
Heme oxygenase-1 as a predictor of sepsis-induced acute kidney injury: a cross-sectional study
Introduction
Bacterial sepsis results in millions of deaths annually (1,2). There is evidence to show that sepsis enables an initial systemic inflammation to progress to an acute local inflammation of multiple organs, which finally leads to organ failure, of which acute kidney injury (AKI) is of particular clinical relevance (3). Sepsis associated AKI have been commonly diagnosed by Kidney Disease: Improving Global Outcomes (KDIGO) standards primarily based on urine volume and serum creatinine (Scr) levels. This has been questioned because of the relatively late discovery. AKI is not reversible in many cases and causes chronic renal failure, end-stage kidney disease and death. Managing sepsis complicated with AKI is a particular challenge because it is associated with high mortality (4). While there is much in the literature regarding the pathogenesis of AKI, the precise mechanisms underlying the progression of sepsis-induced AKI remain unclear. Numerous of experts were concerned with biomarkers for the early diagnosis of sepsis-induced AKI. However, no single marker has sufficient sensitivity or specificity to accurately determine acute kidney injury in sepsis. Once the organism responds to pathological stimulation, the oxidative stress and inflammation exerts mutual reinforcement to further insult the host (5,6). The role of oxidative stress in the development of AKI is being increasingly recognized (7,8). Several studies have suggested that heme oxygenase-1 (HO-1) plays an antioxidant role in the progression of acute organ injury (9-11).
HO-1 is a functional isoform of heme oxygenase and is the rate limiting enzyme of heme catabolism in mammalian cells. Free heme is released upon tissue damage from various heme-containing proteins including hemoglobin, myoglobin (MYO) and cytochromes, and leads to apoptosis, inflammation and oxidative stress (12). Upregulation of HO-1 is associated with increases in cytoprotective molecules such as ferritin, carbon monoxide, and biliverdin (13). The metabolic products of heme are generated after the upregulation of HO-1, which is mainly found in the liver, spleen, and lungs (14-16) and can be monitored with HO-1 serum levels (17,18). In patients with abdominal aortic aneurysm, HO-1 serum levels correlate with arterial HO-1 expression, and both levels are positively associated with disease severity (i.e., aortic rupture) and vaso-protective alterations of oxidative stress (19). Therefore, it is necessary to understand the cytoprotective role of HO-1 in the inflammatory response to organ injury (20,21). Most importantly, the regulation of HO-1 expression and secretion may be a protective response for the development of AKI (22,23).
Studies in animal models of sepsis have shown that HO-1 knock-out leads to an exaggerated inflammatory response including renal dysfunction (24). These results are reason to believe that HO-1 may protect against sepsis-AKI (25,26). Renal thrombotic microangiopathy, as a consequence of septic coagulopathy, is an indicator of the severity of sepsis and can be attenuated in animal models by hemin-triggered induction of HO-1 (27). Also, the anti-inflammatory effects of resveratrol on renal epithelial cells in vitro involve the HO-1 pathway and confirm possible protective effects of increased HO-1 expression on septic AKI (28).
A previous study of sepsis patients during the first 12 hours in intensive care have shown that HO-1 serum levels correlated positively with the levels of interleukin-10, and both levels were indicators of survival (29). Whether HO-1 expression in sepsis is different between critically ill patients with and without AKI has not yet been reported. Therefore, the present study examined sepsis patients from the First Affiliated Hospital of Dalian Medical University to investigate the association between HO-1 and sepsis-induced AKI. The findings indicate that serum HO-1 measurements may a role in the development of HO-1-targeted therapy of sepsis-induced AKI. The aim of this study was to evaluate whether HO-1 can be used for the diagnosis and prediction of sepsis-induced AKI. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4793/rc).
Methods
Subjects and study design
The criterion for selecting sepsis patients was based on the diagnostic criteria for Sepsis 3.0. Patients with cardiovascular disease or cardiac insufficiency, liver failure, serious allergy, rheumatoid arthritis and impaired immune functions were excluded before enrollment. A total of 109 patients who presented with sepsis at the First Affiliated Hospital of Dalian Medical University, China, from June 2018 to January 2019, were enrolled in this study. Patients who died within 24 hours (n=9), did not have blood tests performed within 24 hours (n=6), or presented with chronic nephropathy (n=8) or malignancies (n=3) were excluded from the study. Finally, a total of 83 sepsis patients fulfilled the inclusion criteria, including 36 septic patients with AKI (S-AKI) and 47 sepsis patients without AKI (NS-AKI). According to the definition of septic shock and the global kidney diagnostic criteria described in the KDIGO, these patients were divided into four groups: the sepsis + shock + AKI group (SS-AKI group, n=18), the sepsis + AKI group (S-AKI group, n=18), the sepsis + shock group (SSN-AKI group, n=20), and the sepsis group (SN-AKI group, n=27). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the First Affiliated Hospital of Dalian Medical University (No. YJ-KY-FB-2019-04). Each patient provided written informed consent prior to enrollment.
Laboratory measurements
The venous blood of patients was obtained within 24 hours after the diagnosis of sepsis. Blood samples were placed at room temperature for 15 minutes. After centrifugation, aliquots of the serum were stored at −80 ℃ for subsequent assays. The HO-1 levels of the serum samples were routinely measured using enzyme-linked immunosorbent assay (ELISA). The ELISA assay was evaluated using two different cut-off values. The following physiological parameters were applied for the routine confirmation of sepsis patients admitted to the hospital: HO-1, red blood cell (RBC) count, hemoglobin (HB), hematocrit (HCT), white blood cell (WBC) count, albumin (ALB), alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), total bilirubin (TBil), creatine kinase MB (CK-MB), troponin I (TnI), urea, myoglobin (MYO), Scr, procalcitonin (PCT), amylase (AMY), lipase, activated partial thromboplastin time (APTT), prothrombin time (PT), fibrinogen (FIB), fibrinogen degradation product (FDP), and D-dimer.
Statistical analysis
According to G power calculation, this single-center cross-sectional study was designed a one-way analysis of variance (ANOVA) with four groups and repeated three tests, and the sample size must above 60. The statistical data was generated using SPSS software (22.0, IBM SPSS Statistics, USA). The data are expressed as mean ± standard deviation, or median (25–75th percentile). The unpaired Student’s t-test was used to evaluate differences between the sepsis AKI patient group and the septic patients without AKI group. The one-way ANOVA was conducted to examine the categorical variables in the SS-AKI group, the S-AKI group, the SSN-AKI group, and the SN-AKI group. The difference of indicators between groups were studied with ordinal logistic regression mode. Spearman’s test was performed to assess the correlation between variables. The receiver operating characteristic (ROC) curve was drawn to predict the incidence of sepsis-induced AKI with calculate area under curve (AUC). A P value <0.05 was considered statistically significant.
Results
The clinical characteristics and laboratory data of sepsis patients
Table 1 presents the characteristics of the 83 sepsis patients enrolled in this study. Most of the patients (56.6%) were male. Among these septic patients, 36 (43.4%) presented with sepsis-induced AKI (S-AKI) and 47 (56.6%) did not have sepsis-induced AKI (NS-AKI). The Sequential Organ Failure Assessment (SOFA) scores (5–10 vs. 3–5, P<0.05, unpaired Student’s t-test) and the length of intensive care unit (ICU) stay (20±18 vs. 32±25, P<0.05, unpaired Student’s t-test) were significantly different between S-AKI patients and NS-AKI patients. Table 2 presents the significant differences in HO-1, CK-MB, TnI, urea, MYO, PCT and APTT between S-AKI patients and NS-AKI patients. There were no statistically significant differences in the other parameters between the two groups (P<0.05, unpaired Student’s t-test).
Table 1
| Variable | Total | Acute kidney injury | P value | |
|---|---|---|---|---|
| Yes (n=36) | No (n=47) | |||
| Age (years) | 65.55±17.77 | 66.59±17.88 | 74.73±17.88 | 0.571 |
| Male, n (%) | 47 (56.6) | 20 (55.6) | 27 (57.4) | 0.983 |
| SOFA score | 5.69±3.06 | 7 [5–10] | 4 [3–5] | 0.001 |
| Sepsis focus, n (%) | 0.46 | |||
| Respiratory | 24 (28.9) | 10 (27.8) | 14 (29.8) | |
| Abdominal | 22 (26.5) | 13 (36.2) | 9 (19.1) | |
| Urinary | 4 (4.8) | 3 (8.3) | 1 (2.1) | |
| Skin | 22 (26.5) | 2 (5.5) | 20 (42.6) | |
| Others | 11 (13.3) | 8 (22.2) | 3 (6.4) | |
| MV, n (%) | 61 (73.5) | 29 (80.6) | 32 (68.1) | 0.56 |
| Length of ICU stay (days) | 27±31 | 20±18 | 32±25 | 0.023 |
| ICU mortality, n (%) | 47 (56.63) | 24 (66.67) | 23 (48.93) | 0.093 |
Data are presented as mean ± SD, n (%), or median [ranges]. SOFA, Sequential Organ Failure Assessment; MV, mechanical ventilation; ICU, intensive care unit.
Table 2
| Variable | Total | Acute kidney injury | P value | |
|---|---|---|---|---|
| Yes (n=36) | No (n=47) | |||
| HO-1 (U/L) | 41.15 (34.66–48.27) | 203.5 (40.16–240.39) | 37.45 (27.65–42.69) | 0.001 |
| RBC (109/L) | 3.78±0.93 | 3.92±0.96 | 3.66±0.91 | 0.279 |
| HB (g/L) | 115.48±28.56 | 118.66±31.11 | 113±26.52 | 0.442 |
| HCT (%) | 35.6 (26.9–40) | 37.05 (28.03–42.55) | 34.1 (26.5–38.45) | 0.243 |
| WBC (109/L) | 13.38 (9.54–17.43) | 13.3 (10.87–17.23) | 13.41 (8.28–17.8) | 0.63 |
| ALB (g/L) | 29.97±7.16 | 30.63±7.98 | 29.46±6.5 | 0.541 |
| ALT (U/L) | 40 (29.5–90.5) | 40.5 (29.25–99.75) | 40 (29–89.5) | 0.99 |
| AST (U/L) | 58 (31–104.5) | 49 (34–158.5) | 59 (30–104.5) | 0.626 |
| ALP (U/L) | 76 (51–105.5) | 81.5 (66.5–112.25) | 67 [46–86] | 0.09 |
| γ-GT (U/L) | 39 [18–87] | 31 (20.25–85) | 41 (17.5–99.5) | 0.708 |
| TBil (µmol/L) | 20 (11.8–31.8) | 18.45 (10.4–38.6) | 20 (12.45–27.95) | 0.7 |
| CK-MB (U/L) | 2.7 (1–7.1) | 4.68 (1.75–11.51) | 1.71 (0.78–5.45) | 0.007 |
| TnI (ug/mL) | 0.17 (0.028–0.58) | 0.302 (0.097–0.785) | 0.097 (0.023–0.392) | 0.048 |
| Urea (µmol/L) | 10.81 (7.21–17.61) | 17.61 (11.35–24.04) | 7.63 (5.58–10.41) | 0.001 |
| MYO (ng/mL) | 227.61 (92.84–625.62) | 476.26 (194.72–872.43) | 165.11 (66.43–364.66) | 0.001 |
| Scr (µmol/L) | 104 [70–162] | 184.5 [123–253] | 72 [58–91] | 0.001 |
| PCT (ng/mL) | 2.05 (0.81–7.74) | 7.34 (1.58–32.93) | 1.36 (0.5–2.83) | 0.001 |
| AMY (U/L) | 80 (53.5–155) | 67 (49.5–169) | 84 (53.5–155) | 0.673 |
| Lipase (U/L) | 94 (29.5–268.5) | 99 (31.25–489.5) | 89 (27.5–197) | 0.742 |
| APTT (s) | 33.2 (26.75–44.3) | 37.4 (29.53–53.53) | 31.4 (25.8–36.65) | 0.04 |
| PT (s) | 14.2 (12.7–16.3) | 14.65 (13.13–18) | 13.8 (12.25–15.25) | 0.049 |
| FIB (g/L) | 3.56 (2.43–5.08) | 3.79 (2.79–5.28) | 3.48 (2.14–4.99) | 0.609 |
| FDP (mg/L) | 15.49 (7.93–46.56) | 23.54 (8.8–56.77) | 15.03 (6.64–43.03) | 0.448 |
| D-dimer (µg/L) | 5,270 [2,660–17,970] | 5,130 [2,855–17,920] | 5,410 [2,570–18,105] | 0.597 |
Data are presented as mean ± SD or median (ranges). HO-1, heme oxygenase-1; RBC, red blood cell; HB, hemoglobin; HCT, hematocrit; WBC, white blood cell; ALB, albumin; ALT, alanine aminotransferase; AST, glutamic oxaloacetic transaminase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; TBil, total bilirubin; CK-MB, creatine kinase isoenzyme; TnI, hypersensitive troponin; MYO, myoglobin; Scr, serum creatinine; PCT, procalcitonin; AMY, amylase; APTT, activated partial thromboplastin time; PT, prothrombin time; FIB, fibrinogen; FDP, fibrinogen degradation product.
The septic patients with and without AKI were further categorized into two addition subgroups in total comprising four groups as described in the methods section: sepsis + shock + AKI (SS-AKI), sepsis + AKI (S-AKI), sepsis + shock (SSN-AKI), and the sepsis (SN-AKI). There were significant differences in 7 parameters among these 4 groups, including ALP, urea, MYO, Scr, PCT, APTT, and PT (P<0.05, one-way ANOVA; Table 3).
Table 3
| Variable | SS-AKI | S-AKI | SSN-AKI | SN-AKI | P value |
|---|---|---|---|---|---|
| RBC (109/L) | 4.03±0.98 | 3.8±0.95 | 3.41±0.93 | 3.84±0.86 | >0.05 |
| HB (g/L) | 125.53±33.19 | 110.87±27.6 | 108.76±28.98 | 116±24.83 | >0.05 |
| HCT (%) | 36.25 (30.20–43.70) | 37.25 (30.88–39.63) | 33.50 (24.28–40.18) | 34.7 (29.90–38.5) | >0.05 |
| WBC (109/L) | 12.91 (9.82–17.71) | 14.78 (10.68–17.22) | 2.93 (7.57–19.05) | 13.06 (8.61–17.56) | >0.05 |
| ALB (g/L) | 29.82±8.69 | 31.55±7.29 | 27.61±5.49 | 30.77±6.95 | >0.05 |
| ALT (U/L) | 43 (30.5–150) | 38 [22–68] | 36 [27–109] | 40.5 [31–75] | >0.05 |
| AST (U/L) | 64 (33–224.5) | 45 [34–78] | 59 (26.5–92) | 56.5 (31.25–108.75) | >0.05 |
| ALP (U/L) | 74 (47–113.5) | 83 [75–110] | 66.5 (36.5–77) | 78 [60–99] | 0.043 |
| γ-GT (U/L) | 30 (21.5–89.5) | 40 [17–86] | 21 (13.5–83.5) | 50 (28.5–126) | >0.05 |
| TBil (µmol/L) | 21.1 (12–40.85) | 13.5 (8.7–28.2) | 20.6 (14.75–36.25) | 19.2 (11.28–24.18) | >0.05 |
| CK-MB (U/L) | 3.69 (1.38–12.37) | 5.57 (2.36–12.63) | 1.98 (0.99–4.85) | 1.6 (0.66–5.96) | 0.05 |
| TnI (ug/mL) | 0.33 (0.13–0.99) | 0.26 (0.05–0.77) | 0.14 (0.04–0.58) | 0.069 (0.02–0.36) | >0.05 |
| Urea (µmol/L) | 15.54 (9.7–19.06) | 19.11 (12.61–29.3) | 9.31 (5.59–12.56) | 7.22 (5.94–9.93) | <0.001 |
| MYO (ng/mL) | 570.45 (178.50–819.46) | 471.01 (194.76–1,003.98) | 199.7 (81.52–450.65) | 137.42 (46.75–306.67) | 0.002 |
| Scr (µmol/L) | 160.5 (120–227.25) | 214 [128–294] | 81.5 (69.5–99.75) | 69 [57–88] | <0.001 |
| PCT (ng/mL) | 9.25 (0.73–31.39) | 5.68 (2.57–35.9) | 1.05 (0.4–2.4) | 1.38 (0.59–3.2) | 0.008 |
| AMY (U/L) | 67 (51–125.5) | 67 [47–225] | 82 (39.5–170) | 86.5 (54–149.75) | >0.05 |
| Lipase (U/L) | 68 (18.5–271.5) | 106 [35–748] | 89 [26–141] | 78.5 (33–255.25) | >0.05 |
| APTT (s) | 36.3 (30.15–51.25) | 38.5 (28.4–56.7) | 33.8 (27.15–89.5) | 27.1 (24.7–34.7) | <0.05 |
| PT (s) | 15.8 (14.45–18.4) | 13.2 (12.55–15.05) | 15 (13.15–17.68) | 12.7 (11.8–14.9) | 0.002 |
| FIB (g/L) | 3.48 (2.45–4.43) | 4.55 (2.77–6.35) | 3.15 (1.5–4.54) | 3.48 (2.25–6.5) | >0.05 |
| FDP (mg/L) | 27.4 (17.15–61.7) | 11.22 (4.85–23.76) | 15.1 (5.93–78.2) | 14.5 (6.56–41.98) | >0.05 |
| D-dimer (µg/L) | 12,160 [4,125–18,335] | 3,630 [2,290–9,130] | 5,410 [2,570–19,895] | 5,370 [2,345–14,412.5] | >0.05 |
Data are presented as mean ± SD or median (ranges). SS-AKI, sepsis + shock + AKI group; S-AKI, sepsis + AKI group; SSN-AKI, sepsis + shock group; SN-AKI, sepsis group; RBC, red blood cell; HB, hemoglobin; HCT, hematocrit; WBC, white blood cell; ALB, albumin; ALT, alanine aminotransferase; AST, glutamic oxaloacetic transaminase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; TBil, total bilirubin; CK-MB, creatine kinase isoenzyme; TnI, hypersensitive troponin; MYO, myoglobin; Scr, serum creatinine; PCT, procalcitonin; AMY, amylase; APTT, activated partial thromboplastin time; PT, prothrombin time; FIB, fibrinogen; FDP, fibrinogen degradation product.
Profile analysis of HO-1 levels among the different sepsis groups
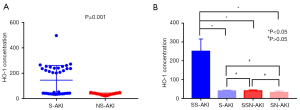
To assess the characteristic role of serum HO-1 in sepsis, the concentration of HO-1 was examined and compared among the distinct sepsis groups. The serum HO-1 levels were significantly higher in the S-AKI group compared to the NS-AKI group (P=0.001; Figure 1A, unpaired Student’s t-test). The serum HO-1 levels in patients with SS-AKI were also detected and compared with the levels in each of the other three groups (S-AKI, SSN-AKI and SN-AKI, one-way ANOVA). The above results are presented in Figure 1B (P<0.05); no significant differences were found between the groups (P>0.05).
Correlation between serum HO-1 levels and laboratory data of sepsis patients
Spearman’s correlation analysis was used to determine the correlation between serum HO-1 levels and other laboratory indicators (Tables 4,5). For all sepsis patients, serum HO-1 was positively correlated with HB, TnI, urea, Scr, APTT, and the SOFA score (P<0.05). In the SS-AKI group, serum HO-1 was positively correlated with the SOFA score, the AKI grade, Scr, γ-GT, and FDP (P<0.05). In the S-AKI group, serum HO-1 was significantly correlated with the AKI grade, Scr, and ALP (P<0.05). However, in the SSN-AKI and SN-AKI groups, serum HO-1 did not have a significant correlation with the SOFA score (P=0.367 and P=0.625, respectively) nor with Scr levels (P=0.12 and P=0.146, respectively).
Table 4
| Variable | HO-1 concentration | |
|---|---|---|
| r | P | |
| HB | 0.247 | 0.024 |
| TnI | 0.246 | 0.025 |
| Urea | 0.295 | 0.008 |
| Scr | 0.489 | <0.001 |
| APTT | 0.233 | 0.035 |
| SOFA score | 0.494 | <0.001 |
SOFA, Sequential Organ Failure Assessment; HO-1, heme oxygenase-1; HB, hemoglobin; TnI, hypersensitive troponin; Scr, serum creatinine; APTT, activated partial thromboplastin time.
Table 5
| Variable | HO-1 concentration | |
|---|---|---|
| r | P | |
| SS-AKI | ||
| SOFA score | 0.548 | 0.018 |
| AKI grade | 0.736 | 0.001 |
| Scr | 0.561 | 0.016 |
| γ-GT | 0.528 | 0.024 |
| FDP | 0.709 | 0.001 |
| S-AKI | ||
| SOFA score | 0.118 | 0.641 |
| AKI grade | 0.548 | 0.019 |
| Scr | 0.566 | 0.014 |
| ALP | 0.49 | 0.046 |
| SSN-AKI | ||
| SOFA score | −0.213 | 0.367 |
| Scr | 0.359 | 0.12 |
| SN-AKI | ||
| SOFA score | −0.099 | 0.625 |
| Scr | −0.406 | 0.036 |
SOFA, Sequential Organ Failure Assessment; AKI, acute kidney injury; HO-1, heme oxygenase-1; SS-AKI, sepsis + shock + AKI group; S-AKI, sepsis + AKI group; SSN-AKI, sepsis + shock group; SN-AKI, sepsis group; Scr, serum creatinine; γ-GT, γ-glutamyl transpeptidase; FDP, fibrinogen degradation products; ALP, alkaline phosphatase.
Potential prognostic value of HO-1
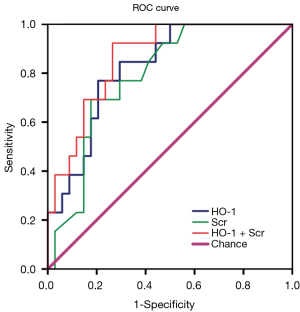
AUC for the incidence of serum HO-1 and Scr was 0.824 [95% confidence interval (CI): 0.703–0.944] and 0.788 (95% CI: 0.658–0.919), respectively (Figure 2). When the cut-off value for serum HO-1 was 40.96 U/L, the sensitivity and specificity for the serum HO-1 prognostic value was 76.9% and 79.4%, respectively. The AUC for the combination of these two indicators was 0.864 (95% CI: 0.761–0.968), and the sensitivity and specificity was 92.3% and 73.5%, respectively.
Discussion
The present cross-sectional study revealed that the levels of serum HO-1 were significantly higher in patients with sepsis-induced AKI compared to septic patients without AKI. Combining serum HO-1 and Scr was significantly more likely to predict the occurrence of AKI during sepsis compared to either indicator alone. For both the SS-AKI and S-AKI groups, the serum HO-1 concentration was positively associated with the AKI grade, suggesting that serum HO-1 levels have a strong relationship with the severity of renal injury. Our study provides clinical evidence for a predictive value of serum HO-1 during progression of sepsis.
Numerous studies have shown that HO-1 can be induced by hemin, which causes oxidative stress and inflammation, especially in relation to the progression of acute organ injury (30-32). Heme, as a ubiquitous compound of human tissue, is involved in sepsis physiology and metabolism. When heme is released from the cell, an oxidation reaction occurs to free the heme. Subsequently, free heme is converted to a ferric state, namely, hemin. Expressed HO-1 acts as a stress response protein in the reticuloendothelial system, where it contributes to decomposition of heme into iron, biliverdin, and carbon monoxide. A positive relationship between oxidative stress and AKI has been reported in the literature (7,33). One characteristics of AKI is tubular epithelial cell oxidative stress, which further causes microvascular dysfunction and inflammation (34,35). Studies in animals have highlighted the favorable effect of HO-1 induction, demonstrating antioxidant and anti-inflammatory effects in kidney disease (36,37). The present study suggests that the increase in serum HO-1 is a positive predictive factor for the extent of AKI (Table 5).
The present observational study showed that the HO-1 concentration had a significant relationship with the Scr levels in both the SS-AKI and the S-AKI groups. However, for the other two groups without AKI, serum HO-1 was not correlated with Scr which is expected since Scr is part of AKI-defining criteria. According to the KDIGO consensus group, Scr increases to ≥0.3 mg/dL or >50% of baseline within the 48-hour period during AKI development. This has enabled the identification of AKI’s clinical importance. Based on the analysis of the area under the ROC curve, values for serum HO-1 and Scr in sepsis patients were 0.824 and 0.788, respectively. The area under the ROC curve for the combination of serum HO-1 and Scr was 0.864, suggesting that the combined analysis of serum HO-1 and Scr may contributed to better prediction of septic AKI compared to either indicator alone. Furthermore, the ROC curve revealed that serum HO-1 by itself may have predictive value in the early stages of sepsis-induced AKI. It can thereby be suggested that serum HO-1 can be regarded as a clinical putative biomarker for the pathogenesis of renal injury, especially for sepsis-induced AKI.
Sepsis is a common condition that contributes to the emergence of systemic inflammation, resulting in hemolytic lesions (38-40). There are several well-known mechanisms underlying hemolysis (41-43). Hemolysis can result from toxins released by pathogens and the fibrin chain that emerges during intravascular coagulation. The complement system intervenes with the activity of RBCs during sepsis (44,45). Interestingly, the present study found that the HO-1 concentration has a positive relationship with HB levels, which is consistent with the phenomenon described above. The present findings suggested that a hemolytic reaction released the heme with the final induction of the HO-1 expression, suggesting that the detection of HO-1 could contribute to the improvement of the hemolytic index (46).
Prior studies have demonstrated the protective potential of HO-1 expression in renal dysfunction, which is associated with the survival rate (47-49). Furthermore, a study has shown that toll-like receptors (TLRs) play an important role in autophagy to protect renal tissues (50). In a sepsis animal model, the deletion of TLR2, but not TLR4, aggravated renal insufficiency and tissue damage. When TLR2+ TLR4− mice were treated with cisplatin, HO-1 expression increased in the heme plus group and renal function improved. However, no similar phenomenon occurred in the TLR4− group (51). This result may be explained by the fact that HO-1 is involved in the promotion of renal function recovery. The survival curves in this study showed that there is a high mortality rate for patients with elevated HO-1 levels. This result might somewhat be limited by the activity of the TLR signal pathway, which leads to the deficiency of HO-1 protective function. Future research examining HO-1 and TLR function is warranted.
There are several limitations to the present study. First, this was a single-center study with a limited sample size for each group. Further studies involving larger cohorts are needed to verify these data. Second, the blood collection was conducted within 24 hours of admission to the ICU, which may be a relatively large time window, especially for an ICU case. Because the conditions of patients in the ICU are more complex and change rapidly, specimen collection and testing should be carried out early to obtain more favorable experimental evidence. In addition, HO-1 expression was not assessed in blood circulating monocytes or leukocytes of which we know that sepsis and systemic inflammation have a systemic impact and may better reflect renal HO-1 expression than serum HO-1 levels.
The present investigation revealed that the concentration of HO-1 in serum increases in sepsis-induced AKI, especially in patients with septic shock and AKI. A significant correlation between serum HO-1 and Scr was identified in these settings, and both indicators have predictive value in disease evolution and diagnosis of sepsis with AKI. Future studies are warranted to further elucidate the role of HO-1 in the pathogenesis and prognosis of sepsis-induced AKI. The evidence from the present study suggested that high serum HO-1 is a putative index, which may contribute to the diagnosis and predictive of sepsis-induced AKI.
Acknowledgments
The authors appreciate the academic support from the AME Sepsis Collaborative Group.
Funding: This work was supported by a grant from the Dalian Science and Technology Innovation Fund (No. 2018J13SN099); and partially supported by grants from the National Natural Science Foundation of China (No. 81703871); the Doctoral Start-up Foundation of Liaoning Province (No. 20170520408); and the “double first-class” construction of colleges and universities in Liaoning Province {Liao Cai Zhi Jiao [2021] No. 87, allocated by Finance Department of Liaoning Province, to Nan Li}.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4793/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4793/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4793/coif). CJW has received fees for speaking and/or consulting from CSL Behring and Biotest. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goodwin AJ. Are Sepsis Outcomes Predetermined? How the Road toward Sepsis May Predict Outcomes. Ann Am Thorac Soc 2019;16:57-9. [Crossref] [PubMed]
- Hodgson CL, Walsh TS, Lone N. The long road home: are outcomes different for patients with sepsis? Intensive Care Med 2018;44:1556-7. [Crossref] [PubMed]
- Gómez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 2017;13:143-51. [Crossref] [PubMed]
- Al-Jaghbeer M, Dealmeida D, Bilderback A, et al. Clinical Decision Support for In-Hospital AKI. J Am Soc Nephrol 2018;29:654-60. [Crossref] [PubMed]
- Yao J, Zheng J, Cai J, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J 2019;33:1695-710. [Crossref] [PubMed]
- Ahmad MI, Zafeer MF, Javed M, et al. Pendimethalin-induced oxidative stress, DNA damage and activation of anti-inflammatory and apoptotic markers in male rats. Sci Rep 2018;8:17139. [Crossref] [PubMed]
- Lu CY, de Albuquerque Rocha N. Oxidative Stress and Metabolism: The NF-Erythroid 2 p45-Related Factor 2:Kelch-like ECH-Associated Protein 1 System and Regulatory T Lymphocytes in Ischemic AKI. J Am Soc Nephrol 2015;26:2893-5. [Crossref] [PubMed]
- Kasuno K, Shirakawa K, Yoshida H, et al. Renal redox dysregulation in AKI: application for oxidative stress marker of AKI. Am J Physiol Renal Physiol 2014;307:F1342-51. [Crossref] [PubMed]
- Jiang L, Jiang Q, Yang S, et al. GYY4137 attenuates LPS-induced acute lung injury via heme oxygenase-1 modulation. Pulm Pharmacol Ther 2019;54:77-86. [Crossref] [PubMed]
- Zhang T, Xiang L. Honokiol alleviates sepsis-induced acute kidney injury in mice by targeting the miR-218-5p/heme oxygenase-1 signaling pathway. Cell Mol Biol Lett 2019;24:15. [Crossref] [PubMed]
- Ohnishi M, Ohshita M, Tamaki H, et al. Shogaol but not gingerol has a neuroprotective effect on hemorrhagic brain injury: Contribution of the α, β-unsaturated carbonyl to heme oxygenase-1 expression. Eur J Pharmacol 2019;842:33-9. [Crossref] [PubMed]
- Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol 2007;18:414-20. [Crossref] [PubMed]
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 2010;50:323-54. [Crossref] [PubMed]
- Waza AA, Hamid Z, Ali S, et al. A review on heme oxygenase-1 induction: is it a necessary evil. Inflamm Res 2018;67:579-88. [Crossref] [PubMed]
- Zhang ZY, Guan J, Li H, et al. Heme Oxygenase-1 Promoter Polymorphism Protects Liver Allograft. Indian J Surg 2016;78:14-9. [Crossref] [PubMed]
- Vasconcellos LR, Siqueira MS, Moraes R, et al. Heme Oxygenase-1 and Autophagy Linked for Cytoprotection. Curr Pharm Des 2018;24:2311-6. [Crossref] [PubMed]
- Wu YH, Chen WC, Tseng CK, et al. Heme oxygenase-1 inhibits DENV-induced endothelial hyperpermeability and serves as a potential target against dengue hemorrhagic fever. FASEB J 2022;36:e22110. [Crossref] [PubMed]
- Sandrim VC, Caldeira-Dias M, Bettiol H, et al. Circulating Heme Oxygenase-1: Not a Predictor of Preeclampsia but Highly Expressed in Pregnant Women Who Subsequently Develop Severe Preeclampsia. Oxid Med Cell Longev 2018;2018:6035868. [Crossref] [PubMed]
- Hofmann A, Müglich M, Wolk S, et al. Induction of Heme Oxygenase-1 Is Linked to the Severity of Disease in Human Abdominal Aortic Aneurysm. J Am Heart Assoc 2021;10:e022747. [Crossref] [PubMed]
- Shalaby YM, Menze ET, Azab SS, et al. Involvement of Nrf2/HO-1 antioxidant signaling and NF-κB inflammatory response in the potential protective effects of vincamine against methotrexate-induced nephrotoxicity in rats: cross talk between nephrotoxicity and neurotoxicity. Arch Toxicol 2019;93:1417-31. [Crossref] [PubMed]
- Park C, Cha HJ, Hong SH, et al. Protective Effect of Phloroglucinol on Oxidative Stress-Induced DNA Damage and Apoptosis through Activation of the Nrf2/HO-1 Signaling Pathway in HaCaT Human Keratinocytes. Mar Drugs 2019;17:225. [Crossref] [PubMed]
- Nath KA. Human AKI and heme oxygenase-1. J Am Soc Nephrol 2012;23:971-4. [Crossref] [PubMed]
- Bolisetty S, Zarjou A, Agarwal A. Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury. Am J Kidney Dis 2017;69:531-45. [Crossref] [PubMed]
- Tracz MJ, Juncos JP, Grande JP, et al. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1-/- mice. Am J Pathol 2007;170:1820-30. [Crossref] [PubMed]
- Jao HC, Lin YT, Tsai LY, et al. Early expression of heme oxygenase-1 in leukocytes correlates negatively with oxidative stress and predicts hepatic and renal dysfunction at late stage of sepsis. Shock 2005;23:464-9. [Crossref] [PubMed]
- Takaki S, Takeyama N, Kajita Y, et al. Beneficial effects of the heme oxygenase-1/carbon monoxide system in patients with severe sepsis/septic shock. Intensive Care Med 2010;36:42-8. [Crossref] [PubMed]
- Kang K, Nan C, Fei D, et al. Heme oxygenase 1 modulates thrombomodulin and endothelial protein C receptor levels to attenuate septic kidney injury. Shock 2013;40:136-43. [Crossref] [PubMed]
- Wang Y, Feng F, Liu M, et al. Resveratrol ameliorates sepsis-induced acute kidney injury in a pediatric rat model via Nrf2 signaling pathway. Exp Ther Med 2018;16:3233-40. [Crossref] [PubMed]
- Ekregbesi P, Shankar-Hari M, Bottomley C, et al. Relationship between Anaemia, Haemolysis, Inflammation and Haem Oxygenase-1 at Admission with Sepsis: a pilot study. Sci Rep 2018;8:11198. [Crossref] [PubMed]
- Wu J, Li S, Li C, et al. The non-canonical effects of heme oxygenase-1, a classical fighter against oxidative stress. Redox Biol 2021;47:102170. [Crossref] [PubMed]
- Lam A, Vetal N, Matalon S, et al. Role of heme in bromine-induced lung injury. Ann N Y Acad Sci 2016;1374:105-10. [Crossref] [PubMed]
- Martins R, Knapp S. Heme and hemolysis in innate immunity: adding insult to injury. Curr Opin Immunol 2018;50:14-20. [Crossref] [PubMed]
- Myrvang H. Acute kidney injury: Obesity is associated with AKI after surgery via oxidative stress. Nat Rev Nephrol 2012;8:433. [Crossref] [PubMed]
- Souza AC, Yuen PS, Star RA. Microparticles: markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and AKI. Kidney Int 2015;87:1100-8. [Crossref] [PubMed]
- Polichnowski AJ. Microvascular rarefaction and hypertension in the impaired recovery and progression of kidney disease following AKI in preexisting CKD states. Am J Physiol Renal Physiol 2018;315:F1513-8. [Crossref] [PubMed]
- Shi S, Lei S, Tang C, et al. Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway. Biosci Rep 2019;39:BSR20181614. [Crossref] [PubMed]
- Behiry S, Rabie A, Kora M, et al. Effect of combination sildenafil and gemfibrozil on cisplatin-induced nephrotoxicity; role of heme oxygenase-1. Ren Fail 2018;40:371-8. [Crossref] [PubMed]
- Greninger AL, Hess JR. Clostridium perfringens sepsis masquerading as a hemolytic transfusion reaction. Transfusion 2017;57:1112. [Crossref] [PubMed]
- Singh L, Malhotra S, Kaur G, et al. Autoimmune hemolytic anemia due to auto anti-N in a patient with sepsis. Transfus Apher Sci 2012;47:269-70. [Crossref] [PubMed]
- Effenberger-Neidnicht K, Hartmann M. Mechanisms of Hemolysis During Sepsis. Inflammation 2018;41:1569-81. [Crossref] [PubMed]
- Sikora J, Orlov SN, Furuya K, et al. Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 2014;124:2150-7. [Crossref] [PubMed]
- Shalel S, Streichman S, Marmur A. The mechanism of hemolysis by surfactants: effect of solution composition. J Colloid Interface Sci 2002;252:66-76. [Crossref] [PubMed]
- Sato Y, Yamakose H, Suzuki Y. Mechanism of hypotonic hemolysis of human erythrocytes. Biol Pharm Bull 1993;16:506-12. [Crossref] [PubMed]
- Lendak D, Mihajlovic D, Mitic G, et al. Complement component consumption in sepsis correlates better with hemostatic system parameters than with inflammatory biomarkers. Thromb Res 2018;170:126-32. [Crossref] [PubMed]
- Zhao X, Chen YX, Li CS. Predictive value of the complement system for sepsis-induced disseminated intravascular coagulation in septic patients in emergency department. J Crit Care 2015;30:290-5. [Crossref] [PubMed]
- Grunenwald A, Roumenina LT, Frimat M. Heme Oxygenase 1: A Defensive Mediator in Kidney Diseases. Int J Mol Sci 2021;22:2009. [Crossref] [PubMed]
- Barakat M, Gabr MM, Zhran F, et al. Upregulation of heme oxygenase 1 (HO-1) attenuates kidney damage, oxidative stress and inflammatory reaction during renal ischemia/ reperfusion injury. Gen Physiol Biophys 2018;37:193-204. [Crossref] [PubMed]
- Lever JM, Boddu R, George JF, et al. Heme Oxygenase-1 in Kidney Health and Disease. Antioxid Redox Signal 2016;25:165-83. [Crossref] [PubMed]
- Fonseca CDD, Watanabe M, Couto SMF, et al. The renoprotective effects of Heme Oxygenase-1 during contrast-induced acute kidney injury in preclinical diabetic models. Clinics (Sao Paulo) 2021;76:e3002. [Crossref] [PubMed]
- Habib R. Multifaceted roles of Toll-like receptors in acute kidney injury. Heliyon 2021;7:e06441. [Crossref] [PubMed]
- Andrade-Silva M, Cenedeze MA, Perandini LA, et al. TLR2 and TLR4 play opposite role in autophagy associated with cisplatin-induced acute kidney injury. Clin Sci (Lond) 2018;132:1725-39. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


