% (RR: 1.043; 95% CI: 0.872–1.247; P=0.648), respectively, with no notable difference between them.
During chemotherapy, the incidence of grade 3–4 vomiting in the megestrol and control groups was 0% (0/60) and 10% (6/60), respectively, and this difference was statistically significant (RR: 1.107; 95% CI: 1.021–1.210; P=0.013). Fewer patients in the megestrol group required the use of rescue drugs compared with the control group (6.7% vs. 35.0%), and this difference was statistically significant (P<0.001) (Table 3 and Figure 3).
Table 3
| Study endpoint | Megestrol group (n=60), n (%) | Control group (n=60), n (%) | RR (95% CI) | χ2 | P |
|---|---|---|---|---|---|
| Delay period (24–120 h) | 46/60 (76.7) | 31/60 (51.7) | 1.586 (1.179–2.134) | 10.276 | 0.001 |
| Acute phase (0–24 h) | 49/60 (81.7) | 47/60 (78.3) | 1.043 (0.872–1.247) | 0.208 | 0.648 |
| Overall period (0–120 h) | 41/60 (68.3) | 28/60 (46.6) | 1.464 (1.063–2.018) | 5.763 | 0.016 |
| Proportion of grade, 3–4 vomiting | 0/60 (0) | 6/60 (10.0) | 1.107 (1.021–1.210) | 6.107 | 0.013 |
P value was calculated by χ2 to test the rate of no vomiting (CR). CI, confidence interval; CR, complete remission rate; RR, risk ratio.

Comparison of adverse reactions between the two antiemetic protocols
During the treatment, the two groups of patients exhibited different degrees of adverse reactions. Among them, the main adverse reactions associated with the antiemetic drugs were fatigue, constipation, hiccups, and vaginal bleeding. In the megestrol group, seven patients reported fatigue (11.7%), 11 had constipation (18.3%), one had hiccups (1.7%), and three had vaginal bleeding (5%); meanwhile, in the control group, three patients reported fatigue (5.0%), five had constipation (8.3%), two had hiccups (3.3%), and no patient reported vaginal bleeding (0%). There was no significant difference in the incidence of adverse effects between the two groups (P>0.05), and the above adverse reactions were mild (I and II degrees) in severity and well-tolerated. Other adverse reactions, such as myelosuppression, peripheral neurotoxicity, and abnormal liver and kidney function, were considered to be chemotherapy drug-related side effects, most of which were of grades I and II; grades III and IV events were relatively less common.
Symptomatic treatment could ameliorate these manifestations, without affecting the chemotherapy. Among them, the proportion of leukopenia in the megestrol and control groups was 13.3% (eight cases) and 28.3% (17 cases), respectively (P=0.043). One case of grade III leukopenia was reported in each group (1.7% vs. 1.7%; P=1.000), and one case of grade III thrombocytopenia was reported in the control group (1.7% vs. 0%; P=0.315). There was no marked difference in the frequency of the other adverse reactions (P>0.05). Neither group of patients discontinued chemotherapy due to serious adverse reactions (Table 4).
Table 4
| Symptoms | Megestrol group (n=60), n (%) | Control group (n=60), n (%) | χ2 | P* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | ||||
| Fatigue | 7 (11.7) | 0 (0) | 0 (0) | 3 (3.5) | 0 (0) | 0 (0) | 1.745 | 0.186 | |
| Constipation | 10 (16.7) | 1 (1.7) | 0 (0) | 5 (8.3) | 0 (0) | 0 (0) | 2.596 | 0.107 | |
| Hiccup | 1 (1.7) | 0 (0) | 0 (0) | 2 (3.3) | 0 (0) | 0 (0) | 0.342 | 0.559 | |
| Vaginal bleeding | 3 (5.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3.077 | 0.079 | |
| Pain | 5 (8.3) | 0 (0) | 0 (0) | 3 (5.0) | 3 (5.0) | 0 (0) | 0.100 | 0.752 | |
| Abnormal liver function | 9 (15.0) | 2 (3.3) | 0 (0) | 19 (31.7) | 1 (1.7) | 0 (0) | 3.523 | 0.061 | |
| Abnormal renal function | 4 (6.7) | 0 (0) | 0 (0) | 4 (6.7) | 0 (0) | 0 (0) | 0.000 | 1.000 | |
| Hypokalemia | 3 (5.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3.077 | 0.079 | |
| Hyperkalemia | 1 (1.7) | 0 (0) | 0 (0) | 2 (3.3) | 0 (0) | 0 (0) | 0.342 | 0.559 | |
| Leukocyte elevation | 4 (6.7) | 0 (0) | 0 (0) | 4 (6.7) | 0 (0) | 0 (0) | 0.000 | 1.000 | |
| Leukopenia | 3 (5.0) | 5 (8.3) | 0 (0) | 10 (16.7) | 6 (10.0) | 1 (1.7) | 4.093 | 0.043 | |
| Thrombocytopenia | 1 (1.7) | 1 (1.7) | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0.000 | 1.000 | |
| Anemia | 11 (18.3) | 9 (15.0) | 1 (1.7) | 5 (8.3) | 7 (11.7) | 1 (1.7) | 2.627 | 0.105 | |
*, the P value was tested using the Pearson c2 test.
Comparison of the QOL between the two groups of patients
One day before treatment, the QOL scores of the megestrol and control groups were 56.17±1.1 and 56.18±0.9 (P>0.05), respectively, and after treatment were 56.68±1.1 and 55.55±1.2, respectively, which was significantly higher in the megestrol group (P<0.05), as shown in Table 5.
Table 5
| Period | Megestrol group (mean ± SD) | Control group (mean ± SD) | t | P* |
|---|---|---|---|---|
| Before treatment | 56.17±1.1 | 56.18±0.9 | 0.100 | 0.920 |
| After treatment | 56.68±1.1 | 55.55±1.2 | 5.721 | 0.000 |
| t | 4.305 | 5.333 | ||
| P | 0.000 | 0.000 |
*, t test was used to calculate the P value.
Discussion
The three-drug combination regimen of a 5-HT3 receptor antagonist combined with dexamethasone and a NK-1 receptor antagonist has been consistently recommended by the American Society of Clinical Oncology (20), the European Society for Medical Oncology (21), the National Comprehensive Cancer Network (22), and other guidelines for the prevention of moderate to high levels of CINV. However, previous phase III studies have shown that the complete remission rate achieved with the triple antiemetic regimen in patients receiving HEC in the overall treatment phase (0–120 h after chemotherapy) was about 60–70%, which indicates that there is still room for further improvement of the antiemetic treatment (22).
In the present study, in the delayed observation period, the proportion of complete remission in the megestrol and control groups were 76.7% and 51.7% (P=0.001), respectively, indicating that there was a significant difference in the main endpoint between the two groups. In the overall observation period, the proportions of complete remission in the megestrol and control groups were 68.3% and 51.7%, respectively (P=0.016), showing significant differences in the control of vomiting. In the acute observation period, the complete remission rates of the megestrol and control groups were 81.7% and 78.3%, respectively (P=0.648). Moreover, it was observed that the complete remission rate of the megestrol group in the delayed period was 76.7%, which was very close to the proportions of standard triple antiemetic regimens (73–74%) (23-25). The control rate of vomiting in the megestrol group was markedly higher than that of the control group in the delayed and overall phases. Furthermore, the complete remission rate of the megestrol group in the delayed phase was 25% higher than that in the control group.
Vomiting can usually be prevented or reduced by the use of preventive antiemetics, but nausea is more difficult to control (26). In a previous well-designed study of olanzapine combined with a standard triple antiemetic regimen, the main research results showed that the complete control rate of nausea of this quadruple regimen was very low (delayed period: 42.4%; overall period: 37.3%) (14). In this study, the two groups of patients developed nausea in three periods. The complete control rates of nausea in the megestrol group were 70.0%, 53.3%, and 40% in the acute, delayed, and overall observation periods, respectively, whereas in the control group were 65.0%, 30.0%, and 15.0% in the same periods. Hence, these results showed that the megestrol combination treatment was considerably more efficient in controlling nausea in the delayed and overall phase (P<0.05). Moreover, in the delayed phase, more than 50% of patients in the megestrol acetate group achieved complete prevention, which further confirmed that megestrol acetate dispersible tablets had significant effects on the prevention of delayed CINV.
Stratified analysis showed that megestrol group patients had no grade 3–4 vomiting (0% vs. 10%; P=0.013) and a reduced use of rescue drugs (6.7% vs. 35.0%; P<0.01). Notably, young women (<50 years old) were more likely to develop CINV when receiving HEC. Moreover, for these patients, the triple antiemetic regimen containing megestrol acetate dispersible tablets exhibited a better vomiting control rate. In terms of adverse reactions, the incidence of fatigue, constipation, and hiccups was high but similar in the two groups (P>0.05), which were mild to moderate in severity and well-tolerated. In addition, previous studies have shown that megestrol can promote granulocytes in the bone marrow to enter the circulating pool, thereby ensuring the presence of white blood cells in the peripheral blood, which could in turn reduce the hemato-toxicity of the chemotherapies (27). In this study, the incidence of leukopenia in the megestrol group was lower than that in the control group (13.3% vs. 28.3%; P=0.043), which was consistent with previous findings (28). Furthermore, among the participants, three patients in the megestrol group reported vaginal bleeding (P>0.05), but there were no other adverse reactions related to megestrol, such as peripheral edema, mental symptoms, or thrombosis. Other observed adverse reactions, such as liver and kidney function damage, and thrombocytopenia, were considered to be chemotherapy drug-related, with no significant difference in terms of incidence between the two groups (P>0.05). After treatment, the QOL score of the megestrol group was significantly higher than that of the control group (P<0.05). This finding was consistent with the results of related studies, which further demonstrated that megestrol acetate dispersible tablets could effectively improve the QOL of patients.
Conclusions
The effect of megestrol acetate dispersible tablets combined with a 5-HT3 receptor antagonist and dexamethasone on HEC-induced CINV, especially delayed CINV, was significantly better than that of a 5-HT3 receptor antagonist combined with dexamethasone alone. Moreover, megestrol acetate tablets could improve the appetite of patients, reduce myelosuppression, and improve the overall QOL of the patients, with only mild and controllable adverse reactions. Preliminary exploration of the efficacy and safety of megestrol acetate dispersible tablets in controlling HEC-induced CINV, especially delayed CINV, provides a new reference for controlling CINV in clinical practice.
The inadequacy of this study is that, this study has not designed a comparison of the efficacy of megestrol and aripipitan. Since this study only explored the effects of megestrol on CINV, future investigations comparing megestrol and arepitant will be performed. These additional clinical trials are expected to comprise a larger sample size and the influence of individual factor differences on the research results will be eliminated through the patients’ own cross-control method, so as to confirm the antiemetic effect of megestrol on acute and delayed CINV caused by HEC drugs.
Acknowledgments
We thank all of the patients and the group members who participated in this research.
Funding: This work was supported by grants from the National Natural Science Fund Youth Fund of China (No. 82003041), the Joint Funds of the National Natural Science Foundation of China (No. U2004132), the Key Scientific and Technological Projects in Henan Province (No. 202102310111), and the Project of Tackling Problems in Medical Science and Technology of Henan Province (No. LHGJ20190631).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4809/rc
Trial Protocol: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4809/tp
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4809/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4809/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Henan Cancer Hospital (ethics batch No. 2017098) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Janelsins MC, Tejani MA, Kamen C, et al. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 2013;14:757-66. [Crossref] [PubMed]
- Toniolo J, Delaide V, Beloni P. Effectiveness of Inhaled Aromatherapy on Chemotherapy-Induced Nausea and Vomiting: A Systematic Review. J Altern Complement Med 2021;27:1058-69. [Crossref] [PubMed]
- Milnes V, Gonzalez A, Amos V. Aprepitant: A New Modality for the Prevention of Postoperative Nausea and Vomiting: An Evidence-Based Review. J Perianesth Nurs 2015;30:406-17. [Crossref] [PubMed]
- Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol 2011;22:30-8. [Crossref] [PubMed]
- Roscoe JA, Heckler CE, Morrow GR, et al. Prevention of delayed nausea: a University of Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. J Clin Oncol 2012;30:3389-95. [Crossref] [PubMed]
- Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients' quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 2006;24:4472-8. [Crossref] [PubMed]
- Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. The Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer 1997;5:307-13. [Crossref] [PubMed]
- Chow R, Chiu L, Navari R, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer 2016;24:1001-8. [Crossref] [PubMed]
- Chiu L, Chow R, Popovic M, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 2016;24:2381-92. [Crossref] [PubMed]
- Glaus A, Knipping C, Morant R, et al. Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 2004;12:708-15. [Crossref] [PubMed]
- Aapro M. CINV: still troubling patients after all these years. Support Care Cancer 2018;26:5-9. [Crossref] [PubMed]
- Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997;15:103-9. [Crossref] [PubMed]
- Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005;23:2822-30. [Crossref] [PubMed]
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2016;375:134-42. [Crossref] [PubMed]
- Yeo W, Mo FK, Suen JJ, et al. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Res Treat 2009;113:529-35. [Crossref] [PubMed]
- Iwata T, Miyauchi A, Suga Y, et al. Neoadjuvant chemo- therapy for locally advanced cervical cancer. Chin J Cancer Res 2016;39:2470-86. [PubMed]
- Lyons E, Line C, Lee JJ. Developing Drugs for Prevention of Chemotherapy-Induced Nausea and Vomiting: Draft Guidance from the FDA. Clin Cancer Res 2021;27:6072-4. [Crossref] [PubMed]
- Zhao J, Li X, He W, et al. Olanzapine combined with megestrol acetate in the treatment of anorexia of advanced cancer. Journal of Modern Oncology 2015;23:1443-6.
- Li Y, Zhang M, Zhao C, et al. Effect of megestrol acetate on quality of life in patients with malignant tumor undergoing radiotherapy. Practical Clinical Medicine 2013;14:35-36,42.
- Qiu T, Men P, Sun T, et al. Cost-Effectiveness of Aprepitant in Preventing Chemotherapy-Induced Nausea and Vomiting: A Systematic Review of Published Articles. Front Public Health 2021;9:660514. [Crossref] [PubMed]
- Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 2010;21:v232-43. [Crossref] [PubMed]
- NCCN National Comprehensive Cancer Network. Antiemesis: Clinical Practice Guidelines in Oncology, Version 1,2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1415
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011;9:188-95. [Crossref] [PubMed]
- Navari RM, Nagy CK, Le-Rademacher J, et al. Olanzapine versus fosaprepitant for the prevention of concurrent chemotherapy radiotherapy-induced nausea and vomiting. J Community Support Oncol 2016;14:141-7. [Crossref] [PubMed]
- Hu Z, Cheng Y, Zhang H, et al. Aprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trial. Support Care Cancer 2014;22:979-87. [Crossref] [PubMed]
- Ng TL, Hutton B, Clemons M. Chemotherapy-Induced Nausea and Vomiting: Time for More Emphasis on Nausea? Oncologist 2015;20:576-83. [Crossref] [PubMed]
- Sgroi DC, Chapman JA, Badovinac-Crnjevic T, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016;18:1. [Crossref] [PubMed]
- Qu W, Zhang E, Liu L, et al. Clinical study of megestrol in the prevention and treatment of tumor chemotherapy vomiting and leukopenia. Modern Tumor Medicine 2010;18:1000-2.
(English Language Editor: A. Kassem)


