
Losartan attenuates upstream vasculopathy in a modified piglet model of pulmonary vein stenosis: contribution of the Hippo pathway
Introduction
Pulmonary vein stenosis (PVS) is an intractable congenital heart disease which generally has a poor prognosis, especially in children with progressive vasculopathy or multivessel involvement (1,2). Patients occasionally develop PVS after surgery involving the pulmonary veins, called postrepair PVS, typically after repairing the total anomalous pulmonary venous connection. The incidence of postrepair PVS is up to 15%, and the 1-year survival is only 55–63% (1,3,4). Stent angioplasty has limitations in the treatment of these patients due to high rates of in-stent stenosis, resulting from in-stent neo-intimal proliferation and the small size of the deployed stent (1,5). Sutureless repair is helpful for patients with focal stenosis adjacent to the venoatrial junction; however, it is not helpful in treating peripheral lesions beyond the first-order division within the pulmonary parenchyma (3,6). Multiple adjuvant therapies are controversial due to their adverse effects, such as vinblastine and methotrexate administration (7), yet systemic sirolimus has been recently reported to have a beneficial effect in PVS (5,8). Addressing diffuse upstream PVS remains a significant challenge.
PVS is characterized by intimal hyperplasia, pulmonary hypertension, and right heart failure, as well as cell accumulation and extracellular matrix (ECM) deposition in venous lesions (1,2,9). Our previous piglet model of PVS recapitulated the clinical and pathological characteristics of PVS patients, and losartan treatment was shown to improve PVS progression and the resultant pulmonary hypertension (10). We also found myofibroblast-like cells in the neointima and alteration of transforming growth factor-β (TGF-β) in the stenotic upstream pulmonary veins (11). Losartan is an angiotensin II type 1 receptor (AT1R) blocker which has been used widely in hypertension and heart failure. The idea of investigating losartan in PVS stems from its application in a mouse model of Marfan syndrome, where it targeted the TGF-β signaling pathway to prevent aortic aneurysm (12). However, losartan treatment did not change the expression of TGF-β, leading us to speculate that an alternative signaling pathway is involved in PVS formation independent of TGF-β signaling (10).
The Hippo pathway regulates cell growth and death. It controls human tumor neoplasia and metastasis, fibrosis, organ development, and regeneration (13,14). As the core components of the Hippo pathway, yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) regulate the expression of downstream genes through their dephosphorylation and nuclear translocation. Multiple extracellular signals induce YAP/TAZ activation, such as mechanical stress, cell-cell contact, and many hormonal factors, most of which act through G protein-coupled receptors (GPCRs) (13). Few studies have focused on the role of the Hippo pathway in vascular remodeling, and the Hippo pathway has not been studied in PVS. AT1R, as one of the GPCR family members, has been reported to mediate the activation of YAP (13,15,16). However, it is still unclear whether YAP activation is involved in PVS formation. Therefore, we hypothesized that Hippo pathway signaling might play an important role in developing PVS and be regulated by losartan treatment. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2621/rc).
Methods
All animal operations were approved by the Ethics Committee of Shanghai Xinhua Hospital (No. XHEC-F-2019-001), in compliance with the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals, 8th edition. To allow for adaptation to the new environment, the animals were operated 2 weeks after their arrival at the animal center of Xinhua Hospital, Shanghai Jiaotong University School of Medicine.
Modified PVS model and tissue harvesting
In the previous PVS model, each neonatal piglet underwent 2-stage bilateral thoracotomies with all the pulmonary veins banded except for the right middle pulmonary vein (10,11). Considering the ensuing surgical trauma and the fact that the main purpose of the study was to assess the mechanism of upstream vasculopathy, the PVS model was modified, in that only a single left thoracotomy was performed with a narrower banding compared with that used in the previous model. After administration of general anesthesia and intubation, the piglets underwent left thoracotomy through the fifth intercostal space. The left upper pulmonary vein and the common trunk of both lower pulmonary veins were dissected, and the circumference of these veins was measured with cotton string. Then, the vein was banded using a 4-mm-wide Gore-Tex vascular stripe (W. L. Gore & Associates, Flagstaff, AZ, USA) with a length equivalent to its physiological circumference (Figure 1A,1B). Progressive PVS occurred as piglets grew.
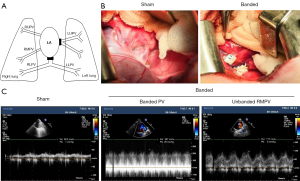
A total of 19 neonatal male piglets (3–4 weeks old, weighing about 5–6 kilograms, with no surgical history) were used in this study. The piglets were randomly divided into 3 groups: sham-operated (Sham, n=7), pulmonary vein banding (Banded, n=6), and banding with losartan treatment (Losartan, n=6). All operations were performed by the same surgeons. The sham group underwent left thoracotomy and dissection of the veins (Figure 1B). The piglets with pulmonary veins banded were randomly allocated to the banded group or the losartan group. The animals in the losartan group were orally administered 1 mg/kg/d losartan (Cozaar, Merck Sharp & Dohme Ltd., London, UK) from the second day after the operation to the end of the study; meanwhile, piglets in the sham and banded groups were given placebos. No adverse events were observed.
At 8 weeks after the operation, the piglets were anesthetized and intubated. Echocardiography was performed to evaluate PVS (stenosis was defined as the pulmonary vein blood flow spectrum showing continuous flow and a mean pressure gradient ≥3 mmHg). Then, the animals underwent sternotomy, and invasive hemodynamic parameters were measured, including pulmonary artery pressure (PAP), aortic blood pressure (ABP), right ventricular pressure (RVP), and left ventricular pressure (LVP). After heparinization, the entire heart and lungs were quickly harvested, rinsed with a large amount of normal saline, and placed in an ice bath to obtain tissues. The banding sites were mobilized to confirm the formation of PVS. We considered the pulmonary vein far from the banding site (greater than 3 cm) as an upstream vein.
Histologic examination and immunofluorescence
Pulmonary vein tissues embedded in paraffin were sectioned and stained with hematoxylin-eosin and Masson trichrome. Quantification of the vessel area was performed as previously reported using Image J 4.3 (National Institutes of Health, Bethesda, MD, USA) (10). The intimal area was between the endothelial layer and the inner elastic layer, and the median area was between the inner elastic layer and the outer border of the smooth muscle layer. The radius was calculated from the layer perimeter.
The quantification of intimal hyperplasia was reported using the ratio of the intima area to the median area (IA/MA). Furthermore, to avoid underestimation of hypertrophy due to regional hyperplasia in the segments, a complementary calibrated index was used to normalize the intimal area to the square of the radius (IA/R2). All measurements were performed under blinded conditions.
For immunofluorescence, paraffin-embedded pulmonary vein sections were deparaffinized in xylene and rehydrated in ethanol. The antigen was re-exposed to sodium citrate buffer, soaked in blocking buffer for 40 min, incubated with primary antibody for 2 h, washed in phosphate-buffered saline (PBS), and labeled with a secondary antibody with fluorophore. The nucleus was stained with 4’,6-diamidino-2-phenylindole and dihydrochloride (DAPI, 1:1,000; Thermo Fisher, Waltham, MA, USA). The endothelial cell marker was CD31 (1:100, Abcam, Cambridge, UK), and the mesenchymal marker was α-smooth muscle actin (1:50, Santa Cruz Biotechnology, Dallas, TX, USA). Other immunofluorescence antibodies included YAP (1:100, Santa Cruz Biotechnology) and AT1R (1:50, Abcam).
Protein extraction and western blotting
The tissues were homogenized, or the cells were lysed and then centrifuged (15 min, 12,000 rpm, 4 ℃); thereafter, the supernatant was collected. Then, the Bradford protein assay was used to quantify the protein concentration. Protein samples were separated using sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane, and blocked with 5% skimmed milk for 1 h. Then, the membrane was probed with YAP (1:1,000, Santa Cruz Biotechnology), AT1R (1:1,000, Abcam), and phosphorylated-YAP [1:1,000, Cell Signaling Technology (CST), Danvers, MA, USA] at 4 ℃ overnight and then incubated with the corresponding secondary antibody. An Immobilon Western Chemiluminescent HRP Substrate Kit (Merck Millipore, Burlington, MA, USA) was used for detection. Molecules α-tubulin and β-actin were used as loading controls to normalize the data. Quantification of western blot data was performed using Image J V4.
Cell culture
Human umbilical vein endothelial cells [HUVECs; American Type Culture Collection (ATCC), Rockville, MD, USA] were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% ampicillin/streptomycin (Sangon Biotech, Shanghai, China) at 37 ℃ with 5% CO2. Human angiotensin II (Ang II) and losartan potassium were obtained from MedChemExpress (Monmouth Junction, NJ, USA).
Cell proliferation
Cell proliferation was evaluated using a cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Rockville, MD, USA) following the manufacturer’s instructions. Briefly, HUVECs (2×103 per well) were plated in 96-well plates overnight under low (10%) serum concentration condition. After cell adherence, they were cultured with Ang II or losartan at various concentrations (from 0.1 to 100 µmol/L). Cells treated with dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) served as the positive controls. After 96 h of treatment, 10 µL of CCK-8 solution was added to each well and incubated for 1 h at 37 ℃. Then, the absorbance of the solvent at 450 nm was measured, and the background was deducted in each well. The relative cell proliferation of each treatment group was expressed as the percentage change relative to the positive control group on the same day.
Quantitative real-time polymerase chain reaction
According to the manufacturer’s instructions, total RNA was extracted from the cultured cells using TRIzol reagent (Invitrogen, Waltham, MA, USA). Reverse transcription was performed from 1 µg of the total RNA using PrimeScriptTM RT Master Mix (Takara, Kusatsu, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out using SYBR Premix ExTaqTM (Takara) and an Applied Biosystems 7500 Fast real-time PCR system (Thermo Fisher). The relative expression of messenger RNA (mRNA) was evaluated using the 2−ΔΔCt method and was normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences are presented in Table 1. All experiments were repeated in triplicate.
Table 1
| Names | Primer sequences |
|---|---|
| CTGF | Forward: CCTGCAGGCTAGAGAAGCAG |
| Reverse: TGGAGA TTTTGGGAGTACGG | |
| CYR61 | Forward: AAGAAACCCGGATTTGTGAG |
| Reverse: GCTGCA TTTCTTGCCCTTT | |
| ANKRD1 | Forward: AGTAGAGGAACTGGTCACTGG |
| Reverse: TGGGCTAGAAGTGTCTTCAGAT | |
| AXL | Forward: GGTGGCTGTGAAGACGATGA |
| Reverse: CTCAGA TACTCCATGCCA | |
| TGM2 | Forward: CCCAGCAGGGCTTTATCTACCA |
| Reverse: GCAGA TGTCTAGGATCCCATCTTCA | |
| LATS2 | Forward: ACTTTTCCTGCCACGACTTATTC |
| Reverse: GATGGCTGTTTTAACCCCTCA | |
| GAPDH | Forward: GGAGCGAGATCCCTCCAAAAT |
| Reverse: GGCTGTTGTCATACTTCTCATG |
CTGF, connective tissue growth factor; CYR61, cysteine-rich angiogenic inducer 61; ANKRD1, ankyrin repeat domain-containing protein 1; AXL, anexelekto, tyrosine-protein kinase receptor UFO; TGM2, transglutaminase 2; LATS2, large tumor suppressor kinase 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
The data of all animals were included. Continuous data were expressed as the mean ± SD. An independent-samples t-test was performed to compare data between the 2 groups. One-way analysis of variance (ANOVA) and a nonparametric test were used to compare the hemodynamic or quantitative data among the 3 groups. Bonferroni comparisons were performed within groups. The Mann-Whitney U test and Kruskal-Wallis H test were used as nonparametric tests for nonnormally distributed data. Column charts and polyline graphs were prepared using GraphPad Prism version 8.3 (GraphPad Software, Inc., San Diego, CA, USA); SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. A P value <0.05 was considered significant.
Results
Losartan treatment improved banding-induced PVS and pulmonary arterial hypertension
As shown in Figure 1C, a continuous blood flow pattern was detected in the banded vein, and the blood velocity was accelerated, in contrast to the low-velocity flow in the sham group. The hemodynamic results of the piglets are shown in Table 2. Compared with the sham group, the body weight of piglets in the banded group was significantly restricted 8 weeks after the operation, along with an increase in the mean PAP (29±4.7 vs. 14±1.3 mmHg, P<0.01). Significant differences were also observed in the ratio of systolic PAP to ABP (0.43±0.09 in the banded group vs. 0.21±0.04, P<0.01 the sham group) and systolic RVP to LVP (0.48±0.09 in the banded group vs. 0.27±0.07, P<0.01 the sham group). In addition, the average heart rate and central venous pressure in the banded group were markedly increased (P<0.01); however, no significant changes were found in aortic blood pressure and left atrial pressure.
Table 2
| Variables | Sham (n=7) | Banded (n=6) | Losartan (n=6) |
|---|---|---|---|
| Preoperative weight (kg) | 6.5±1.1 | 5.7±1.5 | 6±1.2 |
| Weight before death (kg) | 25.5±2.4 | 21.9±1.6* | 22.8±1.9* |
| Heart rate (beats/min) | 80±5.6 | 91±7.1* | 85±6.4 |
| Systolic PAP (mmHg) | 17±2.3 | 38.5±6.5* | 26±7.2*# |
| Diastolic PAP (mmHg) | 11±2.1 | 22.5±3.5* | 14.7±3.7*# |
| Mean PAP (mmHg) | 14±1.3 | 29±4.7* | 20.1±6.2*# |
| Systolic ABP (mmHg) | 83.5±1.5 | 89±10 | 87±5 |
| Diastolic ABP (mmHg) | 53.5±4.5 | 62.5±11.5 | 63.3±3.7* |
| Mean ABP (mmHg) | 64.5±2.5 | 71.5±8.7 | 69.5±6.5 |
| Systolic PAP/ABP | 0.21±0.04 | 0.43±0.09* | 0.3±0.07*# |
| Systolic RVP/LVP | 0.27±0.07 | 0.48±0.09* | 0.36±0.07*# |
| CVP (mmHg) | 2.5±1.3 | 5±2.1* | 4.2±1.8 |
| Mean LAP (mmHg) | 6.5±2.5 | 8.2±3 | 7.3±3.2 |
Data are described as mean ± SD. *P<0.05 vs. sham; #P<0.05 vs. banded. PAP, pulmonary artery pressure; ABP, aortic blood pressure; RVP, right ventricular pressure; LVP, left ventricular pressure; CVP, central vein pressure; LAP, left atrial pressure; SD, standard deviation.
Compared with the banded group, the PAP in the losartan group was significantly reduced after 8 weeks of losartan treatment (P<0.01). A significant decrease was also observed in the systolic PAP/ABP ratio (0.3±0.07 in the losartan treatment group vs. 0.43±0.09 in the banded group, P<0.05) and systolic RVP/LVP ratio (0.36±0.07 in the losartan treatment group vs. 0.48±0.09 in the banded group, P<0.05). Nevertheless, these two ratios in both treatment groups were still greater than those in the sham group (all P<0.05).
Losartan reduced intimal hyperplasia and YAP expression in banded veins
The histological results of pulmonary vein samples from piglets (Figure 2A-2E) demonstrated significant intimal hyperplasia in the banded veins with spindle-like cell accumulation. These neointimal alterations were also found in the losartan group, but were less dominant than those in the banded group. In contrast, there were monolayer endothelial cells in the sham group, and they were parallel to the lumen. Alteration was not seen in the unbanded right middle pulmonary vein (RMPV) in either the banded or losartan group.
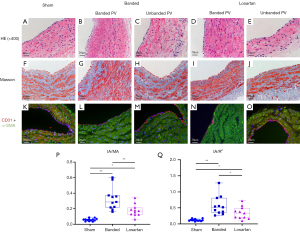
Masson trichrome staining indicated collagen fiber deposition and cell accumulation in the neointima in banded veins, but these were not found in the unbanded RMPV of the same group (Figure 2F-2J). Immunofluorescence, as shown in Figure 2K-2O, demonstrated a reduction in CD31 expression and an increase in α-smooth muscle actin intensity in the neointima of the banded pulmonary vein, which were partially reversed after the use of losartan, yet these changes were not evident in unbanded RMPV.
The results of the quantified intimal hyperplasia are shown in Figure 2P,2Q. Compared with the sham group, there was apparent intimal hyperplasia in the banded and the losartan groups, but the extent of hypertrophy in the losartan group was less than that in the banded group.
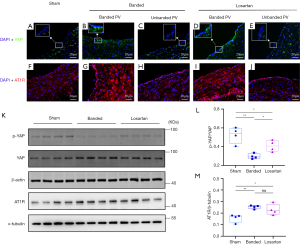
In the banded group, immunofluorescence staining indicated that YAP was upregulated in the banded veins, especially in the endothelial layer, compared with the sham group (Figure 3A-3J). In contrast, the YAP expression decreased after losartan treatment, but it was still more significant than that in the sham group. Quantitative YAP activation, presented as the ratio of phosphorated YAP to total YAP from western blotting demonstrated a significant elevation in the banded group, and this was partially alleviated in the losartan group (Figure 3K,3L). Additionally, AT1R in upstream pulmonary veins had a similar alteration trend as YAP expression among the groups, indicating the enrichment of both YAP and AT1R in the upstream veins (Figure 3K,3M).
Losartan inhibited HUVEC proliferation in vitro
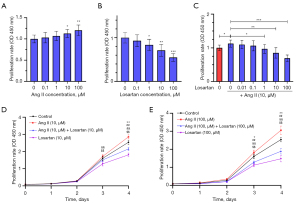
As shown in Figure 4A, Ang II induced HUVEC proliferation at 10 µmol/L. When HUVECs were incubated with losartan, cell proliferation was also significantly inhibited at over 1 µmol/L (Figure 4B). Ang II and losartan both acted in a dose-dependent manner. Furthermore, losartan inhibited the proliferation of HUVECs induced by Ang II (10 µmol/L) when losartan reached over 1 µmol/L (Figure 4C). Concerning the time-dependent effects, HUVECs were incubated with Ang II and losartan at 10 µmol/L. A significant cytostatic effect occurred after 4-day treatment (Figure 4D), and it advanced to the third day when both concentrations were increased to 100 µmol/L (Figure 4E).
Losartan inhibited YAP activation and downstream gene expression in vitro
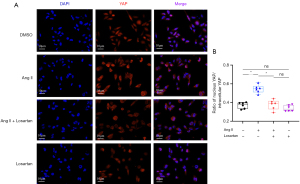
When incubated with Ang II or losartan under serum-free conditions, both had little effect on total YAP in HUVECs. After 4 h of incubation, Ang II and losartan showed significant opposite effects on YAP phosphorylation (Figure 5A-5C). When Ang II plus losartan were administered, losartan effectively reduced the YAP dephosphorylation induced by Ang II in HUVECs (Figure 5D,5E). In terms of downstream gene target of the YAP signaling, Ang II stimulated YAP activation and, therefore, further increased the mRNA levels of YAP/TEAD complex target genes, including CTGF, CYR61, ANKRD1, AXL, TGM2, and LATS2. Losartan treatment inhibited the upregulation of these genes induced by Ang II (Figure 5F,5G). Immunofluorescence results of coincubation with Ang II and losartan are shown in Figure 6. Ang II significantly stimulated YAP transfer from the cytoplasm to the nucleus in HUVECs. In contrast, losartan treatment significantly reduced Ang II-induced YAP nuclear translocation.

Discussion
In this modified PVS model of piglets, pulmonary hypertension, right ventricular hypertension, and diffuse intimal hyperplasia in upstream pulmonary veins were observed, which is consistent with the main clinicopathological characteristics of patients with PVS and previous PVS piglets (10,11). Upregulation of YAP and AT1R expression in banded pulmonary veins indicated their involvement in PVS progression. Losartan administration inhibited intimal hyperplasia and improved pulmonary hypertension. Losartan inhibited YAP dephosphorylation and nuclear translocation in vitro, and this may have contributed to its effect in the PVS model.
Modification of the PVS model of piglets
Given our experience of the previous model, bilateral 2-stage thoracotomy was commonly associated with more surgical bias and slower recovery of animals. Furthermore, previously, only the right middle pulmonary vein was banded during second-stage surgery, which had a limited effect on hemodynamics (10). As the primary purpose of this model is to investigate the underlying mechanisms of PVS, 1-stage surgery encompassing left thoracotomy and banding the left upper pulmonary vein and common trunk of both lower pulmonary veins should be appropriate. Moreover, to create quicker PVS formation, the pulmonary veins were banded with a length equivalent to their physiological circumference, which was narrower than the previous model (by 1.3 times). The unbanded right upper and right middle pulmonary veins may compensate for the adverse effects of pulmonary vein banding and improve the model’s survival. Postoperative hemodynamic measurements revealed evident stenosis formation in the upstream pulmonary veins and pulmonary and right ventricular hypertension. Meanwhile, intimal hyperplasia replicates the main pathological characteristic of PVS, as in the previous model (10,11). This animal model seems to be capable of investigating the mechanism of PVS and has the advantage of a simplified surgical procedure.
Hippo-YAP pathway signaling in the PVS formation
Multiple mechanical signals, such as liquid shear stress, cellular contact alteration, and matrix stiffness, have been reported to activate YAP through Rho GTPase and the Hippo pathway (13,16). As the piglets grew, the stenosis of the banded segments resulted in accelerated blood flow and caused turbulent flow, leading to signaling pathway stimulation. It has been reported that laminar shear stress inhibits YAP activation, but oscillatory shear stress induces YAP activation and vasculopathy (17,18). Thus, the turbulent flow around the banding site can stimulate the YAP at the beginning of the stenosis.
In the present study, the quantification of western blots showed elevated YAP levels in the banded animals; however, YAP levels were unaffected in the in vitro model. The discrepancy of total YAP in vivo and in vitro resulted mainly from the samples. In vivo, the result was obtained from the full thickness of the blood vessel containing multiple types of cells (endothelial cell, smooth muscle cell, fibroblast etc.), which was different from the isolated endothelial cells in vitro. In addition, other factors were involved in the PVS formation, including hemodynamic changes and various cytokines, which were different from the in vitro conditions.
Further to the upregulation of YAP expression in stenotic venous lesions, histology also revealed significant extracellular collagen deposition and matrix stiffness in the neointima. Studies on idiopathic pulmonary fibrosis and myocardial infarction have indicated that matrix stiffness increases YAP/TAZ in fibroblasts and further promotes organic fibrosis and remodeling (19,20). YAP activation also upregulates profibrotic factors, such as connective tissue growth factor and plasminogen activator inhibitor-1, leading to more extracellular protein deposition (13). Enhancement of ECM can result from YAP activation and drive fibroblast activation through YAP and TAZ (21,22). The positive feedback circle between YAP and matrix deposition may partially explain the poor prognosis of children with progressively worsening PVS. Therefore, inhibition of YAP activation seems to be a promising therapeutic strategy in PVS prevention.
Losartan improved intimal hyperplasia and regulated YAP activation
In our study, losartan administration significantly improved PVS formation and resultant pulmonary hypertension in piglets, although the pulmonary pressure was still higher than that of the sham group. Losartan is a receptor blocker of AT1R, which is a typical GPCR family member. It has been reported that AT1R can stimulate multiple signaling pathways involving oxidative stress, ECM synthesis, and tissue fibrosis (23). In addition, the activation of AT1R can directly stimulate Rho-kinase and then promote YAP/TAZ activation and corresponding gene expression (13). Saikawa et al. (24) demonstrated that losartan treatment inhibited YAP activation and attenuated cancer cell growth of cholangiocarcinoma in mice. Niu et al. (21) found that matrix stiffness activated YAP via AT1R in cardiac fibroblasts of myocardial infarction rats.
In the present study, intimal hyperplasia was observed in the banded veins and was effectively reduced after losartan treatment. However, in vitro, an interesting result of incubation with losartan is shown in Figure 4B, where losartan alone inhibited cell proliferation, which suggests that an AT1R independent pathway might be involved in the cytostatic effect of losartan. Furthermore, the in vitro results demonstrated that losartan treatment inhibited YAP dephosphorylation and YAP transferal into the nuclei. All findings suggest that blocking AT1R with losartan may partially cause the amelioration of stenosis progression, probably through inhibiting the Hippo-YAP pathway.
Current research in PVS
Current studies in PVS have been well summarized in several recent reviews (1,25-28). In the past 2 decades, despite great technological advancements, neither catheterization intervention nor surgical repair alone has achieved significant improvement in the outcome of PVS (1). Risk factors for unfavorable outcomes, such as mortality, restenosis, and reintervention, have included multivessel PVS, bilateral PVS, high severity scores, and pulmonary hypertension (28-31). However, patients enrolled in previous studies were quite heterogeneous, and to address this issue, several severity scores were developed to evaluate the baseline scores of patients with PVS (28). Early diagnosis, early intervention, and involvement of multidisciplinary teams are recommended. Most importantly, in recent years, disappointing outcomes have led to a focus on adjunctive therapy.
There have been several studies on adjuvant therapy in addition to optimal interventional and surgical management in children with PVS. Most of them have been based on the pathological findings of pulmonary vein samples from patients with PVS (9,32). Progressive intimal hyperplasia is the main characteristic of PVS, and neointimal lesions include spindle-like cell proliferation, most likely myofibroblasts, and deposition of ECM (1).
Methotrexate and vinblastine, 2 chemotherapeutic agents, were administered in a pilot phase II trial, and Rehman et al. (7) found that the 1-year survival was 38%, and chemotherapy-related toxicities were common. Imatinib, a tyrosine kinase inhibitor, and bevacizumab, an angiogenesis blocker, were given to patients with multiple affected pulmonary veins in a single-arm trial at Boston Children’s Hospital (33). Survival at 72 weeks was 77%, and minimal toxicity specific to tyrosine kinase blockade was found. Vanderlaan et al. from Toronto very recently reported a pilot trial of losartan in patients with multiple affected pulmonary veins (NCT02769130) (1). Finally, a recent landmark study by Patel et al. (8) showed significant survival benefits associated with systemic sirolimus administration in infants and children with moderate-to-severe PVS. Sirolimus is an mTOR inhibitor, and it was also found to be effective in a pig pulmonary vein obstruction model (34). In addition, Callahan et al. (5) found that systemic sirolimus attenuated in-stent stenosis in pediatric pulmonary vein stenosis. Validation of systemic sirolimus administration requires further multicenter clinical trials.
Limitations
First, due to the limitations related to the preservation of blood and tissue samples in our study, we did not assess the concentration of angiotensin in piglet blood or the isolation of primary endothelial cells, which compromised this study, especially in relation to the relevance of differences between in vivo and in vitro experiments. Second, an AT1R-independent mechanism might contribute to PVS progression. Further studies are needed to identify upstream and downstream signaling in PVS. Third, this study only focused on the role of endothelial cells, yet significant ECM deposition was also observed in the neointima, as well as accumulation of fibroblast-like cells. This suggests the importance of fibroblasts in PVS progress. Using HUVECs as tool cells is not sufficient to repeat the scenario in vivo, and, thus, our next step is to investigate the role of fibroblasts. Finally, a translational study from bench to bedside might encounter differences between species, highly variable patient cohorts, more confounding factors, or other issues. Therefore, our results from using an animal model should be interpreted cautiously.
Conclusions
Intimal hyperplasia and pulmonary hypertension were detected after pulmonary vein banding, and they were improved after losartan treatment in a modified piglet model. The activation of the Hippo-YAP pathway plays an important role in the progression of PVS. Losartan can inhibit YAP activation and reduce YAP dephosphorylation and may contribute to alleviation of PVS-related vasculopathy.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81600219 to J Zhu).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2621/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2621/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2621/coif). JZ reports that this work was supported by the National Natural Science Foundation of China (No. 81600219). The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vanderlaan RD, Rome J, Hirsch R, et al. Pulmonary vein stenosis: Treatment and challenges. J Thorac Cardiovasc Surg 2021;161:2169-76. [Crossref] [PubMed]
- Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation 2007;115:103-8. [Crossref] [PubMed]
- Viola N, Alghamdi AA, Perrin DG, et al. Primary pulmonary vein stenosis: the impact of sutureless repair on survival. J Thorac Cardiovasc Surg 2011;142:344-50. [Crossref] [PubMed]
- Prieto LR. In Search of the Holy Grail for Pediatric Pulmonary Vein Stenosis. J Am Coll Cardiol 2021;77:2819-21. [Crossref] [PubMed]
- Callahan R, Esch JJ, Wang G, et al. Systemic Sirolimus to Prevent In-Stent Stenosis in Pediatric Pulmonary Vein Stenosis. Pediatr Cardiol 2020;41:282-9. [Crossref] [PubMed]
- Shi G, Zhu Z, Chen J, et al. Total Anomalous Pulmonary Venous Connection: The Current Management Strategies in a Pediatric Cohort of 768 Patients. Circulation 2017;135:48-58. [Crossref] [PubMed]
- Rehman M, Jenkins KJ, Juraszek AL, et al. A prospective phase II trial of vinblastine and methotrexate in multivessel intraluminal pulmonary vein stenosis in infants and children. Congenit Heart Dis 2011;6:608-23. [Crossref] [PubMed]
- Patel JD, Briones M, Mandhani M, et al. Systemic Sirolimus Therapy for Infants and Children With Pulmonary Vein Stenosis. J Am Coll Cardiol 2021;77:2807-18. [Crossref] [PubMed]
- Kovach AE, Magcalas PM, Ireland C, et al. Paucicellular Fibrointimal Proliferation Characterizes Pediatric Pulmonary Vein Stenosis: Clinicopathologic Analysis of 213 Samples From 97 Patients. Am J Surg Pathol 2017;41:1198-204. [Crossref] [PubMed]
- Zhu J, Ide H, Fu YY, et al. Losartan ameliorates "upstream" pulmonary vein vasculopathy in a piglet model of pulmonary vein stenosis. J Thorac Cardiovasc Surg 2014;148:2550-7. [Crossref] [PubMed]
- Kato H, Fu YY, Zhu J, et al. Pulmonary vein stenosis and the pathophysiology of "upstream" pulmonary veins. J Thorac Cardiovasc Surg 2014;148:245-53. [Crossref] [PubMed]
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006;312:117-21. [Crossref] [PubMed]
- Ma S, Meng Z, Chen R, et al. The Hippo Pathway: Biology and Pathophysiology. Annu Rev Biochem 2019;88:577-604. [Crossref] [PubMed]
- Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015;163:811-28. [Crossref] [PubMed]
- Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012;150:780-91. [Crossref] [PubMed]
- Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov 2020;19:480-94. [Crossref] [PubMed]
- Wang L, Luo JY, Li B, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016;540:579-82. [Crossref] [PubMed]
- Wang KC, Yeh YT, Nguyen P, et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 2016;113:11525-30. [Crossref] [PubMed]
- Francisco J, Zhang Y, Jeong JI, et al. Blockade of Fibroblast YAP Attenuates Cardiac Fibrosis and Dysfunction Through MRTF-A Inhibition. JACC Basic Transl Sci 2020;5:931-45. [Crossref] [PubMed]
- Liu F, Lagares D, Choi KM, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 2015;308:L344-57. [Crossref] [PubMed]
- Niu L, Jia Y, Wu M, et al. Matrix stiffness controls cardiac fibroblast activation through regulating YAP via AT1 R. J Cell Physiol 2020;235:8345-57. [Crossref] [PubMed]
- Calvo F, Ege N, Grande-Garcia A, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 2013;15:637-46. [Crossref] [PubMed]
- Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev 2018;98:1627-738. [Crossref] [PubMed]
- Saikawa S, Kaji K, Nishimura N, et al. Angiotensin receptor blockade attenuates cholangiocarcinoma cell growth by inhibiting the oncogenic activity of Yes-associated protein. Cancer Lett 2018;434:120-9. [Crossref] [PubMed]
- Frank DB, Levy PT, Stiver CA, et al. Primary pulmonary vein stenosis during infancy: state of the art review. J Perinatol 2021;41:1528-39. [Crossref] [PubMed]
- Humpl T, Fineman J, Qureshi AM. The many faces and outcomes of pulmonary vein stenosis in early childhood. Pediatr Pulmonol 2021;56:649-55. [Crossref] [PubMed]
- Vanderlaan RD, Caldarone CA. Pulmonary Vein Stenosis: Incremental Knowledge Gains to Improve Outcomes. Children (Basel) 2021;8:481. [Crossref] [PubMed]
- Backes CH, Nealon E, Armstrong AK, et al. Pulmonary Vein Stenosis in Infants: A Systematic Review, Meta-Analysis, and Meta-Regression. J Pediatr 2018;198:36-45.e3. [Crossref] [PubMed]
- Kalfa D, Belli E, Bacha E, et al. Primary Pulmonary Vein Stenosis: Outcomes, Risk Factors, and Severity Score in a Multicentric Study. Ann Thorac Surg 2017;104:182-9. [Crossref] [PubMed]
- Kalfa D, Belli E, Bacha E, et al. Outcomes and prognostic factors for postsurgical pulmonary vein stenosis in the current era. J Thorac Cardiovasc Surg 2018;156:278-86. [Crossref] [PubMed]
- Feng Z, Mao F, Ma K, et al. Clinical Outcomes Predictors and Surgical Management of Primary Pulmonary Vein Stenosis. Ann Thorac Surg 2022;113:1239-47. [Crossref] [PubMed]
- Riedlinger WF, Juraszek AL, Jenkins KJ, et al. Pulmonary vein stenosis: expression of receptor tyrosine kinases by lesional cells. Cardiovasc Pathol 2006;15:91-9. [Crossref] [PubMed]
- Callahan R, Kieran MW, Baird CW, et al. Adjunct Targeted Biologic Inhibition Agents to Treat Aggressive Multivessel Intraluminal Pediatric Pulmonary Vein Stenosis. J Pediatr 2018;198:29-35.e5. [Crossref] [PubMed]
- Masaki N, Adachi O, Katahira S, et al. Progression of vascular remodeling in pulmonary vein obstruction. J Thorac Cardiovasc Surg 2020;160:777-790.e5. [Crossref] [PubMed]
(English Language Editors: B. Meiser and J. Jones)


