
Non-small cell lung cancer with MET exon 14 skip mutation: case report
Introduction
Lung cancer is the leading cause of death in patients with cancer across both male and female populations (1). However, the survival rate after initial diagnosis has been slowly increasing. It is theorized that this increased survival rate could be due to early detection, targeted therapy, and immunotherapy (2). In fact, it has been shown that targeted therapy can lead to better morbidity and mortality than standard chemotherapy for subtypes of non-small cell lung cancer (NSCLC) (3). The targeted therapy discussed in this case report deals specifically with MET exon-14-skipipng mutations. MET is a tyrosine kinase receptor for hepatocyte growth factor (HGF). The mutation causes reduced degradation of the MET protein which results in it the promotion of uncontrolled cellular growth. Capmatinib is a FDA-approved MET inhibitor used on adult patients with this specific mutation. The NCCN guidelines now state that first-line treatment for metastatic NSCLCs with the MET exon-14-skipping mutation is targeted therapy in lieu of traditional chemotherapy regimens. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-2022-53/rc).
Case presentation
Our patient is a 68-year-old female with a past medical history of hypertension, chronic obstructive pulmonary disease (COPD), and a 46-pack year tobacco history. She initially presented to the emergency department on 4/21/2019 due to worsening shortness of breath (SOB) for 3 weeks. A chest X-ray (CXR) revealed a new lesion in the right upper lung. The patient was instructed to follow-up with her primary care physician (PCP). She met with her PCP who then ordered a CT of the chest. The CT scan was performed on 4/23/2019 which showed a 1.1 cm nodule in the periphery of the right upper lobe. Our patient was then referred to a pulmonologist for further workup.
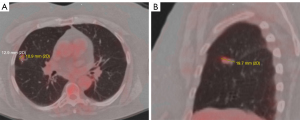
The patient’s pulmonologist ordered a positron emission tomography (PET) scan which was performed on 5/09/2019. It showed an upper lobe nodule (2.0 cm × 1.14 cm × 1.15 cm) (Figure 1A,1B). It had increased tracer uptake of a standardized uptake value (SUV) of 3.08. There was no suspicious adenopathy or regional/distant metastatic disease noted. The patient was then seen by a cardiothoracic surgeon for potential surgical excision of the nodule. PFTs and stress tests were ordered to evaluate if the patient was a good surgical candidate.
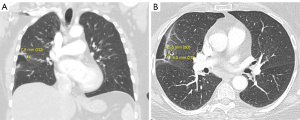
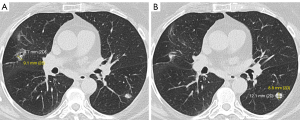
Her PFTs were obtained on 5/23/2019 and revealed FEV1 of 1.7 L and diffusing capacity for carbon monoxide (DLCO) 48 mL/min/mmHg. Although her FEV1 was above 1.5 L, the patient was not deemed to be a good surgical candidate, due to her marginal functional status as shown by ongoing dyspnea on exertion (DOE) with activities of daily livings (ADLs). Interventional radiology was then consulted for a CT-guided biopsy. However, they were unable to perform it due to the risk of pneumothorax associated with the procedure as well as the difficult positioning of the nodule itself. The nodule’s position also made it inaccessible via bronchoscopic biopsy. Therefore, she was seen by radiation oncology on 6/17/2019, who determined that stereotactic body radiation therapy (SBRT) would be the best course of action. The patient was treated with SBRT from 6/24/2019 to 7/10/2019. She tolerated it well. A follow-up CT scan of the chest was performed on 2/4/2020, which showed a reduction in the size of the tumor to 1.56 cm AP × 0.6 cm transverse. However, the CT also revealed lymphadenopathy in the mediastinal, right hilar region, and axilla; it was unclear whether this new lymphadenopathy was neoplastic or reactive (Figure 2A,2B). She was then on surveillance imaging which remained unremarkable until a chest CT scan on 2/12/2021 revealed metastasis to the left lung. The CT showed a lobular left lower lung nodule measuring 1.2 cm × 0.9 cm × 1.1 cm, a second nodule in the left posterior base measuring 0.9 cm, and a third nodule located at the superior aspect of the left lower lobe measuring 0.92 cm. The original nodule remained stable at 0.91 cm × 0.97 cm. Mediastinal lymph nodes were also stable at 2.1 cm × 2 cm (Figure 3A,3B).
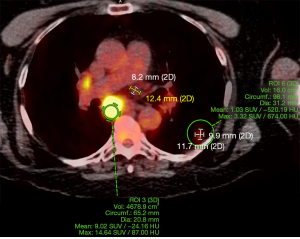
Due to possibility of metastatic disease radiation oncology referred the patient to medical oncology on 4/14/2021. On physical exam the patient noted to have a supraclavicular lymph node. She reported that she had noticed the right supraclavicular lymph node for the last 2 months. She denied any systemic symptoms, such as fevers, chills, night sweats, and weight loss. A new PET scan, brain MRI, and needle biopsy of the supraclavicular lymph node were ordered at this time. The PET scan was completed on 4/20/2021 and it showed multiple metastatic nodes in the left lung, along with the mediastinal and right supraclavicular lymph nodes (Figure 4). Brain MRI was negative for metastasis; however, lymph node biopsy was positive. Next-generation sequencing (NGS) and liquid biopsy were ordered for the patient to find direct targetable therapy.
Unfortunately, pathology was unable to differentiate the subtype of the patient’s NSCLC due to the paucity of tumor sample that was available. The patient had a liquid biopsy of the right supraclavicular node, which was sent for Guardant360 testing. Results showed a MET exon 14 skip mutation; it was negative for PD-L1 mutation and for an additional 83 genes including EGFR, ALK, ROS1, BRAF, MET, HER2, RET, and NTRK. First-line treatment with Capmatinib 400 mg twice a day was initiated on 5/12/2021. She tolerated the treatment well and had a follow-up chest CT on 7/23/2021. CT showed that the right upper lobe nodule increased in size from the previous exam. However, the right hilar mass lesion was no longer visible. The left lower lobe nodule decreased in size as well. Overall, the patient demonstrated a positive response to Capmatinib. There was a shrinkage in most of her cancer sites, apart from the original right upper lobe nodule. She also experienced minimal adverse effect. In fact, the only side effect she experienced was mild edema. Therefore, subsequent targeted radiation therapy was suggested as an adjunct treatment for the right upper lobe nodule. She received 5,400 cGY of radiation in 3 fractions, from 8/23/2021 to 8/30/2021, which she tolerated well. The patient continues to be under surveillance with her medical oncology team.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
NSCLC accounts for 85% of all lung cancers and is the second most common cancer in both males and females and is the most common cause of death in the United States (1). NSCLC is classified histologically into different categories including adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and large cell carcinoma (4). The most important risk factor for NSCLC is tobacco consumption. Other factors include substance or radioactive exposure, family history of cancer, chronic lung disease, and genetic factors (5). Major sites of NSCLC metastases include the brain (47 %), bone (36 %), liver (22 %), adrenal glands (15 %), thoracic cavity (11 %) and distant lymph nodes (10 %) (6).
The mesenchymal epithelium transition factor receptor (MET) gene is located on human chromosome 7 (7q21-q31) and encodes the receptor tyrosine kinase (7). c-MET tyrosine Kinase is expressed in epithelial cells of many organs including liver, pancreas, prostate, kidney, muscle, and bone marrow (7,8). In a highly regulated processes binding of an endogenous ligand, HGF, plays an important role in normal biological functions. It promotes cell proliferation, survival, differentiation, and morphogenesis (9). Studies showed that dysregulated c-MET activation in tumor cells initiates several downstream signaling pathways such as mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), signal transducer and activator of transcription proteins, and nuclear factor-κB which can lead to tumor growth and metastatic progression of cancer cells (10). c-MET gene amplification combined with protein overexpression and kinase activation may lead to several cancers, such as liver cancer, breast cancer, pancreatic cancer, colon cancer, prostate cancer, glioblastomas, and lung cancer (10).
The molecular mechanisms underlying the development of NSCLC are different among patients. c-MET was first discovered in the 1980s. It was later found to be dysregulated in lung cancers in the 1990s (9,11). MET dysregulation occurs through variable mechanisms which includes overexpression of MET and/or its ligand, HGF, and genetic alterations to MET (e.g., mutations, amplification, translocation, or dysregulated transcription), and impaired degradation of MET (12). About 3% to 4% of patients with NSCLC have MET exon 14 skipping mutations which are associated with a poor prognosis (13,14).
Several drugs have been developed to target MET or HGF. Capmatinib is a highly potent c-MET kinase inhibitor. Capmatinib’s efficacy was evaluated in a multicohort study enrolling 364 participants with advanced NSCLC with confirmed MET exon 14 skipping mutation or MET amplification. Patients were assigned to cohorts based on MET status and previous lines of therapy. Results showed that the overall response in patients with MET exon 14 skipping mutation who had not received previous therapy for NSCLC was 68%, the median duration of response was 12.6 months, and the median progression-free survival (PFS) was 12.4 months (13).
Side effects of Capmatinib include Peripheral edema (51%), nausea (45.0%), vomiting (28%), increased blood creatinine (24%), dyspnea (23%), fatigue (22%), decreased appetite (21%) (13,15). The US FDA approved Capmatinib as a first-line treatment for patients with NSCLC carrying a MET exon 14 skipping mutation in May 2020. Other therapeutic agents targeting MET exon 14 skipping mutations are currently in clinical trials (15).
This case demonstrates that targeted therapies, such as Capmatinib are an excellent choice for patients with NSCLC carrying the MET exon 14 skipping mutation. Our patient has achieved partial remission status with no growth of current disease, and no new metastatic disease. She now has an improved prognosis with Capmatinib use and had minimal side effects. Because of our patient’s success, we are now advocating other clinicians to check for MET mutations in patients with NSCLC.
There are also other case reports that have shown favorable responses to targeted therapy against NSCLCs with the MET exon 14 skipping mutation. One case describes the outcome of a patient being treated via Tepotinib. This patient initially had metastatic disease but is now stable for more than 45 cycles and 31 months after starting Tepotinib (16). However, in contrast with our patient, theirs had high levels of PD-L1. Another case describes a patient who was treated with Crizotinib and Cabozantinib. Crizotinib was used as the first-line treatment and showed a PFS of 8 months. However, a chest CT and MRI showed a larger mass in the lung and renal metastases. Cabozantinib was then used as second-line targeted therapy, which showed a reduction in size of the renal metastatic lesions. The patient is still under follow-up and has an overall survival (OS) of >12 months (17).
These cases, in combination with ours, indicate that targeted therapy against MET exon 14 can result in better prognosis. However, it is prudent to note that our patient was also treated with radiation therapy. The combination of targeted therapy and radiation therapy requires further study.
NSCLC is the most common type of lung cancer. With the rise of targeted therapy, it has allowed individualized treatments for patients. In our case, our patient was started on Capmatinib after having metastasis of her cancer. While she has been on this treatment for a short period of time the first CT after treatment showed shrinkage in most of her sites. Further follow-up is needed but early indications are positive. Further development of targeted therapy may result in better prognosis of cancers.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-2022-53/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-2022-53/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-2022-53/coif). MAJ and SD report sitting on an advisory board of New Century Health. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Sholl LM. Biomarkers in lung adenocarcinoma: a decade of progress. Arch Pathol Lab Med 2015;139:469-80. [Crossref] [PubMed]
- Inamura K. Lung cancer: understanding its molecular pathology and the 2015 who classification. Front Oncol 2017;7:193. [Crossref] [PubMed]
- De Mello RA, Neves NM, Amaral GA, et al. The Role of MET inhibitor therapies in the treatment of advanced non-small cell lung cancer. J Clin Med 2020;9:1918. [Crossref] [PubMed]
- Niu FY, Zhou Q, Yang JJ, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer 2016;16:149. [Crossref] [PubMed]
- Fujino T, Suda K, Mitsudomi T. Emerging MET tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Expert Opin Emerg Drugs 2020;25:229-49. [Crossref] [PubMed]
- Skead G, Govender D. Gene of the month: MET. J Clin Pathol 2015;68:405-9. [Crossref] [PubMed]
- Drilon A, Cappuzzo F, Ou SI, et al. Targeting MET in lung cancer: will expectations finally be met? J Thorac Oncol 2017;12:15-26. [Crossref] [PubMed]
- Zhang Y, Xia M, Jin K, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer 2018;17:45. [Crossref] [PubMed]
- Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984;311:29-33. [Crossref] [PubMed]
- Salgia R. MET in lung cancer: biomarker selection based on scientific rationale. Mol Cancer Ther 2017;16:555-65. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-amplified non-small-cell lung cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016;22:3048-56. [Crossref] [PubMed]
- FDA approves first targeted therapy to treat aggressive form of lung cancer. u.s. food and drug administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-treat-aggressive-form-lung-cancer. 2020.
- Roth KG, Mambetsariev I, Salgia R. Prolonged survival and response to tepotinib in a non-small-cell lung cancer patient with brain metastases harboring MET exon 14 mutation: a research report. Cold Spring Harb Mol Case Stud 2020;6:a005785. [Crossref] [PubMed]
- Qin RY, Liu LS, Zhang HY, et al. Responses to crizotinib and cabozantinib in patient with lung adenocarcinoma harboring mesenchymal-epithelial transition factor exon 14 skipping mutation: A case report. Medicine (Baltimore) 2021;100:e24300. [Crossref] [PubMed]


