
Role of conservative therapy prior to surgery in xanthogranulomatous mastitis: a case report
Introduction
Xanthogranulomatous inflammation is a rare chronic inflammatory response that is predominantly detected in the gallbladder or kidneys. The involvement of the breasts is rarely reported due to a combination of low incidence, and the tendency to be clinically asymptomatic (1).
By reviewing the literature on xanthogranulomatous pyelonephritis (XGP) and cholecystitis (XGC), the aetiology of xanthogranulomatous inflammation has been suggested to be primarily obstruction and infection. Obstruction due to stones accounted for almost 85–90.7% of XGC (2,3) whilst majority of XGP cases also had obstruction due to stones or underlying infection (4). Infectious, haemorrhagic, and immunological aetiologies have also been implicated with xanthogranulomatous inflammation.
Despite successful management of xanthogranulomatous mastitis (XGM) with surgical excision with no incidences of recurrence to date (1), we describe a case of XGM with the largest documented dimension that would confer significant surgical risks. We share the details of how long-term antibiotics decreased the dimensions of the lesion suggesting a first-line role of conservative therapy prior to surgical excision in larger masses. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-2022-52/rc).
Case presentation
A 40-year-old Caucasian female presented with self-detected lumps in both breasts. She denied symptoms of pain, nipple discharge or weight loss. She had a past medical history of schizophrenia, which was treated with paliperidone, and no history of trauma, implants, or previously diagnosed cancer. On examination, a palpable right-sided lump at 12 to 3 o’clock with nipple inversion and skin tethering was detected on C-cup sized breasts. Routine blood tests showed no lipid abnormalities.
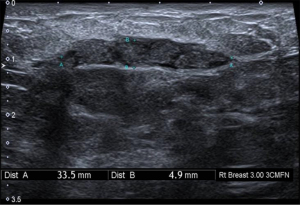
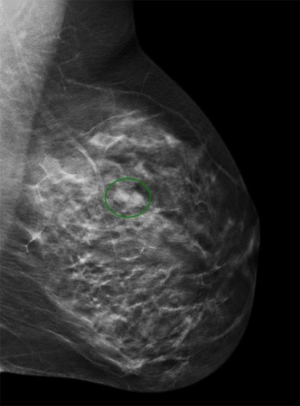
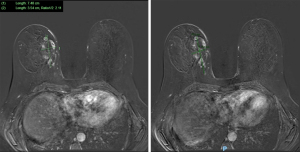
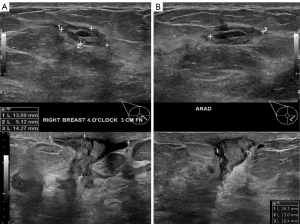
Initial investigations included bilateral ultrasound (Figure 1) and mammography with tomosynthesis (Figure 2) which showed a left sided well-circumscribed lesion consistent with a fibroadenoma. The right sided mass correlated with a scattered heterogeneous focal region measuring 34 mm × 5 mm × 26 mm from 12 to 4 o’clock at 3 cm from the nipple with dilated ducts. Due to clinical and radiological suspicion of malignancy, magnetic resonance imaging of the breasts was done, showing two large areas of extensive ductal enhancement on the right breast suggestive of high-grade ductal carcinoma in situ (Figure 3).
Histological examination of the right breast mass was conducted with nine core needle biopsies (CNB), shown in Figure 4A. There was a mixed inflammatory infiltrate of neutrophils, lymphocytes and prominent foamy macrophages. A significant cystic component was not identified. No emperipolesis and no Touton giant cells were observed. No micro-organisms were identified on Gram, Ziehl-Neelsen or Periodic Acid Schiff-Diastase stains. Immunohistochemistry for AE1/AE3 highlighted only a few background acini and was negative within the abnormal tissue. The features were consistent with an active xanthogranulomatous mastitis.
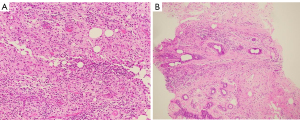
An 8-week course of 100 mg oral doxycycline twice daily was trialled. A conservative approach was sought as there was no histological evidence of a malignant mass and surgical intervention would confer significant risk. Follow up CNB 4 weeks post-antibiotic therapy showed resolution of the xanthogranulomatous inflammation, and instead showed fibrosis and mild chronic inflammation with areas of fat necrosis as seen in Figure 4B.
Subsequent 6-weekly follow up reviews showed clinical improvement with reduction in size on palpation to approximately 3 cm × 3 cm from the 12 to 2 o’clock region. Follow-up ultrasound also revealed reduced size of the lesion but with persistent inflammatory changes as shown in Figure 5A,5B. On review of the literature, this was found to be the first case of XGM that demonstrated clinical improvement with conservative measures.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The aim of this report was to present an alternative treatment method for patients with XGM whilst providing a review of literature on this rare condition. Only 26 cases of XGM have been reported (Table 1) showing an epidemiological prevalence in younger Asian females with a mean age of 45 years. The pathology involves an inflammatory process characterised by the infiltration of foamy macrophages which is more commonly reported in the form of xanthogranulomatous pyelonephritis and xanthogranulomatous cholecystitis (1).
Table 1
| Study | Year | Case | Nationality | Age (years) | Presenting complaint | Location | Malignancy | Size (cm) |
Mode of detection | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Kapoor et al. | 2021 | 1 | – | 18 | Pain | Right | None | 2×1.1×1.8 | US | – |
| 2 | – | 53 | Asymptomatic | Left | None | 0.8 | US | – | ||
| Bamanikar et al. | 2018 | 3 | India | 42 | Pain | Left | None | 3 | MMG | Surgical |
| Leong et al. | 2018 | 4 | Singapore | 41 | Pain | Left | None | – | – | – |
| de Oliveira et al. | 2017 | 5 | Brazil | 47 | Pain, pruritus | Bilateral | None | R: 6.8×4.1, L: 4.2×3.0 |
US | Surgical |
| Dinets et al. | 2016 | 6 | Ukraine | 30 | Asymptomatic | Left | None | 4×5 | – | Surgical |
| Jyoti et al. | 2013 | 7 | India | 19 | Palpable mass | Unknown | None | – | – | Surgical |
| Hussain et al. | 2012 | 8 | UK | 56 | Palpable mass | Left | None | 10 | Px | Surgical |
| Koo and Jung | 2009 | 9 | Korea | 42 | Asymptomatic | Left | None | 0.5 | – | Surgical |
| 10 | Korea | 67 | Asymptomatic | Right | None | 1.3 | – | Surgical | ||
| 11 | Korea | 44 | Asymptomatic | Left | IDC | 1.2 | – | Surgical | ||
| 12 | Korea | 26 | Palpable mass | Right | None | 4.8 | – | Surgical | ||
| 13 | Korea | 36 | Asymptomatic | Left | None | 1.8 | – | Surgical | ||
| 14 | Korea | 36 | Asymptomatic | Right | IDC | 1.5 | – | Surgical | ||
| 15 | Korea | 55 | Asymptomatic | Left | None | 0.5 | – | Surgical | ||
| 16 | Korea | 37 | Asymptomatic | Left | None | 0.8 | – | Surgical | ||
| 17 | Korea | 70 | Palpable mass | Bilateral | None | 3 | – | Surgical | ||
| 18 | Korea | 53 | Asymptomatic | Left | None | 2 | – | Surgical | ||
| 19 | Korea | 48 | Asymptomatic | Right | DCIS | 1.5 | – | Surgical | ||
| 20 | Korea | 37 | Asymptomatic | Right | None | 1.5 | – | Surgical | ||
| 21 | Korea | 47 | Asymptomatic | Left | None | 1.2 | – | Surgical | ||
| 22 | Korea | 46 | Asymptomatic | Left | None | 1 | – | Surgical | ||
| 23 | Korea | 44 | Asymptomatic | Left | None | 1.5 | – | Surgical | ||
| 24 | Korea | 46 | Asymptomatic | Right | None | 1.2 | – | Surgical | ||
| Hwang et al. | 2007 | 25 | Korea | 60 | Asymptomatic | Bilateral | None | R: 3, L: 3 | MMG | Surgical |
| Shin et al. | 2005 | 26 | USA | 74 | Palpable mass | Right | None | 2.2 | – | Surgical |
US, ultrasound; MMG, mammography; Px, physical examination; R, right; L, left; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ.
The trigger for XGM is not completely known although some papers have suggested a traumatic aetiology in the form of implant rupture or prolonged cutaneous scratching (5-7). By reviewing the literature on XGC and XGP, infectious and obstructive aetiologies to this inflammation have also been suggested (3,8). When reviewing previous XGM cases, a potential obstructive cause was found in three cases with co-existent malignant masses, however, this was not implicated in the remaining cases. Despite being characterised by foamy histiocytes, all cases have demonstrated no association to lipid abnormalities whilst stains for bacterial, fungal, and mycobacterial organisms were negative.
The major concern of XGM is its diagnostic challenge to differentiate it from breast malignancy both clinically and radiologically. Most cases of XGM (17/26) were asymptomatic with incidental findings on routine screening whilst a minority of cases presented with pain (4/26) or a palpable lump (5/26). Radiologically there is a tendency for XGM masses to mimic malignancy as in this case warranting a pathological diagnosis either by core or excisional biopsy.
The entity of cystic neutrophilic granulomatous mastitis (CNGM) should be considered as a differential diagnosis for this case. This entity is characterised by rounded clear spaces/vacuoles which are rimmed by neutrophils and epithelioid histiocytes. As a diagnostic feature, Gram positive bacilli should be seen within these spaces, or Corynebacterium species should be grown on culture. In retrospect a culture should have been performed to consider differentials like CNGM, however, due to the good response to antibiotics and classic histological features of XGM its results would have had minor clinical implications. Fat necrosis can show foamy histiocytes due to phagocytosis of necrotic adipocytes, but in this condition, adipose tissue is the predominant component, generally with prominent adipocyte necrosis and haemosiderin deposition, and a neutrophilic infiltrate is not a typical feature. Later stage lesions show variable fibrosis and calcification.
More sinister neoplastic conditions also need consideration. Histiocytoid lobular breast carcinoma has an abundant finely vacuolated cytoplasm that can mimic foamy macrophages, but would be positive for cytokeratins such as AE1/AE3. Histiocytic neoplasms such as Rosai-Dorfman disease (RDD) and Erdheim-Chester disease (ECD) are exceedingly rare in the breast. RDD features large eosinophilic histiocytes showing emperipolesis whereas ECD features foamy histiocytes with Touton giant cells with a sparse lymphoplasmacytic inflammatory infiltrate.
Other forms of chronic inflammatory mastitis such as granulomatous mastitis (GM) often causes diagnostic confusion to XGM amongst clinicians. GM is a chronic inflammatory mastitis characterized by the infiltration of the breast parenchyma by epithelioid histiocytes, multinucleated giant cells and polymorphonuclear leukocytes forming micro-abscesses. It is pathologically distinct to XGM with less predominance of foamy macrophages and tends to occur in young parous females with recent history of childbirth or breastfeeding (9). It is more prevalent within the Middle Eastern, Asian and Hispanic descents. With more documented cases in the literature than XGM, the etiology of GM has been attributed to hormonal factors like pregnancy and breastfeeding and infectious causes. Recently an autoimmune etiology to GM has been becoming more popular due to its good response to steroids and immunosuppressive treatment, T-lymphocyte dominance in immunohistochemical studies and extramammary involvement such as erythema nodosum (10).
The use of antibiotics has been a popular approach during the earlier understanding of GM due to its association with the Corynebacterium species. Antibiotics that had lipophilic and bactericidal properties such as clindamycin were recommended to target these species that survived in lipid-filled vacuoles. Beta lactam was shown to have poor penetration of lipid and hence was not recommended in the treatment of GM (11). As the knowledge of GM is advancing with more publications, the autoimmune etiology of this condition is being favoured. Consequently, the role of antibiotic therapy has been controversial with some studies reporting little to no improvement with antibiotics (12) whilst other papers suggest high rates of complete resolution (13). Akcan et al. summarised that the role of antibiotic therapy is minimal unless there is an etiological or clinical indication such as by positive microbiological cultures, or co-existing abscesses, fistulas or chronic suppuration (14). The histological differentiation of chronic mastitis is therefore essential as the initial approach to the treatment of GM rarely involves the use of antibiotics.
Whilst more publications in the literature have been guiding clinicians in the management of GM, the treatment for XGM has not been well established with all previous cases been predominantly managed with surgical excision (1,5-8,15-18). Excisional biopsy may be diagnostic and therapeutic for small lesions, however, some surgeons may prefer a complete excision due to the suspicion of malignancy. The clinical improvement with antibiotics in this case suggest a conservative initial approach in the treatment of XGM. This was appropriate due to the size of the lesion and the absence of malignancy confirmed through multiple CNB. More data with successful response to antibiotics would be needed to validate a first line conservative approach minimising the risks of operation. The impact on physiological function such as lactating, cosmetic issues especially in younger females, along with infectious and anaesthetic risks that accompanies any operations are factors to consider in surgical management.
Cases with co-existent malignancy, patient preference for surgical removal, or small lesion suitable for an excisional biopsy may opt for an initial surgical approach. Larger XGM lesions with higher surgical risks may be approached conservatively with frequent monitoring for response with consideration of surgical excision if this fails.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-2022-52/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-2022-52/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kapoor H, Zhang Y, Qasem SA, et al. Xantho-granulomatous mastitis preceded by cysts on ultrasound: Two cases with review of literature. Clin Imaging 2021;78:64-8. [Crossref] [PubMed]
- Guzman-Valdivia G. Xanthogranulomatous Cholecystitis: 15 Years’ Experience. World J Surg 2004;28:254-7. [Crossref] [PubMed]
- Yucel O, Uzun MA, Tilki M, et al. Xanthogranulomatous Cholecystitis: Analysis of 108 Patients. Indian J Surg 2017;79:510-4. [Crossref] [PubMed]
- Korkes F, Favoretto RL, Bróglio M, et al. Xanthogranulomatous pyelonephritis: clinical experience with 41 cases. Urology 2008;71:178-80. [Crossref] [PubMed]
- Hussain T, Elahi B, Long E, et al. Xanthogranulomatous inflammation involving latissimus dorsi donor site and implant breast reconstruction: case report and literature review. World J Surg Oncol 2012;10:166. [Crossref] [PubMed]
- Dinets A, Unukovych D, Khrapach V, et al. An unusual case of a ruptured Poly Implant Prothese breast implant associated with xanthoma. Case Reports Plast Surg Hand Surg 2016;3:11-5. [Crossref] [PubMed]
- de Oliveira I. Xanthogranulomatous Mastitis Mimicking Locally Advanced Breast Cancer. Breast J 2017;23:227-9. [Crossref] [PubMed]
- Marinacci LX, Rosales I. Xanthogranulomatous Pyelonephritis. N Engl J Med 2018;378:940. [Crossref] [PubMed]
- Bede K, Valente SA. Idiopathic Granulomatous Mastitis. Ann Breast Surg 2020;4:24. [Crossref]
- Wolfrum A, Kümmel S, Theuerkauf I, et al. Granulomatous Mastitis: A Therapeutic and Diagnostic Challenge. Breast Care (Basel) 2018;13:413-8. [Crossref] [PubMed]
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial Treatment Options for Granulomatous Mastitis Caused by Corynebacterium Species. J Clin Microbiol 2015;53:2895-9. [Crossref] [PubMed]
- Bouton ME, Jayaram L, O'Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg 2015;210:258-62. [Crossref] [PubMed]
- Al-Jarrah A, Taranikanti V, Lakhtakia R, et al. Idiopathic Granulomatous Mastitis. Sultan Qaboos University Medical Journal 2013;13:241-7. [Crossref] [PubMed]
- Akcan A, Oz AB, Dogan S, et al. Idiopathic Granulomatous Mastitis: Comparison of Wide Local Excision with or without Corticosteroid Therapy. Breast Care (Basel) 2014;9:111-5. [Crossref] [PubMed]
- Koo JS, Jung W. Xanthogranulomatous mastitis: clinicopathology and pathological implications. Pathol Int 2009;59:234-40. [Crossref] [PubMed]
- Jyoti K, Preeti B, Sandip S, et al. Comparison of Fine Needle Aspiration Cytology of Non-Neoplastic Lesions of Breast with Histopathology. Sch J Appl Med Sci 2013;1:804-13.
- Bamanikar S, Chandanwale S, Pathak P, et al. A Rare Case of Xanthogranulomatous Mastitis with Intraductal Papilloma. Med J DY Patil Vidyapeeth 2018;11:348-51. [Crossref]
- Hwang SH, Son EJ, Oh KK, et al. Bilateral Xanthogranuloma of the Breast. J Ultrasound Med 2007;26:535-7. [Crossref] [PubMed]


