
Total bilirubin is associated with all-cause mortality in patients with acute respiratory distress syndrome: a retrospective study
Introduction
Acute respiratory distress syndrome (ARDS) is a relatively common and life-threatening clinical syndrome characterized by acute respiratory failure, hypoxemia, and non-hydrostatic pulmonary edema (1,2). Globally, ARDS accounts for 10% of intensive care unit (ICU) admissions and 23% of mechanical ventilations, affecting approximately 3 million individuals annually (3). The hospital mortality for ARDS ranges from 24% to 46% and is positively correlated with the degree of lung injury severity at onset (3,4). A recent prospective cohort study suggested that the prevalence of ARDS in ICU patients with sepsis resulted in increased in-hospital mortality (27–37%) and other poor outcomes (5).
Given the high mortality of ARDS, it is crucial to identify the biomarkers for early identification of ARDS patients who might require aggressive treatment. Previous studies have shown that high levels of inflammatory serum mediators, including interleukin (IL)-2, IL-15, and Decoy receptor (DcR) 3, are associated with mortality in patients with ARDS, while an elevated ratio of T-regulatory cells (Tregs) to lymphocytes in bronchoalveolar lavage fluid independently predicted 30-day ICU mortality (6-8). However, these parameters are not routine lab indices and are obtained via invasive procedures. Various scoring systems have also been used to predict the mortality of ARDS patients; the Lung Injury Score (LIS) is the most commonly used and consists of four criteria: chest radiograph, hypoxemia score, positive end-expiratory pressure (PEEP) score, and respiratory system compliance score (7,9). However, it is unlikely that such scoring systems will be adopted at the beginning of ICU admission since most patients may lack complete examinations.
Bilirubin, an endogenous bile pigment, is derived primarily from hemoglobin catabolism (10). Briefly, when old or damaged erythrocytes are engulfed and degraded by macrophages, hemoglobin will be released and broken down into two main components, the globin, and the hemes. The globin is reused for erythropoiesis, while the hemes are degraded into unconjugated bilirubin (UCB). Next, UCB, a lipid-soluble molecule, is carried to the liver and converted to conjugated bilirubin (CB) with the addition of glucuronic acid (11).
The serum total bilirubin (TBIL), a combination of UCB and CB, is a simple and routine examination indicator known as a biomarker of liver dysfunction. Numerous investigations have suggested that a mild elevation of serum bilirubin within the normal range provides a protective effect on health through its potent antioxidant, anti-inflammatory, immunomodulatory, and anti-excitotoxic properties (12-15). However, when serum TBIL exceeds a specific concentration, it paradoxically exerts a toxic influence on the body by inducing oxidative stress, inflammation, apoptosis/necrosis, and excitotoxicity (16-19). Hyperbilirubinemia, or jaundice, has been associated with mortality and other poor outcomes in critical illnesses, affecting 40% of critically ill patients (20-22). Liver-lung interactions have been well documented previously, suggesting that hepatic dysfunction is a relevant clinical condition that affects the development and progression of ARDS (23,24). A British prospective cohort study found that elevated serum TBIL levels on ICU admission correlated with ARDS development in sepsis (25), and admission TBIL was independently correlated with 60-day ARDS mortality in sepsis-related patients (26). Similarly, a French retrospective study found that TBIL in the initial phase of ARDS was associated with 90-day mortality (27). However, the association between TBIL on ICU admission and mortality in ARDS patients remains largely unknown.
Therefore, the present study aimed to evaluate whether TBIL on ICU admission was associated with mortality in patients with ARDS by using the Medical Information Mart for Intensive Care (MIMIC)-IV database. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1737/rc).
Methods
Data sources
All data were obtained from the MIMIC-IV, an updated version of MIMIC-III, which is a single-center and freely accessible database containing 383,220 admissions from 2008 to 2019 in the ICU of Beth Israel Deaconess Medical Center in Boston, MA, USA (28). We completed the online course and passed the online exams (No. 6182750) to access the database. The establishment of the MIMIC III database was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center (Boston, MA, USA) and the Massachusetts Institute of Technology (Cambridge, MA, USA). One author (ZC) obtained access to the database and was responsible for the data extraction (certification No. 39645558). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Participants
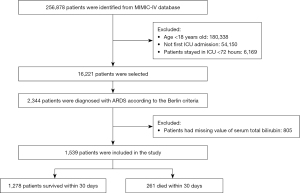
The selection of study participants is shown in Figure 1. We screened all the patients in the database. Our inclusion criteria were as follows: age ≥18 years, an ICU stay of more than 72 hours, an ARDS diagnosis meeting the Berlin criteria at the time of ICU admission, and TBIL levels taken within 24 hours of ICU admission. For patients admitted to the ICU more than once, we only used the data of the first ICU admission. The Berlin criteria included the following: acute onset, arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg, positive end-expiratory pressure (PEEP) ≥5 cmH2O on the first day of ICU admission, bilateral infiltrates on chest radiograph, and absence of heart failure (29). The open-source MIMIC Chest X-Ray (MIMIC-CXR) was used to identify the patients based on the Berlin criteria (30).
According to the Berlin criteria, ARDS severity was classified as mild (>200 mmHg, ≤300 mmHg), moderate (>100 mmHg, ≤200 mmHg), and severe (<100 mmHg) based on the PaO2/FiO2 ratio. Based on a previous study on bilirubin, patients were stratified into three groups according to their TBIL levels 24 hours after admission as follows: (I) serum TBIL level <1.2 mg/dL, (II) serum TBIL level between 1.2 mg/dL and 2 mg/dL, (III) and serum TBIL level ≥2 mg/dL (31).
Data collection and outcomes
We used the Structured Query Language (SQL) software (IBM Corp., Armonk, NY, USA) to extract the data from the MIMIC-IV database. We extracted or calculated the following variables: age, gender, admission type, ventilation status [invasive (including tracheostomy and positive pressure ventilation via endotracheal tube) and non-invasive (including non-invasive positive pressure ventilation, high flow nasal oxygen/cannula, and supplemental oxygen)], baseline measured parameters [including total bilirubin, platelets, white blood cell, alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate aminotransferase (AST), and serum glucose], comorbidity [including pneumonia, hypertension, chronic obstructive pulmonary disease (COPD), renal failure, rheumatic disease, cancer, and diabetes mellitus], vital data taken within 24 hours of ICU admission [heart rate, temperature, respiratory rate, systolic blood pressure (SBP), diastolic blood pressure (DBP)], SpO2, PaO2/FiO2, PEEP, and the Oxford Acute Severity of Illness Score (OASIS) at diagnosis (32). The missing data of these values were less than 5% and replaced using linear interpolation.
The primary outcome in this study was 30-day ICU mortality after admission to ICU. In-hospital mortality was defined as the secondary outcome.
Statistical analysis
The descriptive results were presented as means ± standard error (SE) for continuous variables and frequency (weighted percentages) for categorical variables. The TBIL baseline levels were analyzed both as a continuous and categorical variable (three groups). We tested differences in characteristics between the groups (survival or death within 30 days of admission to the ICU) with Student’s t-tests for the continuous variables and χ2 tests for the categorical variables. The odds ratio (OR) and 95% confidence interval (CI) for mortality per 1 mg/dL increase in TBIL levels in the three TBIL groups were estimated using both univariate and multivariate logistic regression models. Besides the unadjusted model, potential covariates were progressively adjusted in three models: Model 1 was adjusted for age and gender; Model 2 was additionally adjusted for pneumonia, hypertension, COPD, renal failure, cancer, and diabetes mellitus; and Model 3 was further adjusted for admission type, SpO2, PEEP, ALP, ALT, AST, serum glucose, and OASIS score. Restricted cubic spline models were used to estimate the dose-response relationship between TBIL levels and outcomes.
To further explore whether the association between TBIL levels and 30-day ICU mortality was modified by age, gender, admission type, and comorbidities, we performed subgroup analyses by gender (male or female), age group (<60 or ≥60 years), hypertension (yes or no), pneumonia (yes or no), renal failure (yes or no), diabetes (yes or no), ARDS classification (mild, moderate, or severe) and admission type (urgent, emergency, or elective), and examined interactions by likelihood ratio tests. All statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P<0.05 was considered significant.
Results
Patient characteristics
A total of 1,539 ARDS patients were enrolled in this cohort study (Figure 1). The characteristics of the study population are shown in Table 1. The total group had a mean age of 62.3±16.3 years, and most were male (56.7%). Of these patients, 261 (17.0%) died in the ICU within 30 days. Compared to the survivors, cases who died within 30 days were older (66.6±16.0 vs. 61.4±16.2, P<0.001) and more likely to have comorbidities, such as renal failure (18.4% vs. 11.9%, P=0.006) and cancer (20.3% vs. 12%, P<0.001), but less likely to have diabetes mellitus (23.8% vs. 30%, P=0.049). Regarding the baseline vital indexes, non-survivors had lower heart rate levels, temperature, respiratory rate, blood pressure, SpO2, and PaO2/FiO2 but higher PEEP levels. Compared with survivors, those who died showed a higher level of TBIL, ALP, and AST. The rate of invasive ventilation was significantly higher in non-survivors than in survivors.
Table 1
| Variables | Total, N=1,539 | 30-day survivor, N=1,278 | 30-day ICU death, N=261 | P value |
|---|---|---|---|---|
| Age (years) | 62.3±16.3 | 61.4±16.2 | 66.6±16.0 | <0.001 |
| Male gender | 872 (56.7) | 729 (57.0) | 143 (54.8) | 0.548 |
| Admission type | 0.003 | |||
| Urgent | 231 (15.0) | 208 (16.3) | 23 (8.8) | |
| Emergency | 1,086 (70.6) | 881 (68.9) | 205 (78.5) | |
| Elective | 222 (14.4) | 189 (14.8) | 33 (12.6) | |
| Comorbidity | ||||
| Pneumonia | 692 (45.0) | 580 (45.4) | 112 (42.9) | 0.507 |
| Hypertension | 649 (42.2) | 531 (41.5) | 118 (45.2) | 0.306 |
| COPD | 54 (3.5) | 44 (3.4) | 10 (3.8) | 0.9 |
| Renal failure | 200 (13.0) | 152 (11.9) | 48 (18.4) | 0.006 |
| Rheumatic disease | 60 (3.9) | 53 (4.1) | 7 (2.7) | 0.348 |
| Cancer | 206 (13.4) | 153 (12.0) | 53 (20.3) | <0.001 |
| Diabetes mellitus | 446 (29.0) | 384 (30.0) | 62 (23.8) | 0.049 |
| Baseline vital data | ||||
| Body temperature (℃) | 37.1 (36.7, 37.5) | 37.1 (36.7, 37.6) | 36.8 (36.4, 37.1) | <0.001 |
| Heart rate (beats/min) | 101 (86, 118) | 102 (87, 119) | 97 (81, 113) | <0.001 |
| Respiratory rate (breaths/min) | 23 (19, 28) | 23 (19, 28) | 22 (18, 27) | 0.017 |
| SBP (mmHg) | 130.8±26.4 | 132.4±26.4 | 122.7±24.6 | <0.001 |
| DBP (mmHg) | 75.8±21.1 | 76.9±21.0 | 70.3±20.9 | <0.001 |
| MBP (mmHg) | 89.8±21.5 | 91.0±21.6 | 83.9±20.1 | <0.001 |
| SpO2 (%) | 97.5±4.0 | 97.7±3.7 | 96.7±5.2 | <0.001 |
| PaO2/FiO2 (mmHg) | 130.0 (83.3, 190.0) | 130.0 (82.9, 190.0) | 126.7 (86.0, 190.0) | 0.945 |
| PEEP (cmH2O) | 10.7 (7.0, 14.0) | 10.2 (7.0, 13.9) | 12.0 (8.0, 15.0) | <0.001 |
| Baseline measured parameters | ||||
| Total bilirubin (mg/dL) | 1.6±3.2 | 1.5±2.8 | 2.2±4.6 | <0.001 |
| Platelets (×109/L) | 209.0 (136.0, 289.0) | 211.5 (139.0, 293.5) | 198.0 (129.0, 269.0) | 0.086 |
| White blood cell (×109/L) | 12.4 (8.5, 17.4) | 12.4 (8.4, 17.3) | 12.6 (9.0, 18.4) | 0.107 |
| ALP (U/L) | 82.0 (59.0, 119.0) | 79.0 (58.0, 114.0) | 95.0 (67.0, 141.0) | <0.001 |
| ALT (U/L) | 32.0 (18.5, 75.0) | 32.0 (19.0, 71.8) | 34.0 (18.0, 81.0) | 0.259 |
| AST (U/L) | 50.0 (27.0, 117.5) | 47.5 (27.0, 110.8) | 62.0 (32.0, 160.0) | 0.002 |
| Serum glucose (mg/dL) | 157.0 (122.0, 214.0) | 158.0 (124.2, 214.8) | 148.0 (112.0, 211.0) | 0.01 |
| Ventilation status | 0.005 | |||
| Invasive | 1,342 (87.2) | 1,100 (86.1) | 242 (92.7) | |
| Non-Invasive | 197 (12.8) | 178 (13.9) | 19 (7.3) | |
| Severity score | ||||
| OASIS | 40.8±9.0 | 40.2±8.9 | 43.8±9.1 | <0.001 |
Data are means ± SD, n (%), and median (IQR). P values comparing groups are from the Student’s t-test or Mann-Whitney test for continuous data and the chi-squared test for categorical variables. ARDS, acute respiratory distress syndrome; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SpO2, pulse oxygen saturation; PaO2/FiO2, arterial oxygen partial pressure/fraction of inspired oxygen; PEEP, positive end-expiratory pressure; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; OASIS, Oxford Acute Severity of Illness Score.
Outcomes across different levels of TBIL
All cases were stratified into three groups according to their TBIL levels at baseline and displayed different clinical outcomes, as shown in Table 2. Of the 1,539 participants, 258 (16.8%) cases had a TBIL level ≥2 mg/dL. Overall, there were lower rates of in-hospital mortality in cases with higher TBIL levels compared to those with TBIL levels <2 mg/dL (P=0.008). However, the 30-day ICU mortality did not reach statistical significance (P=0.1). Patients with higher TBIL levels had a longer length of stay in the ICU and hospital (P=0.375 and P=0.026, respectively). ICU-LOS appears not different between groups (P=0.375). Furthermore, ICU-LOS in patients with TBIL ≥2 mg/dL is shorter (6.4 days) compared to all other groups (6.8 and 7.4 days, respectively).
Table 2
| Outcomes | All patients | Total serum bilirubin (mg/dL) | P value | ||
|---|---|---|---|---|---|
| <1.2 | ≥1.2, <2 | ≥2 | |||
| Patients, n (%) | 1,539 | 1,077 (70.0) | 204 (13.3) | 258 (16.8) | – |
| 30-day ICU mortality | 261 (17.0) | 170 (15.8) | 36 (17.6) | 55 (21.3) | 0.1 |
| In-hospital mortality | 418 (27.2) | 268 (24.9) | 69 (33.8) | 81 (31.0) | 0.008 |
| LOS in ICU (day) | 6.8 (4.5, 11.6) | 6.8 (4.5, 11.7) | 7.4 (4.7, 11.9) | 6.4 (4.6, 10.2) | 0.375 |
| LOS in hospital (day) | 14.7 (9.0, 22.9) | 14.1 (8.7, 22.1) | 15.5 (9.9, 22.2) | 15.6 (10.0, 25.0) | 0.026 |
Data are displayed as the median (IQR) and n (%). P values comparing groups are the chi-squared test for categorical variables. ARDS, acute respiratory distress syndrome; ICU, intensive care unit; LOS, length of stay.
Primary outcome
Table 3 shows the relationship between TBIL levels and mortality at baseline as continuous and categorical variables, respectively. When the TBIL levels were analyzed as a continuous variable, TBIL was significantly associated with higher 30-day ICU mortality (OR =1.04; 95% CI: 1.01 to 1.06) in the unadjusted logistic regression model. After adjustment for multiple covariates, the association remained significant in the multivariate logistic regression models, indicating that each 1 mg/dL increase resulted in a 4% increase in 30-day ICU mortality (Model 3: OR =1.04; 95% CI: 1.01 to 1.08). When the TBIL levels were treated as categorical variables, patients with TBIL levels ≥2 mg/dL had a higher risk of 30-day ICU mortality in the main model (OR =1.51; 95% CI: 1.02 to 2.22), while those with TBIL levels between 1.2 and 2 mg/dL showed a statistically significant difference according to the lowest TBIL levels in the main model (OR =1.30; 95% CI: 0.85 to 1.99). The OR and 95% CI for each of the covariates in the relationship between TBIL and 30-day ICU mortality are shown in Table S1.
Table 3
| Outcomes | Non-adjusted, OR (95% CI) |
Model 1, OR (95% CI) |
Model 2, OR (95% CI) |
Model 3, OR (95% CI) |
|---|---|---|---|---|
| 30-day ICU mortality | ||||
| TBIL per 1 mg/dL | 1.04 (1.01, 1.06)* | 1.07 (1.04, 1.11)* | 1.07 (1.03, 1.11)* | 1.04 (1.01, 1.08)* |
| TBIL category | ||||
| <1.2 mg/dL | Reference | Reference | Reference | Reference |
| ≥1.2, <2 mg/dL | 1.14 (0.77, 1.70) | 1.14 (0.77, 1.70) | 1.13 (0.75, 1.69) | 1.30 (0.85, 1.99) |
| ≥2 mg/dL | 1.45 (1.03, 2.03)* | 1.64 (1.16, 2.32)* | 1.51 (1.06, 2.15)* | 1.51 (1.02, 2.22)* |
| In-hospital mortality | ||||
| TBIL per 1 mg/dL | 1.03 (1.01, 1.06)* | 1.06 (1.03, 1.10)* | 1.05 (1.02, 1.09)* | 1.04 (1.01, 1.07)* |
| TBIL category | ||||
| <1.2 mg/dL | Reference | Reference | Reference | Reference |
| ≥1.2, <2 mg/dL | 1.54 (1.12, 2.13)* | 1.54 (1.11, 2.13)* | 1.53 (1.10, 2.13)* | 1.65 (1.17, 2.33)* |
| ≥2 mg/dL | 1.38 (1.03, 1.86)* | 1.57 (1.16, 2.14)* | 1.47 (1.07, 2.01)* | 1.41 (1.01, 1.87)* |
Model 1: adjusted for age and gender. Model 2: further adjusted for pneumonia, hypertension, COPD, renal failure, cancer, and diabetes mellitus. Model 3: further adjusted for admission type, SpO2, PEEP, ALP, ALT, AST, serum glucose, and OASIS score. *P<0.05. OR, odds ratio; CI, confidence interval; ICU, intensive care unit; TBIL, total bilirubin; COPD, chronic obstructive pulmonary disease; SpO2, pulse oxygen saturation; PEEP, positive end-expiratory pressure; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase.
The dose-response analysis using a restricted cubic spline model is shown in Figure 2A. There was a nearly linear relationship between the TBIL levels and the odds of 30-day ICU mortality after adjustment for multiple potential covariates in Model 3 (Table 3) (P value for nonlinear =0.373).

Secondary outcome
The association between TBIL and in-hospital mortality was in line with the primary outcome. An increase of 1 mg/dL of serum TBIL led to a 4% increase in in-hospital mortality after adjustment for multiple covariates (Table 3) (Model 3: OR =1.04; 95% CI: 1.01 to 1.07). Patients with TBIL ≥1.2 mg/dL and ≥2 mg/dL had higher in-hospital mortality after adjustments in Model 3 (OR =1.65, 95% CI: 1.17 to 2.33; OR =1.41, 95% CI: 1.01 to 1.87, respectively). A linear relationship was present between TBIL levels and in-hospital mortality after adjustment in Model 3 (Table 3) (P for nonlinear =0.441, Figure 2B). The OR and 95% CI for each of the covariates in the relationship between TBIL and in-hospital mortality are shown in Table S2.
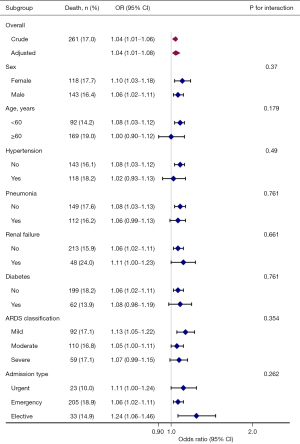
Subgroup analyses
The subgroup analyses of the association between baseline TBIL level as a continuous variable and 30-day ICU mortality are presented in Figure 3. Positive relationships were seen in the different subgroups stratified by gender, age, comorbidities, the severity of ARDS, and admission type, suggesting a consistent association between TBIL and 30-day ICU mortality in all ARDS patients. No statistical significance was found between intra-subgroups in any of the subgroups.
Discussion
The present study showed that a higher serum TBIL level on ICU admission was associated with increased 30-day ICU mortality and in-hospital mortality in ARDS patients. These associations remained consistent even after adjustments for multiple covariates, including pneumonia, renal failure, and biochemical measurements (ALP, ALT, AST, etc.). Our results also showed a nearly linear dose-response relationship. Stratified analyses showed generally consistent associations with the primary outcomes.
Consistent with previous studies (25-27,33), the present study showed that TBIL, a routine laboratory index of liver dysfunction, is independently associated with mortality in certain types of ARDS patients. In 1989, Schwartz et al. investigated 24 ARDS patients during the first week after diagnosis and found that TBIL levels in non-survivors were significantly higher than those in survivors (33). In line with Schwartz et al.’s findings, Zhai et al. reported that non-survivors showed consistently higher serial TBIL levels than survivors of sepsis-related ARDS during a 28-day observation period (25). The prior study documented that for each 1.0 mg/dL increase in serum TBIL levels at ICU admission, sepsis-related 28-day mortality and 60-day mortality increased by 20% and 18%, respectively (25). Also, Sheu et al. investigated 586 ARDS patients using the American-European Consensus Committee (AECC) criteria and showed that levels of TBIL in ICU admission were associated with 60-day mortality (26). A recent post-hoc analysis assessed the associations between TBIL and mortality in ARDS patients with PaO2/FiO2 <150 mmHg and found that TBIL was independently correlated with 90-day mortality (27). A TBIL level ≥33 µmol/L was considered an effective threshold to identify moderate to severe ARDS patients with a higher mortality risk (27).
In this study, 1,539 ARDS patients were identified from a large cohort study. In agreement with Sheu et al.’s findings (26), we found that each 1 mg/dL increase in serum TBIL resulted in a slight increase in 30-day ICU mortality in ARDS patients after adjusting for multiple covariates, showing a nearly linear dose-dependent association. To reduce the bias in survival calculation caused by incomplete observation, the association between TBIL and ARDS in-hospital mortality was considered the secondary outcome. Consistently, serum TBIL levels were associated with in-hospital mortality in ARDS patients. Also, we found that 30-day ICU mortality in patients with serum TBIL levels ≥2 mg/dL was higher than those with serum TBIL between 1.2 to 2 mg/dL or those with TBIL <1.2 mg/dL (25), although statistical significance was not reached. Our results suggested that TBIL is a potential predictor of mortality in ARDS patients, indicating that those with elevated TBIL on ICU admission require a high level of medical attention and vigilance to avoid death.
Unlike previous reports using the AECC definition (26), the Berlin definition was adapted to identify ARDS patients in the present study, and therefore the bias from misdiagnosis of ARDS was minimized (29,34). Although the Berlin definition was used in Dizier et al.’s findings (27), only moderate-to-severe ARDS patients were included in their analysis, whereas the mortality in mild ARDS patients accounts for approximately 25% of all deaths (3). Therefore, to the best of our knowledge, this is the first study with the largest population to report that TBIL levels in ICU admission are independently associated with mortality in patients with ARDS.
The subgroup analysis results were consistent with the main findings. However, we found that the ORs in some specific patients over 60 years old, with hypertension, severe ARDS, or with urgent ICU admission were lower than those in the corresponding subgroups, but this difference did not reach statistical significance. Given that older age, female gender, lower blood pressure, renal failure, and severe ARDS impact ARDS development and mortality, the strength of associations in the subgroup analyses may have been attenuated (1,3). Previous studies have reported that younger patients are predisposed to experience severe ARDS and have a significant deterioration, consistent with our findings (3,29). Additionally, after stratification, only 230 patients with 23 deaths were included in the subgroup of patients who had an urgent ICU admission. Interestingly, subgroup analyses stratified by the severity of ARDS showed that the OR in the mild ARDS subgroup was higher than that in the moderate and severe ARDS groups, suggesting that mild ARDS patients may be more sensitive and vulnerable to higher TBIL. Due to the potential bias caused by the reduced sample size in these subgroups, larger specific populations are needed to validate these results in the future.
Mechanistically, a growing number of studies have suggested that liver dysfunction is the major determinant of the development and mortality in ARDS through the interactions among respiratory host defenses, systemic inflammatory responses, and metabolic processes (23,35,36). In support of our results, a recent study found that higher circulating cell-free hemoglobin (CFH), one of the precursors of bilirubin, is an independent risk factor for acute kidney injury in ARDS patients (37). Similarly, Shaver et al. reported that CFH contributed to ARDS severity by enhancing lung permeability and inflammation in an experimental ARDS mouse model (38). Additionally, in the context of inflammatory diseases such as acute lung injury, excess heme, or heme released in certain pathophysiological contexts may have adverse effects, partly through mechanisms of vascular endothelial dysfunction and activation of programmed cell death pathways (39,40). The functions of bilirubin are considered something akin to a “Janus face” (11). It is reported that mildly elevated levels of TBIL have potent antioxidant and other positive benefits (12). However, hyperbilirubinemia exerts a detrimental effect on organs and causes severe and irreversible damage, especially in ARDS (19,23). Several mechanisms may be involved in this damaging effect. First, available data have suggested that elevated bilirubin results in oxidative stress and the activation of local inflammatory responses in lung tissues, including the infiltration of alveolar macrophages, neutrophils, and the release of cytokines [IL-1, IL-6, and tumor necrosis factor (TNF)-α], which are the pathophysiological features of ARDS (23,41,42). Second, in vitro and in vivo studies have shown that bilirubin directly contributes to alveolar epithelial cell injury (43), leading to the interruption of cell cycles and cell apoptosis (16,44,45). Third, bilirubin's amphiphilic property allows it to bind to the cell membrane, resulting in ATPase inhibition, lipid peroxidation, and other adverse outcomes (46). Fourth, increased serum bilirubin levels have been associated with lung-specific damage by entering the alveolar airspaces and deteriorating the surface tension properties of lung surfactants (47). Overall, these potential mechanisms may explain why TBIL is associated with mortality in ARDS patients.
Despite the inclusion of a large study population with ARDS and adjustment for multiple covariates, our study had some limitations. First, this was a single-center cohort study, which may restrict the generalization of our conclusion. Second, we could not exclude the possibility that some medications may have affected TBIL levels. However, a previous study found that 17 potentially hepatotoxic drugs administered to ICU patients had no significant impact on serum TBIL levels (48). Third, the retrospective study design did not allow us to determine the causality between TBIL and mortality of ARDS patients. Fourth, a large number of patients were excluded because of missing bilirubin values, which may cause some potential bias to the results. Last, the small size of our subgroups was another significant limitation, and a larger population of such subgroups is recommended in the future to draw a more robust conclusion.
Conclusions
In conclusion, the present study shows that a higher level of TBIL on ICU admission is associated with mortality in ARDS patients. Patients with elevated TBIL require careful observation and more intensive treatment. Our results support the need for further large-scale prospective studies to illustrate the specific causality of this relationship.
Acknowledgments
The authors would like to thank all patients, nurses, and physicians who participated in the study. The authors would like to thank Dr. Xiaoling Li for her suggestions on the grammar and vocabulary of this manuscript. We would like to acknowledge AME Editing Service for language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1737/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1737/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1737/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet 2021;398:622-37. [Crossref] [PubMed]
- Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 2019;9:69. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 2014;40:388-96. [Crossref] [PubMed]
- Auriemma CL, Zhuo H, Delucchi K, et al. Acute respiratory distress syn-drome-attributable mortality in critically ill patients with sepsis. Intensive Care Med 2020;46:1222-31. [Crossref] [PubMed]
- Adamzik M, Broll J, Steinmann J, et al. An increased alveolar CD4 + CD25 + Foxp3 + T-regulatory cell ratio in acute respiratory distress syndrome is associated with in-creased 30-day mortality. Intensive Care Med 2013;39:1743-51. [Crossref] [PubMed]
- van der Zee P, Rietdijk W, Somhorst P, et al. A systematic review of biomarkers multivariately associated with acute respiratory distress syndrome development and mortality. Crit Care 2020;24:243. [Crossref] [PubMed]
- Wang Y, Wang H, Zhang C, et al. Lung fluid biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care 2019;23:43. [Crossref] [PubMed]
- Kim BK, Kim S, Kim CY, et al. Predictive Role of Lung Injury Prediction Score in the Development of Acute Respiratory Distress Syndrome in Korea. Yonsei Med J 2021;62:417-23. [Crossref] [PubMed]
- Creeden JF, Gordon DM, Stec DE, et al. Bilirubin as a metabolic hormone: the physiological relevance of low levels. Am J Physiol Endocrinol Metab 2021;320:E191-207. [Crossref] [PubMed]
- Hansen TWR, Wong RJ, Stevenson DK. Molecular Physiology and Pathophysiology of Bilirubin Handling by the Blood, Liver, Intestine, and Brain in the Newborn. Physiol Rev 2020;100:1291-346. [Crossref] [PubMed]
- Adin CA. Bilirubin as a Therapeutic Molecule: Challenges and Opportunities. Anti-oxidants (Basel) 2021;10:1536. [Crossref] [PubMed]
- Žiberna L, Jenko-Pražnikar Z, Petelin A. Serum Bilirubin Levels in Overweight and Obese Individuals: The Importance of Anti-Inflammatory and Antioxidant Responses. Antioxidants (Basel) 2021;10:1352. [Crossref] [PubMed]
- Thomas DT, DelCimmuto NR, Flack KD, et al. Reactive Oxygen Species (ROS) and Antioxidants as Immunomodulators in Exercise: Implications for Heme Oxygenase and Bilirubin. Antioxidants (Basel) 2022;11:179. [Crossref] [PubMed]
- Nocentini A, Bonardi A, Pratesi S, et al. Pharmaceutical strategies for preventing toxicity and promoting antioxidant and anti-inflammatory actions of bilirubin. J En-zyme Inhib Med Chem 2022;37:487-501. [Crossref] [PubMed]
- Watchko JF. Bilirubin-Induced Neurotoxicity in the Preterm Neonate. Clin Perinatol 2016;43:297-311. [Crossref] [PubMed]
- Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage--mechanisms and management approaches. N Engl J Med 2013;369:2021-30. [Crossref] [PubMed]
- Jayanti S, Vítek L, Tiribelli C, et al. The Role of Bilirubin and the Other "Yellow Players" in Neurodegenerative Diseases. Antioxidants (Basel) 2020;9:900. [Crossref] [PubMed]
- Soto Conti CP. Bilirubin: The toxic mechanisms of an antioxidant molecule. Arch Argent Pediatr 2021;119:e18-25. [PubMed]
- Peng M, Deng F, Qi D, et al. The Hyperbilirubinemia and Potential Predictors Influence on Long-Term Outcomes in Sepsis: A Population-Based Propensity Score-Matched Study. Front Med (Lausanne) 2021;8:713917. [Crossref] [PubMed]
- Jenniskens M, Langouche L, Vanwijngaerden YM, et al. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med 2016;42:16-27. [Crossref] [PubMed]
- Qattea I, Farghaly MAA, Elgendy M, et al. Neonatal hyperbilirubinemia and bilirubin neurotoxicity in hospitalized neonates: analysis of the US Database. Pediatr Res 2022;91:1662-8. [Crossref] [PubMed]
- Herrero R, Sánchez G, Asensio I, et al. Liver-lung interactions in acute respiratory distress syndrome. Intensive Care Med Exp 2020;8:48. [Crossref] [PubMed]
- Gacouin A, Locufier M, Uhel F, et al. Liver Cirrhosis is Independently Associated With 90-Day Mortality in ARDS Patients. Shock 2016;45:16-21. [Crossref] [PubMed]
- Zhai R, Sheu CC, Su L, et al. Serum bilirubin levels on ICU admission are associated with ARDS development and mortality in sepsis. Thorax 2009;64:784-90. [Crossref] [PubMed]
- Sheu CC, Gong MN, Zhai R, et al. Clinical characteristics and outcomes of sep-sis-related vs non-sepsis-related ARDS. Chest 2010;138:559-67. [Crossref] [PubMed]
- Dizier S, Forel JM, Ayzac L, et al. Early Hepatic Dysfunction Is Associated with a Worse Outcome in Patients Presenting with Acute Respiratory Distress Syndrome: A Post-Hoc Analysis of the ACURASYS and PROSEVA Studies. PLoS One 2015;10:e0144278. [Crossref] [PubMed]
- Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2000;101:E215-20. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Johnson AEW, Pollard TJ, Berkowitz SJ, et al. MIMIC-CXR, a de-identified publicly available database of chest radiographs with free-text reports. Sci Data 2019;6:317. [Crossref] [PubMed]
- Yang ZX, Lv XL, Yan J. Serum Total Bilirubin Level Is Associated With Hospital Mortality Rate in Adult Critically Ill Patients: A Retrospective Study. Front Med (Lausanne) 2021;8:697027. [Crossref] [PubMed]
- Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of Acute Physiology And Chronic Health Evaluation data elements shows comparable predictive accuracy. Crit Care Med 2013;41:1711-8. [Crossref] [PubMed]
- Schwartz DB, Bone RC, Balk RA, et al. Hepatic dysfunction in the adult respiratory distress syndrome. Chest 1989;95:871-5. [Crossref] [PubMed]
- Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018;319:698-710. [Crossref] [PubMed]
- Yang P, Formanek P, Scaglione S, et al. Risk factors and outcomes of acute respiratory distress syndrome in critically ill patients with cirrhosis. Hepatol Res 2019;49:335-43. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Marsland B. The Gut-Liver-Lung Axis. Modulation of the Innate Immune Response and Its Possible Role in Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Biol 2016;54:161-9. [Crossref] [PubMed]
- Graw JA, Hildebrandt P, Krannich A, et al. The role of cell-free hemoglobin and haptoglobin in acute kidney injury in critically ill adults with ARDS and therapy with VV ECMO. Crit Care 2022;26:50. [Crossref] [PubMed]
- Shaver CM, Upchurch CP, Janz DR, et al. Cell-free hemoglobin: a novel mediator of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2016;310:L532-41. [Crossref] [PubMed]
- Ghosh S, Flage B, Weidert F, et al. P-selectin plays a role in haem-induced acute lung injury in sickle mice. Br J Haematol 2019;186:329-33. [Crossref] [PubMed]
- Ryter SW. Significance of Heme and Heme Degradation in the Pathogenesis of Acute Lung and Inflammatory Disorders. Int J Mol Sci 2021;22:5509. [Crossref] [PubMed]
- Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017;377:562-72. [Crossref] [PubMed]
- Quinton LJ, Jones MR, Robson BE, et al. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect Immun 2009;77:2417-26. [Crossref] [PubMed]
- Cui J, Zhao H, Yi B, et al. Dexmedetomidine Attenuates Bilirubin-Induced Lung Alveolar Epithelial Cell Death In Vitro and In Vivo. Crit Care Med 2015;43:e356-68. [Crossref] [PubMed]
- Bortolussi G, Muro AF. Experimental models assessing bilirubin neurotoxicity. Pediatr Res 2020;87:17-25. [Crossref] [PubMed]
- Qian S, Kumar P, Testai FD. Bilirubin Encephalopathy. Curr Neurol Neurosci Rep 2022;22:343-53. [Crossref] [PubMed]
- Brito MA, Brites D, Butterfield DA. A link between hyperbilirubinemia, oxidative stress and injury to neocortical synaptosomes. Brain Res 2004;1026:33-43. [Crossref] [PubMed]
- Dani C, Martelli E, Tronchin M, et al. Bilirubin influence on oxidative lung damage and surfactant surface tension properties. Pediatr Pulmonol 2004;38:179-85. [Crossref] [PubMed]
- Brienza N, Dalfino L, Cinnella G, et al. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med 2006;32:267-74. [Crossref] [PubMed]


