
Bronchiectasis exacerbation: a narrative review of causes, risk factors, management and prevention
Introduction
Non-cystic fibrosis bronchiectasis (hereafter referred to as bronchiectasis) was, until recently, considered as an orphan disease (1). Over the past 10 years, however, publications have revealed that bronchiectasis is not rare and contributes to a considerable healthcare burden and increased mortality (2-5). Bronchiectasis is a heterogeneous condition that may be a standalone suppurative pulmonary disease and can sometimes complicate other pulmonary diseases, including asthma and chronic obstructive pulmonary disease (COPD) (6). Thus, physicians should recognise this heterogeneous lung condition to appropriately manage patients with bronchiectasis. Increasing recognition has been encouraged by the establishment of large-scale bronchiectasis registries and relevant clinical trials worldwide, improving our understanding of many aspects of bronchiectasis (7-12).
Many patients with bronchiectasis experience frequent acute worsening of symptoms that are referred to as exacerbations. Exacerbation of bronchiectasis is a major adverse event in the natural history of this disease. The number of exacerbations per year independently predicts future mortality risk, with frequent exacerbators having double the mortality rates than those who do not experience exacerbations (13). Consequently, the exacerbation has been a fundamental endpoint in clinical research and a target for most therapeutic interventions in bronchiectasis research (14,15).
Clinical and translational research in recent years has enhanced our understanding of the causes and consequences of bronchiectasis exacerbations. Data from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) registry demonstrated that almost half of the patients with bronchiectasis have two or more exacerbations per year, and approximately one-third of them require one or more hospitalisation per year (7). In this review, we discuss the definition, causes, risk factors, management and prevention of bronchiectasis exacerbations. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3437/rc).
Methods
Source references were identified through a PubMed search of English language abstracts and articles published between January 1990 and March 2022. Randomised controlled trials (RCTs), observational studies, translational studies and systematic and narrative reviews were retrieved. The keywords included “bronchiectasis” and “exacerbation”. These keywords were used alone and in combinations. The reference lists of original articles, narrative reviews, clinical guidelines, and previous systematic reviews and meta-analyses were searched for additional relevant materials. The citations from the articles identified in these searches were also reviewed and included, where appropriate. The search strategy is summarised in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | The initial search was conducted on 1 April 2022 |
| Databases | PubMed |
| Search terms used | Terms used included “bronchiectasis” and “exacerbation” |
| Timeframe | Published between January 1990 and March 2022 |
| Inclusion and exclusion criteria | English language abstracts and articles |
| Selection process | H Choi identified source references. Randomised controlled trials, observational studies, translational studies and systemic and narrative reviews were considered. The reference lists of the original articles, narrative reviews, clinical guidelines and previous systematic reviews and meta-analyses were manually searched to identify additional relevant material. The citation lists from articles identified in these searches were also reviewed and included, where appropriate |
Definition
Clinically, patients recognise exacerbations as a deterioration of respiratory symptoms for which they seek medical help, and which is usually treated with antibiotics. This is how the 2010 British Thoracic Society guidelines defined an exacerbation, using the terminology “a deterioration in respiratory symptoms and/or systemic upset with the need for antibiotic treatment” (16). A problem with this definition is that patients may have a different threshold for reporting a deterioration in symptoms or may perceive normal day to day fluctuations in symptoms as exacerbation. This is particularly a challenge for clinical trials where exacerbations are frequently a primary endpoint. In 2017, an international group of experts on bronchiectasis generated a uniform definition for use in clinical trials. The process was based on a literature review of all published definitions between 2000 and 2015 and a Delphi methodology followed by a round-table discussion (17).
The experts agreed on the final definition of bronchiectasis exacerbation as follows: a person with bronchiectasis with a deterioration of three or more key symptoms for at least 48 hours, in addition to a clinician’s decision that a change in bronchiectasis treatment is required. Key symptoms include (I) cough, (II) sputum volume and/or consistency, (III) sputum purulence, (IV) breathlessness and/or exercise intolerance, (V) fatigue and/or malaise and (VI) haemoptysis (17). The clinician’s decision to change treatment generally refers to the prescription of antibiotics.
This definition was intended for use in clinical trials but is also helpful for conceptualising exacerbations in daily practice. The implications of the definition are that exacerbations are a deterioration in symptoms which is persistent, is greater than the normal day to day variation and can include worsening of respiratory symptoms and systemic upset.
An important observation is that many patients with bronchiectasis experience sustained worsening of symptoms which are not reported as exacerbations. In a study of 21 patients using a novel symptom diary, Artaraz et al. showed that 23 of 52 diary-detected exacerbations were not reported by patients and did not result in antibiotic treatment (18). Considering the detrimental effect of exacerbations in patients with bronchiectasis (19), clinicians should educate their patients to recognise the worsening of symptoms, particularly for those whose exacerbation is insufficiently severe to warrant hospital admission.
Causes of exacerbations
The pathophysiology of bronchiectasis has been poorly understood and this also extends to a lack of mechanistic studies examining the causes of exacerbations. Guidelines recommend that antibiotics are used to treat exacerbations and clinical experience suggests patients often feel better after an antibiotic course. Data suggests, however, that many other factors including viruses, inflammation and external environmental factors can affect the risk of exacerbation (Figure 1).
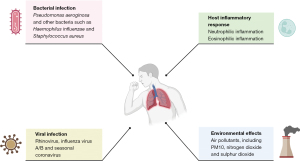
Bacterial infection
Bacterial infection is traditionally regarded as the most probable cause of bronchiectasis exacerbation for two reasons. First, there is a clear relationship in observational studies between chronic bacterial infection and bronchiectasis exacerbation risk (13,20). Second, antibiotic treatment decreases airway bacterial loads and reduces markers of the airway and systemic inflammation in patients with bronchiectasis, leading to clinical improvements in many patients (21). The relationship between bacterial infection and exacerbation risk during stable disease is clear. In a multicentre European study of 2,596 patients with bronchiectasis, chronic P. aeruginosa infection was independently associated with exacerbation frequency (22). In a United States (US) multicentre bronchiectasis cohort study of 830 patients, 94 (11.3%) had Staphylococcus aureus infection. Patients with S. aureus showed a higher exacerbation rate than those without prior S. aureus or glucose-nonfermenting gram-negative bacilli growth in the sputum samples. However, S. aureus growth was not independently associated with the frequency of exacerbations in multivariate analyses (23). Moreover, a US multicentre cohort study of 2,659 patients showed that 134 (5%) patients had Stenotrophomonas maltophilia infection. The prior exacerbation rate was significantly higher in patients with S. maltophilia than in those without pathogens or pathogens other than P. aeruginosa and S. maltophilia (24).
Although it may be logical that microbiological test results differ between the stable and exacerbated states of patients with bronchiectasis, many studies show similar profiles in both stable state and exacerbation. In a study of 40 patients with bronchiectasis, sputum was collected from patients when they were clinically stable and during exacerbations (25). Bacterial abundance and community composition were analysed using culture-based microbiological and molecular-based (16S ribosomal RNA sequencing) techniques. Interestingly, there were no significant intergroup differences between stable and exacerbated states (25), which was also replicated in a Korean study investigating bronchoalveolar lavage samples from 14 patients with bronchiectasis (26). In a more recent microbiome study of 281 patients, individual patient microbiome profiles were relatively stable over time, during exacerbations, and during disease stability (27); however, this study provided further insight. Patients in whom Pseudomonas was dominant in the microbiome profiles (n=35) were at increased risk of all-cause mortality [hazard ratio (HR), 3.12; 95% confidence interval (CI), 1.33–7.36] and had more frequent exacerbations [incident rate ratio (IRR), 1.69; 95% CI, 1.07–2.67] than patients with other dominant genera (n=246). This indicates that a reduction in microbiome diversity, particularly that associated with Pseudomonas dominance, may be closely related to bronchiectasis exacerbation (27).
Nevertheless, several microbiome studies therefore show no consistent changes between the stable and exacerbation states, suggesting conventional acute bacterial infection is not seen consistently at exacerbation. It is likely, therefore, if bacteria are involved in exacerbations that it is changes in the composition of the resident microbes of the lung, rather than invasion of a new pathogen, that is seen in the majority of exacerbations. A study investigating interactions between microbes during exacerbations demonstrated no change in the overall community composition, but an increase in negative interactions between microbes during exacerbations that was partially reversed after antibiotic treatment (28).
This supports a model where bacteria are important in exacerbation, but that the inciting event, the true cause of exacerbation that changes bacterial behaviour, may be external such as a viral infection or a change in the immunological state of the lung.
Viral infection
Viruses are likely to be responsible for a high proportion of exacerbations and this is supported by both direct and indirect evidence. In a prospective study, sputum samples were collected from 108 patients with bronchiectasis every 3–6 months and at the onset of exacerbation, which were sent for bacterial culture and viral polymerase chain reaction. Viral isolation [odds ratio (OR), 3.28; 95% CI, 1.76–6.12] and viral plus bacterial isolation (OR, 2.24; 95% CI, 1.11–4.55) occurred more frequently during the onset of exacerbation (29). Similarly, Gao et al. revealed that respiratory viruses were found more frequently during bronchiectasis exacerbation than during a stable state (49.0% vs. 18.9%, P<0.001) (30). The most common viruses found in patients experiencing exacerbations include rhinovirus, influenza virus A/B and seasonal coronaviruses in these studies from China. Interestingly, virus-positive exacerbations were related to a greater increase in systemic and airway inflammatory markers [serum interleukin (IL)-6 and tumour necrosis factor (TNF)-α; sputum IL-1β and TNF-α] than virus-negative exacerbations (30). A recent Korean cohort study enrolled 792 patients with bronchiectasis who had visited the emergency room or were admitted to the hospital because of exacerbations (31). Respiratory viruses were detected in 25% of patients experiencing bronchiectasis exacerbations. Additionally, viral detection was independently associated with female sex and chronic heart disease as a comorbidity in multivariable analyses (31).
In contrast, some studies have raised questions regarding the role of the virus in bronchiectasis exacerbations. Mitchell et al. performed respiratory viral panels in a cohort of patients with stable bronchiectasis, which showed that the virus was detected in 92% of patients during winter and 33% during summer (32). In addition, a Greek study of 33 patients with bronchiectasis assessed the possible role of atypical bacteria and respiratory syncytial virus in exacerbations. Over a one-year follow-up period, 15 of the 33 patients experienced bronchiectasis exacerbations. Respiratory syncytial virus was detected by polymerase chain reaction during stable visits in four patients; however, it was never detected during exacerbations (33). Hence, respiratory viruses are commonly detected in patients with stable bronchiectasis, and future studies are warranted to define the role of viruses in bronchiectasis exacerbation. These studies were conducted prior to the coronavirus disease 2019 (COVID-19) pandemic and the role of COVID-19 as a cause of bronchiectasis exacerbations has not yet been studied.
Indirect evidence of the importance of viruses is provided by the 48% reduction in exacerbations reported during the COVID-19 pandemic by Crichton et al. Social distancing measures introduced during the pandemic virtually eliminated the transmission of rhinovirus and influenza viruses. There are other possible explanations for the observed reduction in exacerbations, but these data indirectly suggest that viruses cause bronchiectasis exacerbations (34).
Host inflammatory response
Neutrophilic inflammation within the airway is an important risk factor for exacerbation of bronchiectasis. One United Kingdom study measured the sputum neutrophil elastase activity of 381 patients with bronchiectasis at baseline and during exacerbations, and evaluated long-term clinical outcomes over a 3-year follow-up period (35). Elevated sputum elastase activity was associated with a higher frequency of exacerbations (P<0.001). Neutrophil elastase activity also increased during exacerbations (P=0.001) and was reduced by antibiotic treatment (35). Keir et al. performed a proteomic study identifying neutrophil extracellular traps, webs of DNA and granule proteins released from activated neutrophils, as a key mediator of severity and exacerbation risk in the sputum of bronchiectasis patients enrolled in multiple cohorts. Neutrophil extracellular traps predicted the time to the first severe exacerbation (36). These data support the concept of targeting neutrophilic inflammation in bronchiectasis treatment (discussed later). Keir et al. also showed that antibiotic treatment during exacerbation caused extensive protein changes in sputum, but that the response in P. aeruginosa infected patients was minimal, suggesting exacerbations in this group are more severe and less antibiotic responsive.
Some studies have also evaluated systemic inflammation in relation to the exacerbation of bronchiectasis. A Spanish prospective observational study assessed systemic proinflammatory cytokines on days 1, 5, 30, and 60 in 165 patients with exacerbated bronchiectasis (93 of 165 had severe exacerbations). Interestingly, on day 30, severe exacerbations were independently associated with higher levels of IL-17 (OR, 4.58), IL-6 (OR, 4.89), IL-8 (OR, 3.08), and high-sensitivity C-reactive protein (CRP) (OR, 6.7), which were adjusted for age, bronchiectasis severity index, and treatment duration (37). This study suggests that severe exacerbation elicits a higher systemic proinflammatory response sustained until day 30 (37). In addition, Posadas et al. reported the impact of CRP in steady-state bronchiectasis on future exacerbations (n=802) (38). When CRP levels were divided into tertiles, patients with CRP levels in the second (0.4–2.7 mg/L) and third (≥2.7 mg/L) tertiles presented 2.9 (95% CI, 1.4–5.9) and 4.2 (95% CI, 2.2–8.2) times greater probability, respectively, of experiencing a severe exacerbation than the control group (CRP <0.4 mg/L), regardless of bronchiectasis severity or a previous exacerbation history (38). Although systemic inflammation may result from airway inflammation, these studies highlighted a host inflammatory response in bronchiectasis exacerbation.
Although bronchiectasis is historically considered a neutrophilic lung disease, eosinophilic inflammation is increasingly recognised in bronchiectasis. A recent European multicentre study analysing 1,007 patients with bronchiectasis showed that 22.6% of them had blood eosinophil counts of ≥300 cells/µL (39). Furthermore, to examine the relationship between eosinophil counts and exacerbations after adjusting for infection, this study assessed 144 patients who received antipseudomonal antibiotics in a clinical trial. Compared with patients with blood eosinophil counts <100 cells/µL, elevated eosinophil counts of 100–299 cells/µL (HR, 2.38; 95% CI, 1.33–4.25; P=0.003) and 300 cells/µL (HR, 3.99; 95% CI, 2.20–7.85; P<0.001) were associated with a shorter time to exacerbation (39). A further study confirmed and extended these observations by measuring blood eosinophil counts and fractional exhaled nitric oxide to define a type 2 inflammatory endotype (40). Incorporating fractional exhaled nitric oxide increased the proportion of patients with Th2 high bronchiectasis to 30%. In this regard, more studies are needed to characterise eosinophilic bronchiectasis and to develop treatments targeting this population.
Environmental effects
In addition to pathogens and host inflammatory responses, environmental factors such as air pollution may be related to bronchiectasis exacerbations. Goeminne et al. performed a case-crossover analysis that included more than 6,000 exacerbations from approximately 400 patients. This study revealed each 10 µg/m3 increase in ambient particulate matter particles <10 µm in diameter and nitrogen dioxide increased the risk of exacerbation on the same day (by 4.5% and 3.2%, respectively, reaching statistical significance) (14,41). Similarly, a Spanish study showed that a high concentration of sulphur dioxide was significantly associated with hospitalisation due to bronchiectasis exacerbation, even when controlling for the effect of temperature (P=0.008) (42). More data are needed to define which air pollutants are related to bronchiectasis exacerbation and the concentration of each pollutant sufficient to cause exacerbations.
Risk factors and the “frequent exacerbator phenotype”
Previous exacerbations
Considering that exacerbations are key events in the natural history of bronchiectasis, it is meaningful to find specific populations that are more vulnerable to exacerbations. A history of exacerbations should be discussed first. In a European cohort study of 2,572, the baseline exacerbation frequency was categorised into zero, one, two, or more than three per year, and long-term clinical outcomes for up to 5 years were observed (19). Interestingly, frequent exacerbations in the past were the strongest predictors of future exacerbation. The IRR for future exacerbation was 1.73 (95% CI, 1.47–2.02; P<0.001) for one exacerbation per year, 3.14 (95% CI, 2.70–3.66; P<0.001) for two exacerbations per year, and 5.97 (95% CI, 5.27–6.78; P<0.001) for more than three exacerbations per year at baseline (19). Thus, the phenotype was relatively stable over time.
The concept of a frequent exacerbator phenotype in bronchiectasis is similar to that in COPD, which describes a patient group more susceptible to pulmonary exacerbation with no relation to lung function (43). We should pay attention to this bronchiectasis phenotype because frequent exacerbators have more frequent hospitalisations and increased 5-year mortality rates (19). Additionally, exacerbations are the second most concerning aspect of bronchiectasis from the patient’s perspective as reported in a European survey (44). Exacerbations are also associated with poorer quality of life (QoL) (45). Thus, preventing exacerbations is of paramount importance in bronchiectasis management, which will be discussed later.
Chronic Pseudomonas infection
Along with the frequent exacerbator phenotype, the P. aeruginosa phenotype in bronchiectasis is well known for its distinct clinical picture (46). A meta-analysis pooled data from 21 observational cohort studies that compared patients with and without P. aeruginosa colonisation (n=3,683). The study showed that P. aeruginosa was associated with increased exacerbations (mean difference, 0.97 per year; 95% CI, 0.64–1.30; P<0.001), hospital admissions (OR, 6.57; 95% CI, 3.19–13.51; P<0.001) and mortality (OR, 2.95; 95% CI, 1.98–4.40; P<0.001) (47). As P. aeruginosa colonisation is associated with frequent exacerbations and other markers of disease severity (22,47), international guidelines have focused on the eradication and suppression of the pathogen (48,49). The relevant inhaled antibiotic therapy is reviewed below.
Comorbid airway diseases
When bronchiectasis is coupled with airway disease, the risk of exacerbation increases. In a Chinese study of 249 patients with only bronchiectasis and 214 with both bronchiectasis and asthma, the presence of asthma was independently associated with bronchiectasis exacerbation (OR, 2.60; 95% CI, 1.15–5.88; P=0.021) (50). Efforts have been made to define the association between COPD and bronchiectasis, because the interaction between the two diseases is complex (51,52). Despite this complexity, patients with COPD-associated bronchiectasis have an increased frequency of exacerbation compared to those with bronchiectasis not associated with COPD across multiple cohorts (19,53). In a European cohort study of 2,572 patients with bronchiectasis, COPD was independently associated with future exacerbation frequency during up to 5 years of follow-up (IRR, 1.43; 95% CI, 1.22–1.67; P<0.001) (19).
Other risk factors for exacerbations
Patients with bronchiectasis are often elderly and multimorbid, and a higher frequency of exacerbations has been shown to be associated with a higher frequency of co-morbid illnesses (54). Bronchiectasis has many underlying causes and those carrying a higher risk of exacerbation as well as other worse outcomes include rheumatoid arthritis, ulcerative colitis/inflammatory bowel disease, immunodeficiency and allergic bronchopulmonary aspergillosis. The fact that diseases associated with a high inflammatory burden or dysregulation of the immune response points to inflammation as a key driver of exacerbation risk. Gastrooesophageal reflux has been reported to contribute to exacerbation risk as has chronic rhinosinusitis (55). Anxiety and depression are common in this population and is also a risk factor. Exacerbations are a worsening of daily symptoms and so it is not surprising that worse daily symptoms are a risk factor for exacerbation. A study by Gao et al. showed that exacerbation risk increases 10% for each 10 units increase in the St. George’s Respiratory Questionnaire (SGRQ), a validated QoL questionnaire (56).
Management of acute exacerbations
Antibiotic treatment
Antibiotics are the mainstay treatment for bronchiectasis exacerbations and are also recommended by the international bronchiectasis guidelines (48,49). The rationale for using antibiotics is that antibiotic treatment reduces bacterial load, thereby reducing symptoms and airway and systemic inflammation in bronchiectasis (21). In a UK study including 385 patients with bronchiectasis, there was a direct relationship between bacterial load and the risk of subsequent exacerbations (OR, 1.20; 95% CI, 1.11–1.29; P<0.001) and severe exacerbations (OR, 1.11; 95% CI, 1.11–1.21; P=0.02) (21). A UK study evaluating the impact of 14 days of intravenous antibiotics showed that antibiotic treatment reduces 24-hour sputum volume, exercise capacity, symptoms measured on the SGRQ score and reduced systemic inflammation. Interestingly, forced expiratory volume in 1 second is not improved with antibiotics in contrast to cystic fibrosis (57). Based on expert opinion, the length of the antibiotic course is recommended to be 14 days. An antibiotic course of 14 days should always be administered to patients infected with P. aeruginosa. However, shorter courses may suffice in cases of mild exacerbations or a rapid return to baseline condition (48,49).
Because the length of antibiotics has limited evidence, a recent proof-of-concept RCT evaluated this issue (58). This study aimed to assess whether it is feasible to shorten intravenous antibiotics during exacerbations based on bacterial load, and whether 14 days of treatment is superior. A total of 47 patients were in the 14-day group and 43 were in the bacterial load-guided group; 88% of patients in the bacterial load-guided group could stop antibiotics by day 8. The authors concluded that there was a non-significant trend for increased clinical improvement by day 21 with 14 days of antibiotics compared to bacterial load-guided therapy; however, paradoxically, there was a prolonged time to exacerbation in the bacterial load-guided group who received less antibiotics (58). Further studies are needed to elucidate the optimal duration of antibiotics in bronchiectasis exacerbations.
Three points should be considered regarding antibiotics. First, antibiotics should be chosen according to sputum cultures taken either at the start of exacerbation or previously isolated. Empirical antibiotics can be initiated while awaiting sputum microbiology (49). If there is no information on sputum microbiology, clinicians can choose antibiotics based on local data to evaluate common pathogens found in patients with bronchiectasis (11). Second, the British Thoracic Society Guideline for bronchiectasis in adults recommends that suitable patients should have antibiotics to keep at home as part of a self-management plan (49). This strategy guarantees the prompt treatment of exacerbations, but is not universally implemented due to concerns about antibiotic overuse. Finally, it is likely that not all exacerbations require antibiotic treatment as molecular methods do not identify bacteria in all cases. Some symptom worsening may be managed with intensified airway clearance. Further research is needed to clarify whether some exacerbation events could be treated without antibiotics.
Other measures
An RCT exploring the long-term benefits of airway clearance technique (ACT) in bronchiectasis identified its potential role in the management of acute exacerbation, although only one study covered a duration of 12 months (59). Although there is no clear evidence regarding this issue, there are a few systematic reviews of interest. A systematic review including six studies with 120 patients revealed that all ACT appeared safe for adult patients with bronchiectasis experiencing an acute exacerbation, with no adverse reactions reported (60). Another systematic review including seven studies with 105 patients concluded that the role of ACT in acute exacerbation of bronchiectasis is unknown in relation to its clinical effects; however, in view of the chronic nature of bronchiectasis, additional data may be needed to accurately establish its effect (61). Regarding pulmonary rehabilitation, a pilot RCT compared pulmonary rehabilitation (n=9) to standard care (n=18) in exacerbated patients after completing antibiotic treatment. This study revealed that the time to the next exacerbation was not significantly different (HR, 0.83; 95% CI, 0.31–2.19; P=0.7) between the two groups (62). Taken together, ACT and pulmonary rehabilitation may have limited roles in the management of acute bronchiectasis exacerbation, but ACT can be continued safely in patients with bronchiectasis, even in exacerbation.
Prevention
Concept of vicious vortex and treatable traits
The pathophysiology of bronchiectasis has recently been understood as a vicious vortex. Impaired mucociliary clearance and retention of airway secretions along with airway inflammation disrupt normal host defences, rendering the airway susceptible to infection. The three key components—impaired mucociliary clearance, airway infection and inflammation—lead to airway structural damage in a persistent and progressive manner over time (63). Therefore, frequent exacerbations result in disease progression and high mortality (19). Hence, clinicians should intervene in one or all of these key components with the goal of reducing exacerbations. Patients may have multiple risk factors for exacerbation, many of which are treatable. These characteristics are referred to as treatable traits, including pulmonary and non-pulmonary traits. Optimal management includes addressing all possible risk factors in a multidisciplinary manner. Figure 2 summarises managements targeting treatable traits to prevent bronchiectasis exacerbation.
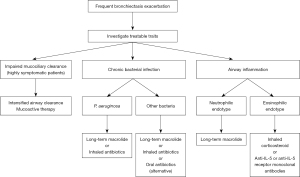
Mucoactive therapy
Regular airway clearance has been shown to reduce exacerbations, most recently in a 12-month RCT. All patients with bronchiectasis should be encouraged to practice airway clearance (59). Clinical trials investigating mucoactive therapy to prevent exacerbations have not given conclusive results. A RCT assessed the impact of inhaled mannitol on the exacerbation rates in patients with bronchiectasis. This study compared 233 patients in the mannitol group (mannitol 400 mg inhaled twice daily for 12 months) with 228 patients in the control group. The exacerbation rate, a primary study endpoint, was not significantly reduced by mannitol [rate ratio (RR), 0.92; P=0.31], although the time to first exacerbation was increased in the mannitol group (HR, 0.78; P=0.022) (64). Another RCT assessed the efficacy of inhaled 6% hypertonic saline (n=20) versus isotonic saline (n=20) administered daily for 12 months in patients with bronchiectasis. The exacerbation rate at 12 months was similar in both groups, although clinically significant improvements in QoL were observed in both groups (65). The lack of a control group makes interpretation difficult because normal saline may have beneficial effects.
Despite the unsuccessful results of clinical trials, we emphasise that a specific proportion of frequent exacerbators may benefit from mucoactive therapy. Gao et al. performed a post-hoc analysis of the aforementioned inhaled mannitol study (56,64). The authors hypothesised that exacerbation reduction would only be evident in highly symptomatic patients using inhaled mannitol. Symptom burden was measured using SGRQ. Interestingly, inhaled mannitol treatment improved the time to first exacerbation (HR, 0.56; 95% CI, 0.40–0.77; P<0.001), and the rate of patients remaining exacerbation-free for 12 months was higher in the mannitol group than in the control group (32.7% vs. 14.6%; RR, 2.84; 95% CI, 1.40–5.76; P=0.003), only in highly symptomatic patients. However, no benefit was evident in patients with lower symptom burdens (56). This supports the treatable traits concept that patients are likely to benefit from mucoactive therapy only if they have severe mucus symptoms.
Inhaled antibiotics
As previously noted, chronic infection, particularly with P. aeruginosa is associated with worse outcomes (13,15,22,47). Thus, the international guidelines recommend inhaled antibiotics to eradicate and suppress P. aeruginosa in frequent exacerbators (48,49). A meta-analysis of 16 trials with 2,597 patients showed that inhaled antibiotics significantly reduced the frequency of all exacerbations (RR, 0.81; 95% CI, 0.67–0.97; P=0.020) and severe exacerbations (RR, 0.43; 95% CI, 0.24–0.78; P=0.005). Furthermore, the time to first exacerbation was significantly prolonged (HR, 0.83; 95% CI, 0.69–0.99; P=0.015) (66). This meta-analysis suggests the efficacy of inhaled antibiotics in preventing exacerbation of bronchiectasis despite heterogeneity between trials.
Individual trials demonstrate conflicting results in the efficacy of inhaled antibiotics. RESPIRE 1, a phase III RCT of ciprofloxacin dry powder inhalation, showed an apparent reduction in exacerbations in the 14-day intervention arm, but not in the 28-day arm (67). In addition, the RESPIRE 2 trial did not show the effect of ciprofloxacin dry powder inhalation in reducing exacerbation of bronchiectasis (68). These conflicting results may be due to patient selection and lack of statistical power (15). Several trial programmes have shown conflicting results between replicate phase III studies. It is likely that only a subset of patients responds to inhaled antibiotics, but no definitive clinical parameter or biomarker has so far been identified. A secondary analysis of two phase III trials of inhaled aztreonam found improved symptoms in patients with high bacterial loads (69). Inhaled antibiotics seem to improve cough and sputum in particular. Symptoms worsen during “off periods” when antibiotics are administered 28-day on and 28-day off suggesting continuous administration may achieve better disease control (70,71).
Long-term macrolide treatment
The international bronchiectasis guidelines recommend long-term macrolides for frequent exacerbators without chronic P. aeruginosa infection (48,49). The recommendations are based on three RCTs showing the impact of long-term macrolides on the reduction of bronchiectasis exacerbations (72-74). Following the publication of the guidelines, an individual patient meta-analysis including the previous three trials (n=341) showed that macrolides reduced the frequency of exacerbation (IRR, 0.49; 95% CI, 0.36–0.66; P<0.001) and prolonged the time to first exacerbation (HR, 0.46; 95% CI, 0.34–0.61; P<0.001) (75). The effect of macrolides was observed in all subgroups; notably, a high level of benefit was observed in patients with P. aeruginosa infection (IRR, 0.36; 95% CI, 0.18–0.72; P=0.004) (75).
Two points regarding long-term macrolide treatment require considerations. First, the benefit of long-term macrolides seemed greater in patients with bronchiectasis with P. aeruginosa infections than in the overall bronchiectasis population. This is thought to be due to the anti-inflammatory properties of macrolides, although it is also possible that macrolides have effects on other constituents of the microbiome or on quorum sensing in P. aeruginosa (75). Future bronchiectasis guidelines may consider macrolides as the first-line drug for frequent exacerbators, regardless of the presence of P. aeruginosa infection. Second, it is important to remember the potential side effects of macrolides, including an increased risk of cardiovascular complications (76) and macrolide-resistant non-tuberculous mycobacterial (NTM) infection (77). Considering that macrolide-resistant NTM pulmonary disease is difficult to treat, it is important to collect sputum samples for NTM culture in patients with bronchiectasis being considered for long-term macrolide treatment (78,79).
Anti-inflammatory therapy
A novel drug that directly targets neutrophilic inflammation in bronchiectasis is currently under development. Increased neutrophil elastase activity in the sputum is closely related to future exacerbation risk in patients with bronchiectasis, which indicates that neutrophilic inflammation in bronchiectasis reflects disease activity (35). Brensocatib is an oral reversible inhibitor of dipeptidyl peptidase 1 (DPP1). By inhibiting DPP1, neutrophils are released from the bone marrow with reduced levels of active neutrophil elastase and other neutrophil serine proteases, thereby reducing inflammation (80). The WILLOW phase II RCT enrolled patients with bronchiectasis in a 1:1:1 ratio to receive placebo (n=87), 10 mg brensocatib (n=82) or 25 mg brensocatib (n=87) for 24 weeks. As a primary endpoint, brensocatib prolonged the time to first exacerbation at 10-mg (HR, 0.58; 95% CI, 0.35–0.95; P=0.03) and 25-mg (HR, 0.62; 95% CI, 0.38–0.99; P=0.046) compared with placebo (81). Significant reductions in sputum neutrophil elasatase were also observed. This therapy is now in phase III trials.
Inhaled corticosteroids (ICS) are the most widely used anti-inflammatory drugs in current clinical practice. However, international guidelines do not recommend ICS in the overall bronchiectasis population, but only in patients with bronchiectasis with specific comorbidities (e.g., asthma, allergic bronchopulmonary aspergillosis) (48,49). As approximately 20% of patients with bronchiectasis were identified as having the eosinophilic endotype (39), we may need to reassess the role of ICS in bronchiectasis. Aliberti et al. performed a post-hoc analysis of a RCT to investigate whether patients with bronchiectasis with high eosinophil counts (≥3% or ≥150 cells/µL) showed improved QoL in the high eosinophil subgroup treated with ICS (82). Although the primary outcome of the study was QoL, future research is warranted to evaluate the role of ICS in preventing exacerbation of eosinophilic bronchiectasis. In addition, anti-IL-5 or anti-IL-5 receptor monoclonal antibodies were used to treat severe eosinophilic bronchiectasis in a German case series, which demonstrated marked improvements in the exacerbation rate, QoL and lung function (83). The recognition of inflammatory endotypes of bronchiectasis has led to several phase III trials of anti-inflammatory drugs in the disease, including brensocatib (ClinicalTrials.gov Identifier: NCT04594369) and benralizumab (NCT05006573) which are ongoing at the time of writing.
Conclusions
The mechanisms underlying bronchiectasis exacerbation are complex. The causes of exacerbations and recurrent events may differ between patients with bronchiectasis and those with multiple episodes in one patient. Although recent studies have characterised frequent exacerbator phenotype and enhanced our understanding of various aspects of exacerbations, future research is needed to better manage and prevent exacerbations in patients with bronchiectasis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3437/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3437/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3437/coif). HC serves as an unpaid Editorial Board Member of the Annals of Translational Medicine from April 2022 to March 2024, and has received consultancy and speaker fees from Boryung Pharmaceutical Co., Ltd. JDC has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Novartis and Insmed; and received consultancy or speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Insmed, Janssen, Novartis and Zambon. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Keistinen T, Säynäjäkangas O, Tuuponen T, et al. Bronchiectasis: an orphan disease with a poorly-understood prognosis. Eur Respir J 1997;10:2784-7. [Crossref] [PubMed]
- Choi H, Yang B, Nam H, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J 2019;54:1900194. [Crossref] [PubMed]
- Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016;47:186-93. [Crossref] [PubMed]
- Diel R, Chalmers JD, Rabe KF, et al. Economic burden of bronchiectasis in Germany. Eur Respir J 2019;53:1802033. [Crossref] [PubMed]
- Choi H, Yang B, Kim YJ, et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci Rep 2021;11:7126. [Crossref] [PubMed]
- Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers 2018;4:45. [Crossref] [PubMed]
- Chalmers JD, Aliberti S, Polverino E, et al. The EMBARC European Bronchiectasis Registry: protocol for an international observational study. ERJ Open Res 2016;2:00081-2015. [Crossref] [PubMed]
- Aksamit TR, O'Donnell AE, Barker A, et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017;151:982-92. [Crossref] [PubMed]
- Lee H, Choi H, Sim YS, et al. KMBARC registry: protocol for a multicentre observational cohort study on non-cystic fibrosis bronchiectasis in Korea. BMJ Open 2020;10:e034090. [Crossref] [PubMed]
- Dhar R, Singh S, Talwar D, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health 2019;7:e1269-79. [Crossref] [PubMed]
- Lee H, Choi H, Chalmers JD, et al. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology 2021;26:619-21. [Crossref] [PubMed]
- Visser SK, Bye PTP, Fox GJ, et al. Australian adults with bronchiectasis: The first report from the Australian Bronchiectasis Registry. Respir Med 2019;155:97-103. [Crossref] [PubMed]
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. [Crossref] [PubMed]
- Perl S, Shteinberg M. Bronchiectasis Exacerbations: Definitions, Causes, and Acute Management. Semin Respir Crit Care Med 2021;42:595-605. [Crossref] [PubMed]
- Abo-Leyah H, Chalmers JD. Managing and preventing exacerbation of bronchiectasis. Curr Opin Infect Dis 2020;33:189-96. [PubMed]
- Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65:i1-58. [Crossref] [PubMed]
- Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017;49:1700051. [Crossref] [PubMed]
- Artaraz A, Crichton ML, Finch S, et al. Development and initial validation of the bronchiectasis exacerbation and symptom tool (BEST). Respir Res 2020;21:18. [Crossref] [PubMed]
- Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the “Frequent Exacerbator Phenotype” in Bronchiectasis. Am J Respir Crit Care Med 2018;197:1410-20. [Crossref] [PubMed]
- Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014;43:1357-67. [Crossref] [PubMed]
- Chalmers JD, Smith MP, McHugh BJ, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012;186:657-65. [Crossref] [PubMed]
- Araújo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018;51:1701953. [Crossref] [PubMed]
- Metersky ML, Aksamit TR, Barker A, et al. The Prevalence and Significance of Staphylococcus aureus in Patients with Non-Cystic Fibrosis Bronchiectasis. Ann Am Thorac Soc 2018;15:365-70. [Crossref] [PubMed]
- Metersky ML, Choate R, Aksamit TR, et al. Stenotrophomonas maltophilia in patients with bronchiectasis: An analysis of the US bronchiectasis and NTM Research Registry. Respir Med 2022;193:106746. [Crossref] [PubMed]
- Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 2013;187:1118-26. [Crossref] [PubMed]
- Byun MK, Chang J, Kim HJ, et al. Differences of lung microbiome in patients with clinically stable and exacerbated bronchiectasis. PLoS One 2017;12:e0183553. [Crossref] [PubMed]
- Dicker AJ, Lonergan M, Keir HR, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med 2021;9:885-96. [Crossref] [PubMed]
- Mac Aogáin M, Narayana JK, Tiew PY, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med 2021;27:688-99. [Crossref] [PubMed]
- Chen CL, Huang Y, Yuan JJ, et al. The Roles of Bacteria and Viruses in Bronchiectasis Exacerbation: A Prospective Study. Arch Bronconeumol 2020;56:621-9. (Engl Ed).
- Gao YH, Guan WJ, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest 2015;147:1635-43. [Crossref] [PubMed]
- Park YE, Sung H, Oh YM. Respiratory Viruses in Acute Exacerbations of Bronchiectasis. J Korean Med Sci 2021;36:e217. [Crossref] [PubMed]
- Mitchell AB, Mourad B, Buddle L, et al. Viruses in bronchiectasis: a pilot study to explore the presence of community acquired respiratory viruses in stable patients and during acute exacerbations. BMC Pulm Med 2018;18:84. [Crossref] [PubMed]
- Metaxas EI, Balis E, Papaparaskevas J, et al. Bronchiectasis exacerbations: The role of atypical bacteria, respiratory syncytial virus and pulmonary function tests. Can Respir J 2015;22:163-6. [Crossref] [PubMed]
- Crichton ML, Shoemark A, Chalmers JD. The Impact of the COVID-19 Pandemic on Exacerbations and Symptoms in Bronchiectasis: A Prospective Study. Am J Respir Crit Care Med 2021;204:857-9. [Crossref] [PubMed]
- Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am J Respir Crit Care Med 2017;195:1384-93. [Crossref] [PubMed]
- Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med 2021;9:873-84. [Crossref] [PubMed]
- Menéndez R, Méndez R, Amara-Elori I, et al. Systemic Inflammation during and after Bronchiectasis Exacerbations: Impact of Pseudomonas aeruginosa. J Clin Med 2020;9:2631. [Crossref] [PubMed]
- Posadas T, Oscullo G, Zaldivar E, et al. C-Reactive Protein Concentration in Steady-State Bronchiectasis: Prognostic Value of Future Severe Exacerbations. Data From the Spanish Registry of Bronchiectasis (RIBRON). Arch Bronconeumol 2021;57:21-7. (Engl Ed). [PubMed]
- Shoemark A, Shteinberg M, De Soyza A, et al. Characterization of Eosinophilic Bronchiectasis: A European Multicohort Study. Am J Respir Crit Care Med 2022;205:894-902. [Crossref] [PubMed]
- Oriano M, Gramegna A, Amati F, et al. T2-High Endotype and Response to Biological Treatments in Patients with Bronchiectasis. Biomedicines 2021;9:772. [Crossref] [PubMed]
- Goeminne PC, Cox B, Finch S, et al. The impact of acute air pollution fluctuations on bronchiectasis pulmonary exacerbation: a case-crossover analysis. Eur Respir J 2018;52:1702557. [Crossref] [PubMed]
- Garcia-Olivé I, Radua J, Sánchez-Berenguer D, et al. Association between environmental factors and hospitalisations for bronchiectasis in Badalona, Barcelona, Spain (2007-2015). Med Clin (Barc) 2018;150:257-61. [PubMed]
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. [Crossref] [PubMed]
- Aliberti S, Masefield S, Polverino E, et al. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. Eur Respir J 2016;48:632-47. [Crossref] [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Inflammatory Responses, Spirometry, and Quality of Life in Subjects With Bronchiectasis Exacerbations. Respir Care 2015;60:1180-9. [Crossref] [PubMed]
- Aliberti S, Lonni S, Dore S, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016;47:1113-22. [Crossref] [PubMed]
- Finch S, McDonnell MJ, Abo-Leyah H, et al. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann Am Thorac Soc 2015;12:1602-11. [PubMed]
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50:1700629. [Crossref] [PubMed]
- Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019;74:1-69. [Crossref] [PubMed]
- Mao B, Yang JW, Lu HW, et al. Asthma and bronchiectasis exacerbation. Eur Respir J 2016;47:1680-6. [Crossref] [PubMed]
- Traversi L, Miravitlles M, Martinez-Garcia MA, et al. ROSE: radiology, obstruction, symptoms and exposure - a Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC Airways Working Group. ERJ Open Res 2021;7:e00399-2021. [Crossref] [PubMed]
- Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J 2018;52:1800328. [Crossref] [PubMed]
- Ni Y, Shi G, Yu Y, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2015;10:1465-75. [Crossref] [PubMed]
- McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016;4:969-79. [Crossref] [PubMed]
- Handley E, Nicolson CH, Hew M, et al. Prevalence and Clinical Implications of Chronic Rhinosinusitis in People with Bronchiectasis: A Systematic Review. J Allergy Clin Immunol Pract 2019;7:2004-2012.e1. [Crossref] [PubMed]
- Gao YH, Abo Leyah H, Finch S, et al. Relationship between Symptoms, Exacerbations, and Treatment Response in Bronchiectasis. Am J Respir Crit Care Med 2020;201:1499-507. [Crossref] [PubMed]
- Murray MP, Turnbull K, Macquarrie S, et al. Assessing response to treatment of exacerbations of bronchiectasis in adults. Eur Respir J 2009;33:312-8. [Crossref] [PubMed]
- Bedi P, Cartlidge MK, Zhang Y, et al. Feasibility of shortening intravenous antibiotic therapy for bronchiectasis based on bacterial load: a proof-of-concept randomised controlled trial. Eur Respir J 2021;58:2004388. [Crossref] [PubMed]
- Muñoz G, de Gracia J, Buxó M, et al. Long-term benefits of airway clearance in bronchiectasis: a randomised placebo-controlled trial. Eur Respir J 2018;51:1701926. [Crossref] [PubMed]
- Phillips J, Lee A, Pope R, et al. Effect of airway clearance techniques in patients experiencing an acute exacerbation of bronchiectasis: a systematic review. Physiother Theory Pract 2020;36:1300-15. [Crossref] [PubMed]
- Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev 2015;CD008351. [PubMed]
- Chalmers JD, Crichton ML, Brady G, et al. Pulmonary rehabilitation after exacerbation of bronchiectasis: a pilot randomized controlled trial. BMC Pulm Med 2019;19:85. [Crossref] [PubMed]
- Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018;392:880-90. [Crossref] [PubMed]
- Bilton D, Tino G, Barker AF, et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial. Thorax 2014;69:1073-9. [Crossref] [PubMed]
- Nicolson CH, Stirling RG, Borg BM, et al. The long term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med 2012;106:661-7. [Crossref] [PubMed]
- Laska IF, Crichton ML, Shoemark A, et al. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta-analysis. Lancet Respir Med 2019;7:855-69. [Crossref] [PubMed]
- De Soyza A, Aksamit T, Bandel TJ, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018;51:1702052. [Crossref] [PubMed]
- Aksamit T, De Soyza A, Bandel TJ, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018;51:1702053. [Crossref] [PubMed]
- Sibila O, Laserna E, Shoemark A, et al. Airway Bacterial Load and Inhaled Antibiotic Response in Bronchiectasis. Am J Respir Crit Care Med 2019;200:33-41. [Crossref] [PubMed]
- Crichton ML, Lonergan M, Barker AF, et al. Inhaled aztreonam improves symptoms of cough and sputum production in patients with bronchiectasis: a post hoc analysis of the AIR-BX studies. Eur Respir J 2020;56:2000608. [Crossref] [PubMed]
- Chalmers JD, Cipolla D, Thompson B, et al. Changes in respiratory symptoms during 48-week treatment with ARD-3150 (inhaled liposomal ciprofloxacin) in bronchiectasis: results from the ORBIT-3 and -4 studies. Eur Respir J 2020;56:2000110. [Crossref] [PubMed]
- Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:660-7. [Crossref] [PubMed]
- Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251-9. [Crossref] [PubMed]
- Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309:1260-7. [Crossref] [PubMed]
- Chalmers JD, Boersma W, Lonergan M, et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med 2019;7:845-54. [Crossref] [PubMed]
- Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ 2013;346:f1235. [Crossref] [PubMed]
- Choi H, Kim SY, Lee H, et al. Clinical Characteristics and Treatment Outcomes of Patients with Macrolide-Resistant Mycobacterium massiliense Lung Disease. Antimicrob Agents Chemother 2017;61:e02189-16. [Crossref] [PubMed]
- Choi H, Kim SY, Kim DH, et al. Clinical Characteristics and Treatment Outcomes of Patients with Acquired Macrolide-Resistant Mycobacterium abscessus Lung Disease. Antimicrob Agents Chemother 2017;61:e01146-17. [Crossref] [PubMed]
- Yang B, Ryu J, Kim T, et al. Impact of Bronchiectasis on Incident Nontuberculous Mycobacterial Pulmonary Disease: A 10-Year National Cohort Study. Chest 2021;159:1807-11. [Crossref] [PubMed]
- Giam YH, Shoemark A, Chalmers JD. Neutrophil dysfunction in bronchiectasis: an emerging role for immunometabolism. Eur Respir J 2021;58:2003157. [Crossref] [PubMed]
- Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N Engl J Med 2020;383:2127-37. [Crossref] [PubMed]
- Aliberti S, Sotgiu G, Blasi F, et al. Blood eosinophils predict inhaled fluticasone response in bronchiectasis. Eur Respir J 2020;56:2000453. [Crossref] [PubMed]
- Rademacher J, Konwert S, Fuge J, et al. Anti-IL5 and anti-IL5Rα therapy for clinically significant bronchiectasis with eosinophilic endotype: a case series. Eur Respir J 2020;55:1901333. [Crossref] [PubMed]


