
The beneficial effects of Fomitopsis pinicola chloroform extract on a dextran sulfate sodium-induced ulcerative colitis mice model
Highlight box
Key findings
• The chloroform extract of Fomitopsis pinicola (Swartz.: Fr) Karst (FPKc) can protect mice from dextran sulfate sodium (DSS)-induced ulcerative colitis (UC) injury.
What is known and what is new?
• FPKc exerts excellent anti-inflammatory effect.
• FPKc can inhibit the inflammatory response in UC.
What is the implication, and what should change now?
• FPKc may be a potential UC therapeutic drug and the specific components of FPKc needs to be further confirmed.
Introduction
Ulcerative colitis (UC) is an intestinal non-specific inflammatory lesion, which mainly affects the rectum and sigmoid colon, but can also affect the entire colon and terminal ileum (1,2). The clinical manifestations of UC include abdominal pain, persistent or recurrent diarrhea, mucus pus, blood in the stool, and various systemic symptoms (3). Currently, the pathogenesis of UC remains unclear, and as a result, there is a lack of targeted treatments for UC (4). The complications of UC include toxic megacolon, intestinal stricture, intestinal perforation, lower gastrointestinal bleeding, and colorectal cancer, which not only threaten the health of patients, but also place great mental pressure on patients (5,6). Due to the relapsing attacks, UC becomes a high-risk factor for cancer (7). Current treatments for UC mainly relieve the symptoms of the disease, that include medicines such as 5-aminosalicylic acid (5-ASA), sulfasalazine (SASP), and glucocorticoids (8-10). More research needs to be conducted to establish novel therapeutic strategies for UC.
Compared to Western medicines, which tend to cause a variety of toxic effects and other side effects, traditional Chinese herbal medicines have characteries of multiple targets for treatment of human diseases, which exert better curative effects and better safety. In recent years, traditional Chinese herbal medicines have received more and more attention in the treatment of multiple human diseases, including UC (11-13). For example, Huang Lian (Rhizoma coptidis) is discovered to alleviate inflammatory damage of colon tissue in UC rats via activating PPARγ gene and inactivating p38MAPK and NF-κB (14). Fu et al. (15) found that Ligularia fischeri root extracts played protective effects on UC in mice via activation of Bcl-2/Bax signalings. Fomitopsis pinicola (Swartz.: Fr) Karst (FPK) is a wood-decay fungus and a common medicinal fungus widely distributed in the temperate Northern Hemisphere (16). Previous studies have reported that FPK extracts have multiple biological effects, such as anti-cancer (17), anti-bacteria (18), anti-oxidation (19), anti-hypoglycemic (20) and anti-inflammation effects (21).
Inflammation is the defensive response to infection and injury (22). Triterpenoids isolated from Traditional Chinese medicines exert predominant anti-inflammatory effects by lowering pro-inflammatory factor levels. Previous studies have shown that FPK extracts contain triterpenoids (16,23). More importantly, Liu et al. (24) and Kuo et al. (21) discovered that the triterpenoids isolated from the fruiting bodies of FPK also have excellent anti-inflammatory effects. Until now, there is no any literature could be searched concerning the effects of FPK extracts on UC. It is not yet clear whether FPK extracts can alleviate the symptoms of UC.
In the current research, an in-vivo acute UC mice model was established by allowing the mice free access to a 4% dextran sulfate sodium (DSS) aqueous solution for 1 week. By setting the SASP treatment as a positive control, we firstly explored the possible beneficial effect of the chloroform (CHCl3) extract of FPK (FPKc) on UC. Our findings may provide an experimental basis to further explore whether FPKc can be used as a novel medicine to treat UC in the future. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5143/rc).
Methods
Preparation of FPKc
Fresh FPK was collected from Pingheliang, south of the Qinling Mountains, in the Shaanxi Province of China (altitude 2,193~2,353 km, 33°27'N, 108°30'E) and identified by Professor Yaping Xiao (Shaanxi Normal University, Shaanxi Province, China). After washing, drying and crushing, the sporocarp powder of FPK was dissolved into ethanol. The ethanol extraction of FPK was harvested by ultrasonic shock and condensed. To obtain FPKc, the ethanol extraction of FPK was fractionated by CHCl3. FPKc was dissolved in dimethyl sulfoxide solution to a storage concentration of 350 mg/mL and diluted using sunflower oil to a working concentration of 35 mg/mL.
Establishment of an in-vivo UC model and drug intervention
A total of 50 healthy Kunming mice (25 males and 25 females, weighing 25–30 g) were provided by the Experimental Animal Center of Air Force Military Medical University [license number: SCXK (Shaan) 2019-001]. After feeding in our facility for 1 week (a 12 h light/dark cycle, with free access to drink and food), all the mice were randomly divided into the normal control (CT) group, UC + Oil group, UC + Normal saline (NS) group, FPKc group, and SASP (H31020557, Shanghai Xinyi Tianping Pharmaceutical Co., Ltd., Shanghai, China) group (with 10 mice per group). With the exception of the mice in the CT group, the other mice were given free access to a drink with 4% DSS for 1 week to establish an acute UC model as previously reported (25). For mice in the UC + Oil group, UC + NS group, FPKc group, and SASP group, 0.2 mL of sunflower oil, 0.2 mL of physiological saline solution, 0.2 mL of FPKc (35 mg), and 0.2 mL of SASP (35 mg), respectively, were administered via gavage every day, for 3 weeks. The dosage selection of FPKc was based on pre-toxicity test (the LD50 of FPKc is much greater than 35 mg) and the maximum dosage of FPKc that can dissolve in oil for gavage). Animal experiments were performed under a project license (No. 2021-02) granted by ethics committee of Xi’an University, in compliance with national guidelines for the care and use of animals. A protocol was prepared before the study without registration.
DAI score
The body weights of the mice were measured at a fixed time every day. Their food intake, water intake, mental state, and hair gloss were also recorded. The stool occult blood (OB) kit (BA-2020B, Zhuhai Beisuo Biotechnology Co., Ltd. Guangdong Province, China) was used to detect the stool hematochezia condition of the mice. The disease activity index (DAI) score represents the average weight loss (%), fecal trait score, and fecal hematochezia score. In relation to the fecal trait score, a score of 0 indicates a normal fecal trait, a score of 2 indicates a loose fecal trait, and a score of 4 indicates a watery fecal trait (diarrhea).
CMDI score
After the experiment, all of the mice were anesthetized with isoflurane and sacrificed by cervical dislocation. Next, the colon tissues were removed, and the length of the colon was measured. To observe the colon injury of the mice, the fatty tissue and mesenteric impurities on the outer wall of the colon were eliminated. The colon was then cut along the longitudinal axis, and the intestinal wall of the colon was rinsed with physiological saline solution. Under the colonic mucosa damage index (CMDI), a score of 0 indicates no damage to the colon mucosa, a score of 1 indicates mild congestion and edema of the colon, but no erosion or ulceration, a score of 2 indicates congestion and edema of the colon, and localized epithelial defects or adhesions in the intestinal wall, a score of 3 indicates severe congestion and edema of the colon, necrosis and ulcers on the surface of the mucosa, a maximum longitudinal diameter of the ulcer <1 cm, a thickening of the intestinal wall or necrosis and inflammation on the surface, and a score of 4 indicates on the basis of 3 score, a maximum longitudinal diameter of the ulcer >1 cm or total intestinal wall necrosis.
H&E staining
The colon tissues of the mice were fixed in 10% formalin, embedded with paraffin and cut into 6-µm thick sections. Following de-paraffinizing in xylene solution and hydrating in decreasing gradient ethanol solution, the sections were stained by hematoxylin and eosin (H&E) solutions, respectively. Subsequently, after undergoing the process of dehydration in increasing gradient ethanol solution and transparency in the xylene solution, the sections were blocked with neutral resin. The results were observed and captured by microscope.
Measurement of the thymus and spleen indexes
After anaesthetization and sacrifice, the thymus and spleen tissues were excised from the mice and weighed. The following formula was used to calculate the organ index (%): organ index (%) = wet organ weight (g)/mouse body weight (g) ×100%. The thymus index and spleen index were both calculated.
Test of inflammatory factor levels in the serum
After anaesthetization and heart blood sampling, the interleukin (IL)-6, IL-8 and tumor necrosis factor-α (TNF-α) levels in the serum were tested using a mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kit (CK-E20012M), a mouse IL-8 ELISA kit (CK-E20533M), and a mouse TNF-α ELISA kit (CK-E20220M, Suzhou Calvin Biotechnology Co., Ltd., Jiangsu Province, China), respectively.
Detection of liver function
After anaesthetization and heart blood sampling, the aminotransferase (AST) and alanine aminotransferase (ALT) levels in the serum were detected using an AST assay kit (C010-2-1) and an ALT assay kit (C009-2-1, Nanjing Jiancheng Bioengineering Institute, Jiangsu Province, China), respectively.
Statistical analysis
SPSS 25.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The data are presented as the mean ± standard deviation (SD). Significant differences between the groups were examined using the Duncan method. a–c means P<0.05.
Results
FPKc improved weight loss, hematochezia, and DAI score
First, as Figure 1A shows, the body weight of the mice in the CT group gradually increased during the experiment. During the process of acute UC model establishment (1–8 days), the body weight of the mice in UC + Oil and UC + NS groups obviously decreased, indicating that the 4% DSS treatment caused a decline in the body weight of the mice. Additionally, relative to the UC + Oil group, the weight loss (%) of the mice in the FPKc group improved. Relative to the UC + NS group, the weight loss (%) of the mice in the SASP group also increased. These results indicated that the FPKc and SASP treatment reversed the weight loss of mice caused by the 4% DSS treatment.
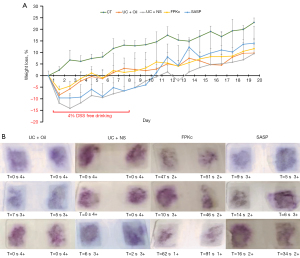
Further, as Figure 1B shows, the mice in the UC + Oil and UC + NS groups had severe hematochezia, but FPKc or SASP treatment alleviated the hematochezia of the mice. The effect of FPKc appeared to be better than that of SASP on hematochezia. Notably, the feces of the mice in the FPKc group took a longer time to turn purple and had a lower fecal hematochezia score.
Additionally, the DAI scores of the mice in the UC + Oil and UC + NS groups were both significantly increased (see Table 1). The FPKc and SASP treatment significantly improved the DAI scores of the mice (which mainly manifested as an improvement in weight loss, P<0.05). These outcomes showed that the mice UC model was established successfully, and both FPKc and SASP improved the body weight loss and hematochezia of the mice with UC.
Table 1
| Groups | Weight loss (%) | Stool traits | Stool hematochezia | DAI |
|---|---|---|---|---|
| CT | 0 | 0 | 0 | 0 |
| UC + Oil | 10.84±1.80bc | 2.38±0.62 | 1.86±0.81 | 5.02 |
| UC + NS | 15.84±2.60a | 2.14±0.47 | 1.76±0.66 | 6.58 |
| FPKc | 9.84±2.49c | 2.49±0.79 | 1.78±0.76 | 4.70 |
| SASP | 12.15±4.77b | 2.05±0.71 | 1.90±0.71 | 5.37 |
a–c means P<0.05. DAI, disease activity index; SD, standard deviation; CT, computed tomography; UC, ulcerative colitis; NS, normal saline; FPKc, Chloroform (CHCl3) extract of Fomitopsis pinicola (Swartz.: Fr) Karst; SASP, sulfasalazine.
FPKc improved the CMDI scores
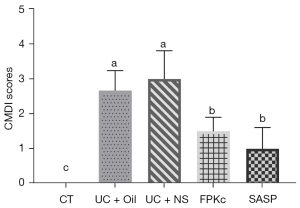
During the experiment, 1 mouse died in the UC + Oil group. The morphology of the colon tissues revealed that the colons of the mice in the UC + Oil and UC + NS groups showed severe congestion and edema, which was accompanied by a large area of ulcers on the mucosal surface. Relative to the UC + Oil and UC + NS groups, the degrees of colon congestion, edema, and ulcers on the mucosal surface of the mice in the FPKc and SASP groups were all reduced. The CMDI score results illustrated that the mice in the UC + Oil and UC + NS groups had high CMDI scores compared to the mice in the CT group (see Figure 2; P<0.05). However, compared to the mice in the UC + Oil and UC + NS group, the CMDI scores of the mice in the FPKc or SASP groups were significantly reduced (P<0.05). These outcomes showed that both FPKc and SASP improved the CMDI scores of the mice with UC.
FPKc improved the microstructural and length changes of colon tissues
To observe the microstructural changes in the colon tissues of the mice in each group, H&E staining was performed. As Figure 3A shows, the colonic crypt structure appeared normal in the CT group. Conversely, the colonic crypt structures of the mice in the UC + Oil and UC + NS groups were completely destroyed, and had leaky sieve shapes and became the focus of the ulcers. Both FPKc or SASP treatment led to the partial recovery of the colonic crypt structures of the mice and reduced the inflammatory response. Additionally, as Figure 3B shows, relative to the CT group, the colonic lengths of the mice in UC + Oil and UC + NS groups were significantly decreased (P<0.05). The FPKc and SASP treatments significantly improved the decreases in colonic length (P<0.05). The colonic length of the mice in the FPKc group approached that of the mice in the CT group (but the results were not significate). These outcomes show that both FPKc and SASP improved the microstructural and length changes in the colon tissues of the mice with UC.

FPKc reduced the thymus and spleen indexes
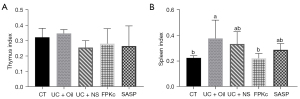
The thymus and spleen indexes of the mice in each group were measured to evaluate the effects of the FPKc and SASP treatments on the immune organs of the mice. As Figure 4A shows, there was no significant difference in the thymus indexes of the mice in each group. As Figure 4B shows, relative to the CT group, the spleen indexes of the mice in the UC + Oil and UC + NS groups were significantly increased (P<0.05). The FPKc treatment significantly alleviated the increase of spleen of the in mice (P<0.05), and its value was similar to that of the CT group. There was no significant difference in the spleen indexes between the UC + NS group and the SASP group. These outcomes show that FPKc reduced the spleen index and thus improved the damage to the immune organs of the mice with UC.
FPKc decreased the inflammatory factor levels in the serum
The IL-6, IL-8, and TNF-α levels in the serum were also examined to explore whether FPKc improved UC by activating immune regulation. As Figure 5A-5C show, compared to the CT group, the IL-6, IL-1β, and TNF-α levels in the serum of the mice in the UC + Oil and UC + NS groups were significantly increased (P<0.05). The FPKc treatment significantly reduced the IL-6 and IL-1β levels in the serum (P<0.05) but had no significant effect on the TNF-α levels in the serum. The SASP treatment also decreased the IL-6 and IL-1β levels in the serum (P<0.05) but had no significant effect on the TNF-α levels in the serum. The effects of FPKc on the IL-6 and IL-1β levels were better than the effects of SASP. These outcomes show that FPKc and SASP relieved the symptoms of UC might be via reducing the inflammatory factors (i.e., the IL-6 and IL-1β levels) in the serum.

FPKc decreased the AST and ALT levels in the serum
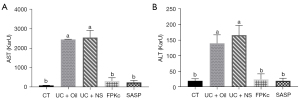
Finally, the AST and ALT levels in the serum were also examined. As Figure 6A shows, relative to the CT group, the AST levels in the UC + Oil and UC + NS groups were significantly increased (P<0.05). The FPKc or SASP treatments significantly reduced the AST levels in the serum (P<0.05). Similar results were found concerning the ALT levels in the serum. As Figure 6B shows, compared to the CT group, the ALT levels in the serum of the mice in the UC + Oil and UC + NS groups were also significantly increased (P<0.05). However, relative to the UC + Oil and UC + NS groups, the ALT levels of the mice were significantly reduced in the FPKc and SASP groups (P<0.05). These outcomes show that FPKc and SASP relieved the symptoms of UC also might be via protecting the liver function of mice.
Discussion
As a non-specific inflammatory and relapsing disorder of the intestine, UC is very difficult to cure (5). Currently, the molecular etiology of UC is still not clear; however, it might involve genetic, microorganic, environmental, and other unknown factors (26). In recent years, great efforts have been made to establish a UC animal model and to search for effective and safe therapeutic medicines for UC (12,27). DSS is a polyanionic derivative of dextran, formed by the esterification reaction of dextran and chlorosulfonic acid (28). A large number of studies have shown that the animal model of UC established by DSS chemical induction is similar to human UC and has similar histological characteristics, clinical manifestations, onset location, and pro-inflammatory cytokine expressions (27,29). The DSS-induced UC animal model has been widely used to investigate the pathogenesis of UC and search for therapeutic drugs for UC (30,31).
In the present study, healthy Kunming mice were given free access to a drink containing 4% DSS for 1 week to establish the UC model. The DAI score and the CMDI score are the 2 main indexes to reflect the severity of UC (32). We found that the UC model was established successfully in the mice as evidenced by decreases in body weight, hematochezia, reductions in CMDI scores, and changes to the microstructural and length of colon tissues. These findings suggest that the mice UC model established in our research by providing 4% DSS drinking water.
Recent years, lots of studies confirmed the efficacy and safety of Traditional Chinese Medicine in UC treatment, which including some clinical evidence (33,34). FPK is a traditional Chinese herbal medicine (20). As one of the most popular medical fungi, FPK has been shown to have a number of effects, including expelling wind, dehumidifying, and anti-bacterial effects, and to regulate the central nervous system and lower blood sugar. It has often been used to treat wind-cold dampness, joint pain, and hyperglycemia (16). In recent years, many molecular biology studies have shown that the FPK extract has many other biological effects (17,19,21). Among them, the anti-inflammatory effect of the FPK extract has attracted widespread attention (21,24).
Given that the inflammatory response is responsible for UC development (1), we hypothesized that FPK would exert beneficial effects on UC. In our experiment, we showed that FPKc significantly improved the decreases in body weight, hematochezia, reductions in CMDI score, and changes in the microstructural and length of the colon tissues in the 4% DSS-induced UC mice. Additionally, in terms of the weight loss, hematochezia, CMDI score, and colon length of the mice with UC, FPKc appeared to have better effects than SASP, which is a common medicine for UC therapy (9). These findings suggest that FPKc had a beneficial effect on the 4% DSS-induced UC mice, and might be a valuable novel drug for UC therapy; however, further in vitro and in vivo experiments need to be conducted in the future.
Previous research showed that the dysregulation of the immune response contributed to the onset and development of UC (35). The excessive response of T cells to antigenic stimulus causes intestinal tissue injury (36). Additionally, the defects of mucosal immunity have been shown to promote UC progression (37). The thymus and spleen are 2 important immune organs responsible for the regulation of body’s immune response. Zhao et al. (38) found that the spleen weight was significantly increased in a DSS-induced UC rat model. In our experiments, we discovered that compared to the UC + Oil group, the spleen index was decreased in the FPKc group, which implied that FPKc exerted a beneficial effect in the 4% DSS-induced UC mice might be via modulating the immune functions of organs.
As an inflammatory disease, UC development is accompanied by the release of pro-inflammatory factors (4). IL-6, IL-1β, and TNF-α are key pro-inflammatory cytokines in UC (39). Among them, TNF-α is the earliest and most important pro-inflammatory factor in the inflammatory response (40). It can activate neutrophils and lymphocytes, increase the permeability of vascular endothelial cells, regulate the metabolic activity of other tissues, and promote the synthesis and release of other cytokines (40). IL-6 can induce B cells to differentiate and produce antibodies, which can serve as a trigger for the inflammatory response (41). IL-1β can stimulate the chemotaxis of neutrophils, T lymphocytes, and eosinophils, cause endothelial cell damage, result in the blood flow stasis of microcirculation, tissue necrosis, and organ function impairment (42).
We discovered that the IL-6, IL-1β, and TNF-α levels in the serum were all increased in the 4% DSS-induced UC mice, which was inconsistent with previous reports (43). FPKc treatment lowered the IL-6 and IL-1β levels in the serum. Additionally, the effect of FPKc on the pro-inflammatory cytokines in the serum seemed to be better than SASP. These findings suggested that FPKc had great inhibitory effects on the serum pro-inflammatory cytokines in the 4% DSS-induced UC mice.
The liver is the main site of drug metabolism. AST and ALT are 2 indicators used to evaluate liver function, and are also important indicators used to assess drug safety (44). AST and ALT are considered the main markers for evaluating liver fibrosis and cirrhosis (45). UC is usually accompanied by parenteral lesions, affects multiple organs functions, and may result in liver damage (46). Research has shown that the liver damage caused by UC can also aggravate UC progression by affecting the translocation of intestinal flora and impairing intestinal barrier (47).
In the current research, the AST and ALT levels in the serum were increased in the UC model groups. The FPKc treatment decreased the AST and ALT levels in the serum, which suggests that as a relatively safe drug, FPKc might exert a beneficial effect on UC and also alleviate liver damage. Due to the unstable data caused by experimental errors, an insufficiently small sample size, or large individual differences in the mice, the differences in the data between the groups were not significant. Thus, further studies need to be conducted in the future to confirm the effects of FPKc on UC-induced liver injury.
The intestinal tract is rich in lymphoid tissue. Intestinal mucosal immune system, which is composed of intraepithelial lymphocytes, lamina propria lymphocytes, Peyer’s patch and other gut related lymphoid tissues, is an important part of the body’s immune system and plays key role in preventing and resisting the invasion of bacteria, viruses and toxin (48,49). If the intestinal mucosal barrier is damaged, pathogenic microorganisms in the intestine will invade to human body, causing inflammatory response, including UC (50). Considering that the key role of intestinal mucosal immunity in UC, we propose that FPKc might exert protective role in intestinal mucosal immunity injury of UC. In the future, we will study the possible beneficial role of FPKc in intestinal mucosal immunity injury of UC. We believe that with the intensive study, we will clarify the internal molecular mechanism of FPKc in relieving UC symptoms.
Conclusions
This research showed the beneficial and safe effects of FPKc in a DSS-induced UC mice model. FPKc significantly improved hematochezia and weight loss, and restored the colon length and crypt structure. The effects of FPKc on UC might be related to the activation of immune regulation and the downregulation of pro-inflammatory cytokines. FPKc might be a potential and valuable UC therapeutic drug, in spite of further pre-clinical experiments still need to be conducted.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (Nos. NSFC81972883, NSFC32270530, NSFC82202067 and NSFC32100389), the Natural Science Basic Research Project of Shaanxi Province (No. 2017JQ8040), the Special Research Projects of the Xi’an Science and Technology Bureau (No. 2021XDJH38), the Shaanxi Province University Student Innovation and Entrepreneurship Training Program (No. S202011080054), and the Special Foundation of President of The College of Biological and Environmental Engineering (No. YZJJ202108).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5143/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5143/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5143/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. 2021-02) granted by ethics committee of Xi’an University, in compliance with national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3:390-407. [Crossref] [PubMed]
- Li H, Mo Y, Huang C, et al. An MSCT-based radiomics nomogram combined with clinical factors can identify Crohn's disease and ulcerative colitis. Ann Transl Med 2021;9:572. [Crossref] [PubMed]
- Wang J, Zhang Q, Deng Y, et al. Efficacy and safety of heat-sensitive moxibustion in the treatment of ulcerative colitis: A protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e24078. [Crossref] [PubMed]
- Fries W, Comunale S. Ulcerative colitis: pathogenesis. Curr Drug Targets 2011;12:1373-82. [Crossref] [PubMed]
- Panaccione R, Sutherland LR. Clinical course and complications of ulcerative colitis and ulcerative proctitis. In: Targan SR, Shanahan F, Karp LC, editors. Inflammatory Bowel Disease: From Bench to Bedside. Boston, MA: Springer, 2003.
- Strong SA. Expert Commentary on Prevention, Diagnosis, and Treatment of Complications of the IPAA for Ulcerative Colitis. Dis Colon Rectum 2018;61:536-7. [Crossref] [PubMed]
- de Campos Silva EF, Baima JP, de Barros JR, et al. Risk factors for ulcerative colitis-associated colorectal cancer: A retrospective cohort study. Medicine (Baltimore) 2020;99:e21686. [Crossref] [PubMed]
- Luo J, Wang Y, Lan D, et al. Differential expression of serum microRNAs in glucocorticoid-resistant patients with ulcerative colitis. Int J Clin Exp Pathol 2018;11:936-46. [PubMed]
- Shin MR, Park HJ, Seo BI, et al. New approach of medicinal herbs and sulfasalazine mixture on ulcerative colitis induced by dextran sodium sulfate. World J Gastroenterol 2020;26:5272-86. [Crossref] [PubMed]
- Miyoshi J, Matsuoka K, Yoshida A, et al. 5-Aminosalicylic acid aggravates colitis mimicking exacerbation of ulcerative colitis. Intest Res 2018;16:635-40. [Crossref] [PubMed]
- Guo D, Murdoch CE, Liu T, et al. Therapeutic Angiogenesis of Chinese Herbal Medicines in Ischemic Heart Disease: A Review. Front Pharmacol 2018;9:428. [Crossref] [PubMed]
- Sałaga M, Zatorski H, Sobczak M, et al. Chinese herbal medicines in the treatment of IBD and colorectal cancer: a review. Curr Treat Options Oncol 2014;15:405-20. [Crossref] [PubMed]
- Zhang C, Jiang M, Lu A. Considerations of traditional Chinese medicine as adjunct therapy in the management of ulcerative colitis. Clin Rev Allergy Immunol 2013;44:274-83. [Crossref] [PubMed]
- Jiang X, Liu C, Zhu Y. Effects of the total alkaloids of coptis chinensis on intestinal mucosal injury and p38-PPARγ/NF-κB pathway in rats with ulcerative colitis. Chin Pharmacists 2019;21:2188-93.
- Fu YP, Yuan H, Xu Y, et al. Protective effects of Ligularia fischeri root extracts against ulcerative colitis in mice through activation of Bcl-2/Bax signalings. Phytomedicine 2022;99:154006. [Crossref] [PubMed]
- Wang Y, Cheng X, Wang P, et al. Investigating migration inhibition and apoptotic effects of Fomitopsis pinicola chloroform extract on human colorectal cancer SW-480 cells. PLoS One 2014;9:e101303. [Crossref] [PubMed]
- Bishop KS. Characterisation of Extracts and Anti-Cancer Activities of Fomitopsis pinicola. Nutrients 2020;12:609. [Crossref] [PubMed]
- Liu XT, Winkler AL, Schwan WR, et al. Antibacterial compounds from mushrooms II: lanostane triterpenoids and an ergostane steroid with activity against Bacillus cereus isolated from Fomitopsis pinicola. Planta Med 2010;76:464-6. [Crossref] [PubMed]
- Reis FS, Pereira E, Barros L, et al. Biomolecule profiles in inedible wild mushrooms with antioxidant value. Molecules 2011;16:4328-38. [Crossref] [PubMed]
- Lee SI, Kim JS, Oh SH, et al. Antihyperglycemic effect of Fomitopsis pinicola extracts in streptozotocin-induced diabetic rats. J Med Food 2008;11:518-24. [Crossref] [PubMed]
- Kuo PC, Tai SH, Hung CC, et al. Antiinflammatory triterpenoids from the fruiting bodies of Fomitopsis pinicola. Bioorg Chem 2021;108:104562. [Crossref] [PubMed]
- Krejsek J. Defensive and damaging inflammation: basic characteristics. Vnitr Lek 2019;65:76-80. [Crossref] [PubMed]
- Rösecke J, König WA. Constituents of various wood-rotting basidiomycetes. Phytochemistry 2000;54:603-10. [Crossref] [PubMed]
- Liu Y, Liu W, Li M, et al. Lanostane triterpenoids from the fruiting bodies of Fomitopsis pinicola and their anti-inflammatory activities. Phytochemistry 2022;193:112985. [Crossref] [PubMed]
- Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993;69:238-49. [PubMed]
- Du L, Ha C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol Clin North Am 2020;49:643-54. [Crossref] [PubMed]
- Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther 2013;7:1341-57. [PubMed]
- Tazi LM, Jayawickreme S. Determination of residual dextran sulfate in protein products by SEC-HPLC. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1011:89-93. [Crossref] [PubMed]
- Yan YX, Shao MJ, Qi Q, et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol Sin 2018;39:1633-44. [Crossref] [PubMed]
- Yeganeh PR, Leahy J, Spahis S, et al. Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis. J Nutr Biochem 2018;57:56-66. [Crossref] [PubMed]
- Zhu Y, Zhuang Z, Wu Q, et al. CD39/CD73/A2a Adenosine Metabolic Pathway: Targets for Moxibustion in Treating DSS-Induced Ulcerative Colitis. Am J Chin Med 2021;49:661-76. [Crossref] [PubMed]
- Zhang H, Song G, Song H, et al. P20 The novel compound HLY effectively relieves ulcerative colitis by xbp1-myh9 pathway. Biochem Pharmacol 2017;139:131-2. [Crossref]
- Chen M, Ding Y, Tong Z. Efficacy and Safety of Sophora flavescens (Kushen) Based Traditional Chinese Medicine in the Treatment of Ulcerative Colitis: Clinical Evidence and Potential Mechanisms. Front Pharmacol 2020;11:603476. [Crossref] [PubMed]
- Yan ZX, Liu YM, Ma T, et al. Efficacy and safety of retention enema with traditional Chinese medicine for ulcerative colitis: A meta-analysis of randomized controlled trials. Complement Ther Clin Pract 2021;42:101278. [Crossref] [PubMed]
- Adamczyk A, Gageik D, Frede A, et al. Differential expression of GPR15 on T cells during ulcerative colitis. JCI Insight 2017;2:e90585. [Crossref] [PubMed]
- Seo GS, Choi SC. Pathophysiology of ulcerative colitis - Relationship with genetics and immunity. Korean J Med 2009;76:643-8.
- Targan SR, Karp LC. Defects in mucosal immunity leading to ulcerative colitis. Immunol Rev 2005;206:296-305. [Crossref] [PubMed]
- Zhao P, Dong L, Luo JY, et al. Establishment of dextran sulfate sodium-induced ulcerative colitis model in rats. Available online: 38. http://en.cnki.com.cn/Article_en/CJFDTOTAL-DSJY200519006.htm
- Doszhan A, Bektayeva R, Galiyeva A, et al. Determination of cytokine profile and fecal calprotectin in patients with inflammatory bowel disease of different activity degree. Journal of Clinical Medicine of Kazakhstan 2019;2:23-9. [Crossref]
- Murdaca G, Spanò F, Contatore M, et al. Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf 2015;14:571-82. [Crossref] [PubMed]
- Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis 2007;13:1016-23. [Crossref] [PubMed]
- Mitsuyama K, Toyonaga A, Sasaki E, et al. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exp Immunol 1994;96:432-6. [Crossref] [PubMed]
- Fan H, Shen L, Tang Q, et al. Effect of Wumeiwan on cytokines TNF-alpha, IL-6, IL-8, IL-10 and expression of NF-kappaBp65 in rats with ulcerative colitis. J Huazhong Univ Sci Technolog Med Sci 2009;29:650-4. [Crossref] [PubMed]
- Lee AR, Yim JM, Kim WI. Influence of prescribed herbal and Western medicine on patients with abnormal liver function tests: a retrospective quasi-experimental study. J Pharmacopuncture 2012;15:34-9. [Crossref] [PubMed]
- Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci 1999;44:1249-53. [Crossref] [PubMed]
- Mancina RM, De Bonis D, Pagnotta R, et al. Ulcerative Colitis as an Independent Risk Factor for Hepatic Steatosis. Gastroenterol Nurs 2020;43:292-7. [Crossref] [PubMed]
- Cengiz M, Altuner Y, Sahintürk V, et al. Lycopene Protects Liver Against Ulcerative Colitis. Current Drug Therapy 2012;7:24-9. [Crossref]
- Pizarro TT, Dinarello CA, Cominelli F. Editorial: Cytokines and Intestinal Mucosal Immunity. Front Immunol 2021;12:698693. [Crossref] [PubMed]
- Maldonado-Contreras AL, McCormick BA. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res 2011;343:5-12. [Crossref] [PubMed]
- Zou J, Liu C, Jiang S, et al. Cross Talk between Gut Microbiota and Intestinal Mucosal Immunity in the Development of Ulcerative Colitis. Infect Immun 2021;89:e0001421. [Crossref] [PubMed]


