Which clinical significance has automatic detection of very low levels of nucleated red blood cells in the peripheral blood?
The direct evaluation through light microscopy (LM) of May-Grünwald-Giemsa (MGG) stained blood smears is still considered the “gold standard” for laboratory identification and count of nucleated red blood cells (NRBC) in peripheral blood (PB). However, the LM procedure is affected with high imprecision and low sensitivity (1). The Sysmex XN-9000 (Sysmex Co., Kobe, Japan) automated hematological analyzer is a modern system able to carry out NRBC count with excellent performance in PB samples, even at very low concentrations (NRBC <2%) (2).
In October 2014, a 76-year-old man was admitted to our hospital emergency room for suspect heart failure. Blood serum tests showed a slight increase in troponin-I levels (0.15 ng/mL) but a remarkable increase in B-type natriuretic peptide (BNP) levels (2,310 ng/mL).
A complete blood count (CBC) performed on XN-9000 provided the following results: red blood cell (RBC): 4.5×1012/L; white blood cell (WBC): 11.55×109/L; hemoglobin (Hb): 132 g/L; mean corpuscular volume (MCV): 88/fL; RBC distribution width (RDW): 23%; platelets (PLT): 185×109/L. The automated differential leukocyte count (DIFF count), gave the following results: neutrophils (NE): 8.07×109/L; lymphocytes (LY): 1.60×109/L; monocytes (MO): 1.76×109/L; eosinophils (EO): 0.08×109/L; and basophils (BA): 0.04×109/L. Notably, the XN-9000 showed the presence of NRBC with an absolute value of 0.02×109/L and a percentage value of 0.2%. No morphological flag was presents.
Since the analyzer revealed both the presence of mild monocytosis and circulating NRBC, LM reviews in PB was made. The PB smear was prepared by SP-10 automated slide maker and stainer (Sysmex, Kobe, Japan) with MGG staining (Carlo Erba, Italy). The LM was performed by a laboratory specialist in hematology, according to CLSI standard H20-A2 (1) and other criteria described in the “International Council for Standardization in Hematology” document (3). The LM confirmed the results of DIFF count observing the presence of NRBC with an estimated percentage of 0.5% together with a RBC marked anisopoikilocytosis with dacryocytes.
Although all these LM findings were detailed in the CBC report, no further test was performed by clinicians, as in the meantime, due to the sudden appearance of acute ischemia, the patient underwent an emergency aortocoronary bypass surgery. During the hospitalization, the presence of NRBC was confirmed. Spleen was not palpable. After 30-days, the patient was discharged with a diagnosis of ischemic heart disease with heart failure.
After a long interval of 14 months from the first finding of NRBC in PB (during this period, no CBC was performed), the patient returned to our hospital emergency room with a new acute exacerbation of chronic heart failure. However, the CBC analysis showed: severe anemia (RBC: 3.7×1012/L; Hb: 85 g/L), leukocytosis (WBC: 12.83×109/L) and thrombocytosis (PLT: 1,032×109/L). The XN-9000 DIFF count gave the following results: NE: 9.74×109/L, LY: 1.40×109/L, MO: 1.46×109/L, EO: 0.12×109/L, BA: 0.11×109/L and NRBC: 0.06×109/L with the two morphological flags: “immature granulocytes (IG)” and “Blasts?” (Figure 1). The LM examination confirmed NRBC (0.5%), RBC abnormal morphology with marked anisopoikilocytosis and dacryocytes, together with the presence of blast cells (6%) and some dysplastic NE (Figure 1).
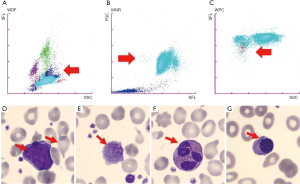
This laboratory picture and the presence of palpable spleen (3 cm below the costal margin) prompted clinicians to proceed with a bone marrow (BM) examination. BM evaluation showed: (I) hypocellularity with reduced representation of the erythroid and neutrophilic series; (II) giant megakaryocytes with marked nuclear atypia and PLT clusters; (III) fibrosis of grade MF-2. Genetic analysis showed a heterozygous JAK2V617F mutation whereas the BCR/ABL assay was negative. All the data described above confirmed a diagnosis of primary myelofibrosis (PMF), in accordance with current WHO standards (4).
Myelofibrosis is a BM disorder characterized by several hematological features, such as abnormal proliferation of monoclonal myeloid cells, BM fibrosis and osteosclerosis, extramedullary hematopoiesis, pancytopenia, hepatosplenomegaly, PB leukoerythroblastosis and constitutional symptoms. When myelofibrosis develops without another previous BM disease, it is called PMF (5,6).
Myelofibrosis usually develops slowly. In its very early stages, signs or symptoms may be absent. As the disease progresses, patients refer unspecific symptoms including persistent fatigue, fever and chills, night sweats, itching and unexplained weight loss. Splenomegaly is frequent. In advanced stages, the myelofibrosis-associated anemia can become severe, causing palpitations, tachycardia and dyspnea and requiring systematic RBC transfusions. Hemorrhagic and thrombotic disorders are also common in myelofibrosis, due to altered PLT production (5,6). The only curative treatment is allogeneic BM transplant, but, despite recent advances in pharmacological therapies, the prognosis of this disease remains dismal in most patients (7).
Notably, the XN-9000 automated analyzer can detect by default NRBC in PB sample through its white nucleated red (WNR) cell channel, whereas many other instruments do this as a reflex test only.
Furthermore, in this study were evaluated 240 healthy subjects (HS) (120 males and 120 females) randomly selected among healthy blood donors (first donation) of the same geographic area as healthy controls to compare the patient’s results. The NRBC median was 0.00×109/L (IQR 0.00–0.00) and reference intervals, detected according to CLSI document EP28-A3c (8), by robust biweight quantile test were: lower limit of 0.00×109/L [confidential interval (CI) 90%, 0.00–0.00], upper limit of 0.00×109/L (CI 90%, 0.00–0.00). The XN-9000 performs NRBC count as demonstrated by our case, revealing a very low count (0.02×109/L). Data confirmed by LM review. This is a very low concentration since, in order to confirm the same NRBC count by LM, the hematology specialist should perform a differential count over an extended WBC number at least equal to 1,000 cells (1).
The excellent performances shown by XN-9000 in NRBC count are well documented in literature, and are of valuable help in routine analysis of blood samples for the screening of many relevant hemopathies in early sub-clinical stage (2). As reported by Danise et al., peripheral NRBCs are a common finding in most myeloproliferative disorders that are characterized by altered or inefficient hematopoiesis (9,10). There may also be other non-myeloproliferative conditions that are commonly associated with the presence of NRBC in PB, e.g., thalassemia and BM involvement by metastatic solid tumors (9,10).
In our case report, the XN-9000 analysis and LM examination showed the presence of NRBC in PB, together with altered RBC morphology, and monocytosis more than one year before the diagnosis of PMF. All these features were suggestive signs of an erythropoietic disease at early stage that was simply overlooked by clinicians without further examination. However, the patient would likely not have undergone a specific treatment at that time, since there are no effective therapies for PMF at early stage (7). Clinicians may also have considered that following-up the patient at regular times in order to anticipate a sudden exacerbation of the disease could have been the best strategy to properly manage his condition.
In any case it is worth noting that XN-9000 offers the valuable opportunity to identify the presence of circulating NRBC in PB at very low concentrations (i.e., 0.02×109/L). This leads the Authors of the present data to conclude that further studies are required to better understand the role of NRBC a very low concentrations in PB as a premonitory sign of malignant hemopathies, as well as the potential benefits of their automated count, besides the warning indicator role, on the clinical outcome.
Acknowledgements
We wish to acknowledge the help provided by Dr. Finazzi and Dr. Vavassori.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- CLSI. Reference Leukocyte (WBC) Differential Count (Proportional) and Evaluation of Instrumental Methods; Approved Guideline-Second Edition. CLSI document H20-A2.Wayne, PA: Clinical and Laboratory Standards Institute; 2007. Available online: http:clsi.org
- Tantanate C, Klinbua C. Performance evaluation of the automated nucleated red blood cell enumeration on Sysmex XN analyser. Int J Lab Hematol 2015;37:341-5. [Crossref] [PubMed]
- Palmer L, Briggs C, McFadden S, et al. ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int J Lab Hematol 2015;37:287-303. [Crossref] [PubMed]
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937-51. [Crossref] [PubMed]
- Mesa RA, Green A, Barosi G, et al. MPN-associated myelofibrosis (MPN-MF). Leuk Res 2011;35:12-3. [Crossref] [PubMed]
- Mughal TI, Vaddi K, Sarlis NJ, et al. Myelofibrosis-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. Int J Gen Med 2014;7:89-101. [PubMed]
- Tefferi A. Myeloproliferative neoplasms: A decade of discoveries and treatment advances. Am J Hematol 2016;91:50-8. [Crossref] [PubMed]
- CLSI. Defining, establishing and verifying reference intervals in the clinical laboratory; Approved Guideline-Third edition. CLSI document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute, 2008. Available online: http:clsi.org
- Danise P, Maconi M, Barrella F, et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin Chem Lab Med 2011;50:357-60. [PubMed]
- Buoro S, Vavassori M, Pipitone S, et al. Evaluation of nucleated red blood cell count by Sysmex XE-2100 in patients with thalassaemia or sickle cell anaemia and in neonates. Blood Transfus 2015;13:588-94. [PubMed]


