
Narrative review—focal therapy: are we ready to change the prostate cancer treatment paradigm?
Introduction
In the United States, prostate cancer is the most diagnosed malignancy and the second leading cause of death in men (1). PCa is unique because it remains one of the few malignancies routinely screened by a serum laboratory test [prostate-specific antigen (PSA)]. Subsequently, the guidelines for detection and screening have been a popular target for criticism and review. The leading argument poses that the natural course of PCa is indolent, and the risks of overdiagnosis (and treatment) lead to an uncertain benefit. The current gold standard for PCa is divided between surgical (radical prostatectomy) and radiation therapy for patients who decide to pursue treatment. While different in onset and timing, both therapies are subject to procedural risks and significant adverse effects of urinary incontinence and sexual side effects (e.g., erectile dysfunction) (2).
For clinicians and patients alike, the shared-decision and counseling process in PCa management options is often clear for high-risk disease; however, a gray area exists for patients with very low/low to intermediate-risk prostate cancer. While these patients have a diagnosis of malignancy, the previously mentioned adverse side-effects of treatment are balanced with close monitoring and signs of progression; also known as active surveillance (AS). Traditionally, AS offers the most favorable risk-benefit profile for low-risk prostate cancer (3).
Robotic surgery and organ-sparing radiation treatment have improved quality of life, urinary symptoms, and sexual/erectile dysfunction (4,5). Cancer diagnosis often provokes patient anxiety and the desire for treatment, despite consultation. Few options for patients that would classically be placed on AS avoid the risk of treatment-related morbidity for patients that may never progress to clinically significant or metastatic disease. An attractive, emerging option for these patients may be accomplished with the field of focal therapy.
Focal treatment of PCa utilizes advents in imaging to employ minimally invasive therapy options that specifically target the region of interest while avoiding surrounding tissue to prevent the adverse effects of previously mentioned radical techniques. Several treatment options are currently being investigated for their PCa application and have demonstrated promising short-term outcome data regarding oncologic and quality of life outcomes. Much of this shift is primarily attributed to the success, and mainstream application of multi-parametric magnetic resonance imaging (mpMRI) guided screening/detection and subsequent ultrasound-fusion biopsy of prostate lesions (6,7). Previous application of various ablation techniques (i.e., whole gland cryoablation) have been used; however, an unfavorable side-effect profile mirrors similar outcomes to more radical therapy (2,4,5). While a paucity of long-term outcome studies exist, a boom for focal therapy is gaining popularity for the primary treatment of PCa.
Compared to standard-of-care treatment options such as radical surgery and radiation therapy, FT reigns superior for reducing complications with a clear benefit in reducing morbidity. While FT’s safety profile and short-term cancer control is promising, the biggest hurdle for mainstream adaption lies in evidence for long-term oncologic control. Previous groups have shown Higher International Society of Urologic Pathology (ISUP) grade group, the number of positive cores at biopsy, and bilateral disease as the main risk factors for disease recurrence after focal therapy (8).
FT success largely revolves around appropriate patient selection and is paramount for optimal success. The ideal case for focal therapy should be an MRI visible significant lesion (PIRADS score ≥3), with a positive targeted biopsy for significant cancer (Gleason grade group 2–3), and insignificant or absent disease in the nontarget random biopsy areas (9). This could theoretically be applied to multiple areas of interest within the same gland if amenable.
By isolating lesions of interest, various mechanisms have been theorized and designed to treat localized prostate cancer. While many of these novel therapies have shown promising pathologic and quality-of-life outcomes, most still lack the clinical evidence to change management guidelines. This review aims to identify existing published data for the most exciting and emerging PCa focal therapy modalities. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2337/rc).
Methods
This study was a narrative review to identify and search the current literature for relevant investigations related to focal therapy (FT) modalities as they apply to the treatment of prostate cancer. A MEDLINE/PubMed literature search was performed using combinations of the terms “Prostate cancer” AND “focal therapy” AND “Irreversible Electroporation” OR “cryotherapy, cryoablation” OR “nano, nanoparticle” OR “photodynamic therapy” OR “laser interstitial thermal therapy, focal laser” OR “phototherapy” OR “high intensity focused ultrasound, HIFU” OR “focal ablation.” Multiple groups have reported their FT for PCa outcomes and several systematic reviews were identified. Given the broad nature of our topic, we included articles that were the most up-to-date and relevant articles discussing the specific application of focal therapy to treating targeted lesions. We acknowledged both the novelty and host of reported articles surrounding FT, and therefore we focused to acknowledge studies that addressed the descriptive ablation mechanism in combination with reported outcomes and safety profile. Systematic review and meta-analysis articles were included if the authors demonstrated a succinct methodology and detailed structure for comparison regarding FT as it relates to the future of PCa. Studies that included primarily whole- or hemi-gland ablation, older cohorts and/or had repetitive outcome findings were excluded per internal review.
The primary objective of our review was to identify the most promising FT modalities and evaluate clinical outcomes (if applicable), mechanism, safety, and future directions for each PCa FT option. Table 1 summarizes the search strategy used for our review. The heterogeneity of reported study populations limited statistical analysis and was summarized in a narrative review of pertinent findings.
Table 1
| Items | Specification |
|---|---|
| Date of literature search | 7/1/2021–08/26/2022 |
| Databases and other sources searched | MEDLINE/PubMed |
| Search terms used | “Prostate cancer” AND "focal therapy” AND “Irreversible Electroporation” OR “cryotherapy, cryoablation” OR “nano, nanoparticle” OR “photodynamic therapy” OR “laser interstitial thermal therapy, focal laser” OR “phototherapy’ OR “high intensity focused ultrasound, HIFU” OR “focal ablation” |
| Timeframe | August 2007–June 2022 |
| Inclusion and exclusion criteria | Inclusion: Primary investigational, clinical trial, meta-analysis, systematic review |
| Exclusion: articles not published in English languages | |
| Selection process | Articles were selected and reviewed by Urology attendings, fellows, and senior residents. One attending is a board member of the Focal Therapy Society |
| Any additional considerations, if applicable | Additional relevant references were included during the primary literature search |
Discussion
Cryoablation
Cryoablation was one of the first focal therapy modalities to be applied with clinical relevance. After cryotherapy, theoretical tumor eradication occurs secondary to freeze-thaw cycles that lead to protein denaturation and vascular damage, culminating in localized tissue destruction. Initially used as a dermatologic agent in the form of liquid nitrogen, cryotherapy has since been adapted with several indications for genitourinary malignancies (10). In 1995, Uchida et al. demonstrated probe-based percutaneous cryoablation of the kidney for renal cell carcinoma (11). Since then, cryoablation has become a minimally invasive alternative to standard extirpative strategies in managing both kidney and prostate cancer. The primary delivery mechanism predominately uses high-pressure argon gas and helium through a series of conduction and convention transferred to the cryoprobe, resulting in overall tissue cooling and thawing.
As one of the first focal therapy options, various studies have published exceptional outcomes for cryoablation’s application to treat localized prostate cancer. In addition, few studies have looked at treating high risk disease; therefore, cryotherapy is primarily used today for low-intermediate risk/volume disease. Applications currently range from focal (targeted), hemi-/whole-gland, and salvage therapy. In a retrospective review by Cheetham et al., long-term outcomes (defined as ≥10 years) were studied in relation to overall mortality and cancer-specific deaths; secondary end-points were disease recurrence and overall progression (12). Most of these patients underwent salvage treatment after failed primary radiation therapy (n=51, 67.1%) compared to primary treatment (n=25, 32.9%). All patients underwent whole-gland therapy. Of note, 40 patients (52.6%) were deemed D’Amico high-risk (risk group classification to estimate the risk of biochemical recurrence following treatment for localized prostate cancer. The median follow-up was 10.1 years with 87% overall prostate-cancer-specific survival. While this study represents whole-gland rather than focal therapy, their results exhibit cryoablation mechanistic success with long-term data in a cohort that includes high-risk and salvage patients.
Barqawi et al. published their focal, targeted sectoral cryoablation results in 62 men with low-risk prostate cancer (13). On a 24-month follow-up, 71% of patients had biochemical progression-free survival (BPFS), with a positive biopsy in 19.4% of patients. There were no significant changes in the scores of International Index of Erectile Function (IIEF) from baseline characteristics. Our review found no comparative studies for primary cryoablation with radical prostatectomy or radiation therapy; however, some focal/partial- versus whole-gland cryotherapy comparisons have been conducted. Accessing the Cryo-Online Data (COLD) registry, Mendez et al. looked at patients with low-risk prostate cancer after focal (FT)- and whole-gland (WT) cryotherapy using a matched-pair analysis (14). A total of 634 men were included (317 FT: 317 WT). On a 60-month evaluation, there was no difference in BPFS. Additionally, they found that patients who underwent partial ablation had significantly better rates of erectile recovery compared to those who underwent whole-gland cryotherapy (68.8% vs. 46.8%, respectively). Both treatment groups had similar urinary continence, retention, and fistula rates.
High-intensity focused ultrasound (HIFU)
The idea of HIFU has been around in the clinical landscape for quite some time, with various therapeutic options spanning benign to malignant disease (e.g., fibroid ablation, vascular blood clot disruption). Looking specifically at HIFU’s role in relation to prostate cancer, early applications were initially proposed for whole-gland treatment but have recently resurfaced as a focal treatment therapy. HIFU involves pulsed ultrasound beams focused on target tissue to produce thermal and mechanical energy. Due to their inherent properties, when sound waves propagate through a medium (i.e., cellular tissue), the sound wave’s amplitude will decay over distance due to absorption and scatter, a phenomenon known as attenuation. As a result of this absorption, some energy is released as heat at a greater rate than heat dissipation (15). In addition to the generated thermal energy, mechanical energy is created through oscillation and cavitation forces (16). In the context of urologic surgery, applications of mechanical ultrasonic energy are already commonly employed during percutaneous nephrolithotripsy and shockwave lithotripsy. Several intracavitary devices have been developed for the transrectal and transurethral treatment of prostate lesions.
Ultrasound is classically acknowledged for its safe and inexpensive diagnostic capabilities; therefore, it makes sense to capitalize on this growing field of innovation in both the therapeutic and diagnostic settings. HIFU’s safety profile and duality of energy output make it a realistic option for targeting localized prostate cancer therapy. Compared to diagnostic ultrasound (maximum time-averaged intensity of 0.72 W/cm2), HIFU has intensities in the range of 100 to 10,000 W/cm2 (10). As tissue necrosis is expected to occur, it is imperative to ensure focal targeting leaves the unwanted surrounding tissue intact. Limitations hindered previous applications of HIFU in imaging modalities; however, recent advancements in magnetic resonance and ultrasound diagnostics have afforded researchers the ability to evaluate and target specific lesions accurately. HIFU complications are minor but must arise from inadvertent ultrasonic energy distribution to non-targeted, adjacent tissue. Specific to PCa, care must be taken to identify and protect against damage to the rectum/bowel, ureters, urethra, and bladder. While safeguards for most protocols have been instituted to help prevent such injury, some reported GU complications can include urinary retention or incontinence, urethral stricture, urinary fistula, pelvic/perineal pain, and erectile dysfunction (17).
Multiple studies have recently attempted to elucidate the role of HIFU in treating localized prostate cancer as a focal therapy. In the largest series with the longest follow-up data on HIFU for PCa treatment, Reddy et al. analyzed the HIFU Evaluation and Assessment of Treatment (HEAT) registry from 13 UK centres [2005–2020] (18). 1,379 patients with ≥6 mo of follow-up (5+ years of follow-up was available for 325 (24%) patients). Primary outcomes looked at failure-free success (FFS), defined by their group as no evidence of disease to require salvage whole-gland or systemic treatment, or metastases or prostate cancer-specific mortality, amongst varying D'Amico risk groups. The majority of patients were classified as D'Amico intermediate risk (65%, 896/1,379) followed by high risk (28%, 386/1,379). The study’s overall median follow-up was 32 [17–58] months. At 7-year follow-up, metastasis-free survival and prostate cancer-specific mortality were 100%, and overall survival was 97. There was 69% FFS. Of those with treatment failure (residual or recurrent cancer). On interim analysis, 69% of patients met the criteria for FFS; 252 patients underwent repeat FT, and 92 required salvage whole-gland treatment. This study shows clinical relevance in that they majorily included intermediate and high-risk patients while displaying favorable overall survival results with acceptable treatment success for cancer control.
In a study by Crouzet et al., they published their 2-year follow-up for targeted primary treatment with curative intent in a cohort of patients with PCa after treatment with HIFU across six Urology departments (19). A total of 803 patients met the study’s inclusion criteria. Risk stratification was determined as low (40.2%), intermediate (46.3%) and high-risk (13.5%) based on D’Amico guidelines. Mean follow-up time was calculated at 42 months, with control biopsies found to be negative in 85% of all cases. At 8 years, overall and cancer-specific survival rates were 89% and 99%, respectively. The metastasis-free survival rate at an 8-year follow-up was 97%. As expected, when stratified for pre-treatment risk groupings, outcomes decreased linearly. The 5- and 7-year biochemical-free survival rates were 83–75%, 72–63%, and 68–62% (P=0.03) and the additional treatment-free survival rates were 84–79%, 68–61%, and 52–54% (P<0.001) for low-, intermediate-, and high-risk patients, respectively.
Spanning over 5-years, Guillaumier et al. published their prospective study’s outcomes for localized PCa after HIFU (20). Focal HIFU was offered for patients who met the following criteria: nonmetastatic prostate cancer, Gleason score 6–9, stage T1c–3bN0M0, and prostate-specific antigen of < or = 30 ng/mL. Gleason 6 disease required a minimum of 4 mm of cancer. Follow-up included PSA measurement, mpMRI, and biopsy. A total of 625 patients met inclusion with a mean follow-up time of 56 months. 84% of patients were deemed intermediate or high risk. The median follow-up was 56 months. Failure-free survival, defined as lack of local salvage procedures, systemic therapy, metastasis, and cancer-specific mortality, was 99% at 1 year, 92% at 3 years, and 88% at 5 years. For the whole patient cohort, metastasis-free, cancer-specific, and overall survival at 5 years was 98% (95% CI: 97–99%), 100%, and 99%, respectively. Overall complications were low, with only 2% of patients reporting urinary incontinence (any pad use). This study shows promising outcomes with minimal side effects at the 5-year mark in patients treated for clinically significant PCa.
Given these promising results, the role of HIFU as salvage or adjuvant therapy became popularized as a topic of therapeutic intervention. In a study published by Crouzet et al., 418 patients were enrolled for salvage HIFU after failed external beam radiotherapy (EBRT) for localized prostate cancer recurrence (21). This multi-institutional, retrospective study took place across 9 different centers over 14 years. The goal was to assess oncologic outcomes of salvage HIFU for locally recurrent prostate cancer after EBRT. Primary outcomes assessed biochemical failure-free survival (bFFS) based on the ‘Phoenix’ criteria of biochemical recurrence (PSA nadir + 2 ng/mL). In addition, overall survival, cancer-specific survival, and metastasis-free survival were evaluated. The mean follow-up for their cohort was 3.5 years. When stratified according to risk profiles, bFFS was 58%, 51%, and 36% for pre-EBRT low-, intermediate- and high-risk patients, respectively, at 5-years follow-up. Additionally, the degree of recurrence as measured by pre-salvage-HIFU showed significant differences with bFFS rates of 67%, 42%, and 22% for PSA levels ≤4, 4–10, and ≥10 ng/mL, respectively. Notably, 196 patients (46.9%) received androgen deprivation therapy (ADT) after salvage-HIFU. OS, CSS, and MFS rates at 7 years were 72%, 82%, and 81%, respectively. On multivariate analysis, the history of ADT, pre-S-HIFU Gleason score, and pre-S-HIFU PSA levels were significantly linked to biochemical recurrence. During the study period, post-RT safety parameters were introduced and subsequently decreased complication rates; however, overall, Moderate-severe incontinence (32%), AUS implantation (15%), bladder outlet obstruction or stenosis (30%), recto-urethral fistula (9%) and pubic bone osteitis (3%) highlight severe morbidities shown in this cohort. This study highlights the role of salvage HIFU after EBRT with favorable survival rates; however, differences per risk-stratification groupings, patient comorbidities, and significant adverse events must be thoroughly discussed before therapy.
Irreversible electroporation
Irreversible Electroporation (IRE) is a novel ablation approach that uses short pulses of direct-current electricity to create irreversible pores that destabilize the target cell membrane, ultimately leading to cell death and tissue necrosis. IRE differs from other ablative techniques because it uses nonthermal energy and inherently carries a significantly decreased risk to surrounding tissue (22). Two thin electrodes, with probes approximately 10–20 mm apart, produce electric fields encompassing the lesion of interest. Post-IRE histologic specimens have shown this electricity to create micropores and cause cellular apoptosis while preserving extracellular matrices and surrounding connective tissue architecture (23). Tissue electrical current dynamics are fine-tuned and individually adjusted for optimum effective field strength. Before the intervention, target lesions are identified and localized using MR/US fusion guidance with biopsy. Subsequently, using fusion software and real-time transrectal ultrasonography, 3 to 6 needle electrodes are transperineally guided into the target lesion. Nanoknife® (AngioDynamics Inc., Latham, NY, USA) is an IRE system programmed to deliver direct current pulses at 1,500 Volts/cm with a pulse length of approximately 90µs. One consequence of the electrical field generation is nearby muscle contraction; therefore, patients require both general anesthesia and complete neuromuscular blockade to prevent probe migration and accurate lesion targeting.
Several early studies have published their results on patients who underwent IRE as a focal treatment for prostate cancer. Between 2013 and 2016, van den Bos et al. evaluated a cohort of 63 men treated with primary IRE for localized PCa (24). Outcomes highlighted both oncologic control and quality-of-life validated questionnaires. All patients had low-intermediate (Gleason score ≤7) PCa and had a preoperative mpMRI with MR/US-fusion guided targeted biopsy. No peri-procedural or postoperative serious adverse events (CTCAE ≥3) were reported. On clinical evaluation, there were no significant changes from baseline in physical, mental, bowel, or urinary QoL domains; however, compared to baseline, there was a mild decrease in Expanded Prostate Cancer Index Composite (EPIC) sexual domain scores (P<0.001). Oncologic control was stratified into in-field and out-of-field failure as evidenced by follow-up lesion on MRI and subsequent biopsy. At the 6-month follow-up, 55 patients had no evidence of residual disease. Upon 12-month completion, 45 patients had follow-up biopsies, with 11 men showing residual disease.
Valerio et al. describe a prospective study of 16 patients who underwent IRE focal ablation of prostate cancer (25). All lesions were mapped using concordant MR/US mapping and biopsy, then localized using a transperineal template (brachytherapy grid and modular stepper). Primary outcomes looked at side effect profile, domain-specific toxicity profile, and early oncologic disease control at 12 months. Patients were limited to Gleason 3+3 (42%, n=8) and 3+4 (58%, n=11). At 6-month follow-up, repeat mpMRI with biopsy revealed 33.3% in-field clinically significant disease. At the end of their study assessment (12 months), 11 patients (61.1%) had no residual disease, one patient (5.6%) had clinically insignificant disease (1 of 9 cores Gleason 3+3), and six men (33.3%) harbored clinically significant disease (GS 3+4=7, median maximum cancer core length of 4 mm). There were no serious adverse events (grade 3+) recorded at the primary end-point, and all men had pad-free/leak-free continence. Using UCLA-EPIC and I-PSS validated questionnaires, there was a statistically significant improvement in urinary symptoms (P=0.039 and 0.001, respectively). Additionally, erectile function remained stable on IIEF-15 (P=0.572). While this study demonstrated the safety and low toxicity of IRE, the authors and editorial commentary state that the relatively high number of residual disease found may be attributed to narrow margin selection and needs further follow-up and protocol adjustment.
In a slightly more extensive study, Ting et al. reviewed short-term functional and oncologic outcomes of focal IRE in 25 patients with low (n=2, 8%) and intermediate (n=23, 92%)-risk PCa (26). Overall safety was again demonstrated; however, one Clavien Grade 3 complication was reported (non-ST elevation myocardial infarction). There were no significant changes in AUA urinary symptom score, sexual, or bowel function. Contrasted to the above study, there were no suspicious MRI lesions and no in-field positive biopsy results. For areas adjacent to the treatment zone, there were 5 (21%) suspicious MRI lesions and 4 (19%) significant, positive biopsies. Out-of-field had 2 (8%) suspicious findings on mpMRI and 1 (5%) significant finding on biopsy. Significant biopsies for these 5 patients were all Gleason 7 or 8 PCa. Of these, 4/5 (80%) occurred within the first 12 patients with a margin of 5mm that was subsequently increased to 10mm for the final 13 patients of this series. The authors again establish promising functional outcomes with the potential for adequate oncologic control in this short-term study.
In a matched-pair analysis by Scheltema et al., 50 men who underwent focal IRE or robotic-assisted radical prostatectomy were compared (27). As predicted, IRE showed superior pad-free continence and erectile preservation; however, 13 of those who underwent IRE had significant residual PCa compared to no biochemical recurrence/failure in the RALP group at 12 months. While the above trials display promise for IRE as a prominent focal therapy contender, significant infield residual or positive biopsy rates still exist. Future studies should experiment to maintain safety margins with protocol changes and extension of the ablation margin. Longer studies and better oncologic control will be paramount to IRE's future for focal PCa primary treatment.
Vascular-activated photodynamic therapy
Photodynamic therapy (PDT) refers to tissue ablation by utilizing a photosensitive agent, given in its inactive form, that is selectively activated by light in the presence of oxygen (28). The drug may be administered topically, orally, and intravenously but remain inert until activated. Ideally, tumor cells will preferentially uptake the agent after administration of the intravascular agent, and low-power near-infrared laser light of a specific wavelength is used to activate the agent. Activation requires the combination of a specific wavelength of light and oxygen for a given amount of time. Cellular destruction occurs through a variety of multiple mechanisms. Some energy is released as heat and light; however, the predominant destructive component occurs after drug conversion into its intermediate state, which generates superoxide and hydroxyl radicals that become highly reactive with proteins, lipids, and nucleic acids, ultimately leading to cell death (10). Previously hindered by systemic distribution and unfavorable uptake time into sensitive tissue (skin and retinas), vascular-activated photodynamic (vaPDT) agents act to solve these problems. These agents are highly soluble and remain confined to the vasculature, rapidly cleared by renal and hepatic systems.
As of recent, PDT has been studied in the context of prostate cancer treatment, mainly in the setting of clinical trials. Multiple drug compositions vary in efficacy and activation wavelengths, including 5-ALA, motexafin lutetium, AlS2Pc, WST-09, WST-11, and mTHPC (28). Preoperative planning is paramount to success and accuracy. Again, developments in high-resolution mpMRI and US fusion software make focal therapy with PDT an option. Visualization is accomplished with intraoperative transrectal ultrasound, and targeting is achieved through a brachytherapy grid attached to the stepper. Per prior preoperative mapping, transperineal needle cannulae are placed to the level of the target area. The PDT-specific laser fiber is put through each cannulae and fired to activate the vaPDT agent accumulated within the target tissues.
Gill et al. published their 4-year intermediate results of a prospective clinical trial (PCM301) in which men with low-risk prostate cancer were randomized to partial gland ablation with vaPDT or AS protocols (29). Patient inclusion criteria were specific for low-risk PCa (Gleason score 6 or less, GG1), clinical-stage T2a or less, and PSA 10 ng/mL or less, with 2 or 3 positive cores, prostate volume 25–70 cc. 266 patients were included, 147 in the vaPDT arm and 119 in the AS arm. Repeat biopsy was mandated at 12 and 24 months. Mid-assessment review results showed that conversion from either study arm to radical therapy (surgery or radiation) was less likely in the ablation cohort compared to the surveillance at 7% vs. 32% at 2 years, 15% vs. 44% at 3 years, and 24% vs. 53% at 4 years (HR 0.31, 95% CI: 0.21–0.46), respectively. Cancer progression rates on repeat biopsy were significantly lower in the ablation cohort (HR 0.42, 95% CI: 0.29–0.59). Evidence of clinically significant (Gleason 7 or higher) cancer was less likely in ablation groups overall (16% vs. 41%). The authors show a meaningful reduction in cancer volume and treatment burden; however, editorial responses and limitations highlight the study's use of end-points that cannot be clinically used as realistic surrogates for disease-specific survival. A phase III clinical trial conducted by Azzouzi et al. showed similar results (30). They enrolled 413 men with low-risk prostate cancer and randomized them to PDT or AS. They found that at 24 months, only 28% of the PDT cohort was found to have disease progression compared to 58% of the AS cohort.
Gold nano-particle directed therapy
The use of nanoparticles for ablation is a relatively novel idea that spans the realm of diagnostic to therapeutic possibilities. Gold nanoparticles are composed of a dielectric core and metallic outer shell (SiO2-Au materials; gold-silica nanoshells, AuroShells). Similar to the idea of PDT, particles are inherently inert and can be activated via near-infrared laser emission to absorb light and convert this energy into thermal output. Gold nanoparticles are approximately 150 nm and are characterized by surface plasmon resonance. When excited by specific frequencies of light, collective oscillation and excitation of surface electrons are converted into thermal energy.
The administration of AuroShells is given systemically and must ultimately rely on sufficient accumulation within the target tissue of interest. It was initially hypothesized that tumors, including prostate cancer, often encompass fenestrated vessel walls with abhorrent neovascularity and lymphatic drainage, allowing AuroShells to preferentially accumulate to significant volumes within tumors (31). Accurate targeting relies on preoperative mpMRI imaging and subsequent MR/US fusion-guided biopsy of the target lesion. To allow for adequate accumulation time, patients undergo intravenous infusion with the AuroShell solution at a concentration of 7.5 mL/kg one day before the procedure. Under general or local anesthesia, trocars are transperineally inserted and advanced to the desired location using MR/US fusion mapping and real-time transrectal ultrasonography. Once positioned, a laser fiber is introduced and activated to excite the AuroShells causing thermal ablation and cell death.
In the initial clinical safety study, Stern et al. enrolled 22 patients who underwent nanoshell infusion and subsequent radical prostatectomy (32). Fifteen of these patients were irradiated by single-fiber laser ablation in each prostate hemisphere 5-days before prostatectomy. On serial assessment through 6 months post-infusion, there were no device-related changes in blood/hematology/urinalysis assays. No serious adverse events were recorded. Following this documentation of safety and minimal toxicity, therapeutic efficacy, and feasibility study for gold nanoparticle ablation (Rastinehad et al.) looked at men aged 58–79 years with clinical stage of ≤ T2a disease and Gleason score ≤7 (31). Patient follow-up included subsequent multi-parametric magnetic resonance imaging (mpMRI) on post-ablation days 2 and 3. Repeat mpMRI and MR/US fusion-biopsy were repeated at 3- and 12 months post-treatment. Immediate post-ablation mpMRI showed T2-weighted edema and nonspecific changes with acceptable ablation zones on dynamic contrast-enhanced images in all but 2 of the 15 men who completed the study. At 3 months, scar contraction was seen in the ablation zones and a loss of lesion enhancement in the majority of cases. Post-ablation biopsy showed no evidence of disease (NED) in 62.5% of patients at 3 months and 87.5% of samples at 1-year follow-up. Clinical indices were excellent without any change from baseline in International Prostate Symptom Score (IPSS), Sexual Health Inventory for Men (SHIM), or urinary quality of life scores at 3-months post-treatment. With overall exceptional results, an open-label multicenter clinical trial for nanoparticle focal ablation for treatment of low- and intermediate- prostate cancer (NCT02680535) is currently underway and pending results analysis.
Focal laser ablation
Laser interstitial thermal (LITT), or focal laser ablation (FLA), uses a diode laser placed interstitially through a cooling catheter to cause precise tissue destruction. Once appropriately positioned, laser excitation of surrounding tissue generates thermal energy and localized coagulative necrosis. FLA has recently been studied as a modality with the potential for treating prostate cancer due to the prostate gland’s optical absorption rate, relatively low vascularity, and accessibility via transperineal or transrectal access (10). Most of those mentioned above contemporary focal therapy options in prostate cancer rely on imaging provided by transrectal ultrasound guidance; however, FLA is unique in that the equipment is MR compatible and affords the opportunity of supplementary intraoperative imaging. Real-time MR thermometry can monitor temperature changes for the ablation zone and surrounding tissues (Figure 1). This can increase the chances of in-field success with targeting adjustments and provides an additional safeguard to ensure that critical structures (i.e., urethra, rectum, etc.) are protected from the inadvertent thermal spread.
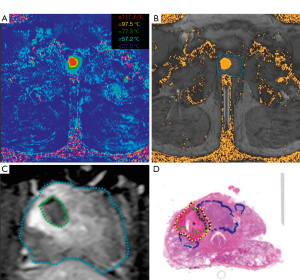
Typical techniques involve trocar and guide placement using a modified template grid (MR-compatible) under ultrasound guidance to a pre-determined depth. Once the correct placement is confirmed, treatment is delivered for approximately 60–120 seconds at 6–25 W. Some limitations for FLA are large tumor volume and difficult locations (e.g., anterior), as repositioning may compromise safety and in-field treatment success.
In a phase II evaluation, Eggener et al. looked at a 12-month follow-up for 27 patients treated with FLA for PCa (34). Treatment was conducted using a 14-gauge titanium introducer and 15W Visualase® laser with 5mm ablation margins. The mean procedure duration was noted at 197 minutes. Patients underwent serial assessment with mpMRI and biopsy of ablation zone(s) at 3 months, followed by a 12-month repeat mpMRI and systematic biopsy. 12-month biopsy detected positive cancer in 10 patients (37%). These were categorized as 3 in-field (11%) and 8 (30%) out-of-field. On clinical assessment for urinary function and erectile dysfunction, there was no significant change from baseline in I-PSS or SHIM scores at 12-month follow-up. Of note, the majority of men were low-risk patients (85%) with clinical stage T1c (n=25, 93%), with the rest being T2a (n=2, 7%).
Several other groups have reported studies with their experience and outcomes with FLA of prostate cancer. In a cohort of 25 men, Lepor et al. evaluated oncologic and clinical outcomes after intra-operative MRI-guided FLA (35). They reported no significant differences in baseline AUA-SS or SHIM scores at 3-month. On short-term analysis at 3 months, 96% of all ablation zones showed no evidence of residual cancer with a mean decrease in PSA of 40%. Natarajan et al. (36) reported their outcomes for 8 patients with intermediate-risk PCa who underwent FLA treatment. They re-demonstrated excellent clinical outcomes related to incontinence and erectile dysfunction, with no changes in IPSS or IIEF5 scores. At a 6-month follow-up, they found in-field detection in 3 men (37.5%) and out-field detection in 6 men (75.0%), often near or just outside the ablation margin. Per author's discussion, as with all novel techniques and therapy, defining effective protocols and a learning curve may explain a relatively high failure rate. Like many other focal therapy options, FLA affords a high safety profile with preferable sexual and urinary outcomes but must ultimately address or prove equivocal/superior oncologic control outcomes.
Future directions
The above review highlights novel focal therapy modalities with realistic potential for primary treatment. As outlined above, early research has taken the first steps for numerous focal therapies, reporting exceptional safety, low toxicity, and acceptable oncologic outcomes. Table 2 outlines a summary of the discussed modalities. Advances in imaging, namely mpMRI, have allowed the progression of FT due to the accuracy of detection and enablement for precise targeted biopsies. In addition to primary treatment, the application of focal salvage therapy after biochemical recurrence has shown promise with minimal side effects; however, the number of patients treated with salvage PCa focal therapy remains low and needs further elucidation (37).
Table 2
| Focal therapy modality | Mechanism | Clinical outcomes | Advantages and future directions |
|---|---|---|---|
| Cryoablation | Alternating freeze-thaw cycles lead to protein denaturation and vascular damage, culminating in localized tissue destruction | Overall prostate-cancer-specific survival of 87% at 10.1 years (whole-gland cryoablation) (10) | One of the most established modalities with large body of evidence in multiple specialties |
| 71% BPFS and no change in IIEF at 24 months (11) | Long-term data for focal cryoablation shows durability in oncologic outcomes while preserving the minimal side effect profile | ||
| No difference in BPFS between focal and whole-gland cryoablation (12) | |||
| HIFU | Pulsed ultrasound beams focused on target tissue to produce thermal and mechanical energy. | Overall and cancer-specific survival rates were 89% and 99%, respectively, with 97% metastasis-free survival at 8-years (16) | Minimal side-effect profile using ultrasound technology |
| Metastasis-free, cancer-specific, and overall survival at five years was 98% (95% CI: 97–99%), 100%, and 99%, respectively (17) | Long-term follow-up data needed to confirm oncologic durability and minimal side-effect profile | ||
| IRE | Short pulses of direct-current electricity to create irreversible pores that destabilize the target cell membrane, ultimately leading to cell death and tissue necrosis. | No significant changes from baseline in physical, mental, bowel, or urinary QoL domains (21) | Nonthermal energy and inherently carries a significantly decreased risk to surrounding tissue |
| 33.3% in-field clinically significant disease at 6-month follow-up (22) | Better demonstration of parameters to control both in-field and out-of-field recurrence needed | ||
| No significant changes in AUA urinary symptom score, sexual, or bowel function (23) | |||
| Compared to RALP, superior pad-free continence and erectile preservation in IRE group; however, higher incidence of PCa compared to no biochemical recurrence/failure in the RALP group at 12-months (24) | Further comparative and long-term studies between RALP and IRE. | ||
| Vascular-activated photodynamic therapy (PDT) | Utilizes a photosensitive agent, given in its inactive form, that is selectively activated by light in the presence of oxygen. | Compared to Active Surveillance, cancer progression rates on repeat biopsy were significantly lower in the ablation cohort. Clinically significant (Gleason 7 or higher) cancer was less likely for ablation groups overall (16% vs. 41%) (26) | Agreement for identifying the most appropriate PDT agent best suited for prostate cancer |
| Long-term follow-up data needed to confirm oncologic durability and minimal side-effect profile | |||
| Gold nano-particle focal therapy | Gold nanoparticles are composed of a dielectric core and metallic outer shell (SiO2-Au materials; gold-silica nanoshells, AuroShells). Particles are inert and can be activated via near-infrared laser emission to absorb light and convert this energy into thermal output. | No device-related changes in blood/ hematology/urinalysis assays. No serious adverse events were recorded (28) | Novel mechanism that utilizes minimally invasive and confirmed safety profile |
| Post-ablation biopsy showed no evidence of disease in 62.5% of patients at 3 months and 87.5% of samples at 1-year follow-up. Clinical indices were excellent without any change from baseline in IPSS, SHIM, or urinary quality of life scores at 3-months post-treatment (27) | Clinical trial underway and pending results/follow-up | ||
| Long-term follow-up data needed to confirm oncologic durability and minimal side-effect profile | |||
| focal laser ablation (FLA) | FLA uses a diode laser placed interstitially through a cooling catheter to cause precise tissue destruction. Once appropriately positioned, laser excitation of surrounding tissue generates thermal energy and localized coagulative necrosis | 12-month biopsy detected positive cancer in 10 patients (37%). These were categorized as 3 in-field (11%) and 8 (30%) out-of-field. On clinical assessment for urinary function and erectile dysfunction, there was no significant change from baseline in IPSS or SHIM scores at 12-month follow-up (29) | High safety profile with preferable sexual and urinary outcomes |
| Needs better elucidation of margins to improve in-/and out-of-field results | |||
| Protocol tweaking | |||
| No significant differences in baseline AUA-SS or SHIM scores at 3-months. On short-term analysis at 3-months, 96% of all ablation zones showed no evidence of residual cancer with a mean decrease in PSA of 40% (30) | Long-term follow-up data needed to confirm oncologic durability and minimal side-effect profile | ||
| No changes in IPSS or IIEF5 scores. At 6-month follow-up, they found in-field detection in 3 men (37.5%) and out-field detection in 6 men (75.0%) (31) |
HIFU, high-intensity focused ultrasound; IRE, irreversible electroporation; PDT, photodynamic therapy; FLA, focal laser ablation; BPFS, biochemical progression free-survival; QoL, quality of life; AUA, American Urological Association; RALP, robotic-assisted radical prostatectomy; IPSS, International Prostate Symptom Score; SHIM, Sexual Health Inventory for Men.
Coupled with an increasing life expectancy, access to screening and improved diagnostics have created a treatment dilemma for a largely believed, indolent pattern of disease progression. While the advantages of FT in avoiding sexual and urinary side-effects of radical surgery or radiation are apparent, there must be further evidence for equivocal oncologic control that is durable over time and comparable to current gold-standard treatment options. Many contemporary trials are single-arm and limited to low- or intermediate-risk prostate cancer. As more research arises, future directions should attempt to expand inclusion criteria and focus on head-to-head comparisons, specifically with those of radical prostatectomy and primary radiation therapy.
The premise of focal therapy relies on adequate identification and targeting of an index lesion. While most modalities discussed above excel at targeted eradication, there is a concern about leaving undetected or micro-disease that may ultimately progress to clinical significance. Further exploration into adjuvant- or neo-adjuvant treatment with FT may provide a solution for addressing oncologic control of the theoretical underlying disease. Adding anti-androgens or androgen deprivation therapy as a combinatorial approach could provide additive control on satellite or multi-focal lesions. Another strategy may be to combine Stereotactic Body Radiation Therapy (SBRT) or brachytherapy as a boost to primary focal therapy. Research in this area has been conducted previously, showing acceptable results with minimal toxicity (38,39); however, further emphasis and longer follow-up are necessary to validate this approach. The introduction of genetic markers (i.e., Decipher®, Oncotype DX®, etc.) to predict tumor phenotype and aggressiveness is an exciting tool for counsel and management of prostate cancer. Incorporating predictive nomograms into patient selection may serve as an adjunct to promoting FT success.
As evidenced by the above review, many novel, different focal therapies exist to treat prostate cancer. Inherent in the heterogeneous study characteristics and relatively short follow-ups, it isn't easy to crown superiority when comparing each modality. To add to the confusion, no strict guidelines offer guidance for patient selection. Most reported studies target low-risk patients, and further data should include higher-risk patient cohorts. While this modality was not explicitly discussed in our review, Ehdaie et al. Recently published their outcomes for a phase 2b multicentre study using MRI-guided focused ultrasound energy for treatment of patients with intermediate-risk prostate cancer. At 24 months, 78 of 89 men (88%) had no evidence of grade 2 or higher PCa in the treated area (40). No serious treatment-related adverse events were observed, again confirming the safety profile of a novel FT modality. As more widespread patient inclusion data unfolds, defining appropriate patient selection criteria will help better delineate guidelines to predict oncological success when considering FT to treat PCa.One hurdle with FT’s novelty can be found in intra-observer imaging interpretation. Many studies included post-ablation imaging findings after focal therapy. Used to compare pre- and post-ablation success, follow-up imaging findings are often nonspecific and require time-decay to determine the ablation’s effect. The 2015 Joint International Consultation on Urological Diseases (ICUD)/Societ Internationale de Urologie (SIU) defined image-guided focal therapy success as:
- Within the treated or infield area as the:
- Eradication of the tumor focus on the short term.
- Absence of clinically significant cancer in the intermediate to long-term
- Within the untreated or out-field area for the development of clinically significant cancer. In the short term, this out-field cancer focus likely represents selection failure; in the intermediate- to long-term, this may mean de novo cancer (41).
There are few if any experts in interpreting, let alone correlating ablation success, the post-ablation FT changes. Adding to the conundrum is the fact that each modality likely will create an individual imaging signature that will undoubtedly vary based on the mechanism used to induce necrosis (i.e., thermal, mechanical, etc.). Further experience, pattern recognition, and repetition will be critical in standardizing post-treatment outcomes as measured by DCE-mpMRI findings.
Another essential consideration and piece for where focal therapy fits into the treatment paradigm is the financial cost-effectiveness on the long-term scale when compared to surveillance or radical whole-gland treatment. For patients on active surveillance, up to one-half of all men will ultimately pursue radical intervention (42). After focal therapy, the risk of untreated satellite lesion progression or in-field recurrence is possible and must be considered for retreatment. Additionally, surveillance in the form of repeat biopsy and imaging is expected indefinitely and bears unknown financial ramifications. Ramsay et al. compared the balance of relative clinical effectiveness with cost-effectiveness for ablative FT (cryotherapy, brachytherapy, HIFU, amongst others) with non-ablative therapy (radical prostatectomy, external beam radiotherapy, and active surveillance) for primary treatment of localized prostate cancer. While this was an exhaustive review of over 120 included studies, given the heterogeneity of reviews, there was insufficient evidence to form any clear recommendations for clinical effectiveness and cost-effective superiority (43). These considerations should be considered moving forward and future efforts should include RCTs to generate standardized outcomes.
Conclusions
Focal therapy for the treatment of prostate cancer spans multiple modalities with promising early data to support its use as a primary treatment. FT offers the potential for disease eradication while limiting the side effects of whole-gland, radical intervention. Current management with radical prostatectomy or radiation therapy is considered the gold standard for oncologic outcomes; however, both yield a significant risk of post-treatment incontinence, sexual/erectile dysfunction, and proctitis. Most of the existing evidence supporting FT is heterogeneous, short-term, and with strict inclusion. Future studies should explore adjuvant, combinatorial approaches that may improve outcomes. The durability of treatment success over a long-term follow-up is paramount to establishing guidelines. Patient selection, improvement in imaging, and fine-tuning ablation margins will be critical to improving some of the unfavorable reported in- and out-field recurrence rates. While still in its infancy, this review sheds light on the current progress and creates optimism for focal therapy’s role in the prostate cancer management paradigm.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2337/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2337/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2337/coif). ARR receives the consulting fees from Nanospectra Biosciences and Philips Healthcare, holds stock in Nanospectra Biosciences. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Andriole GL, Crawford ED, Grubb RL 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310-9. [Crossref] [PubMed]
- Glass A, Punnen S, Carroll PR. Active Surveillance for Prostate Cancer. AUA Update Series. 2013;32:130-5.
- Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012;62:405-17. [Crossref] [PubMed]
- Tavukçu HH, Aytac O, Atug F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig Clin Urol 2016;57:S172-84. [Crossref] [PubMed]
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390-7. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Bakavicius A, Marra G, Macek P, et al. Available evidence on HIFU for focal treatment of prostate cancer: a systematic review. Int Braz J Urol 2022;48:263-74. [Crossref] [PubMed]
- Jaipuria J, Ahmed HU. Clinical and pathologic characteristics to select patients for focal therapy or partial gland ablation of nonmetastatic prostate cancer. Curr Opin Urol 2022;32:224-30. [Crossref] [PubMed]
- Smith JA, Howards SS, Glenn M. Preminger, and Frank Hinman. Hinman's Atlas of Urologic Surgery. Philadelphia: Elsevier/Saunders, 2012.
- AUA Cryosurgery core curriculum. Available online: https://university.auanet.org/core/uroradiology/cryosurgery/index.cfm
- Cheetham P, Truesdale M, Chaudhury S, et al. Long-term cancer-specific and overall survival for men followed more than 10 years after primary and salvage cryoablation of the prostate. J Endourol 2010;24:1123-9. [Crossref] [PubMed]
- Barqawi AB, Stoimenova D, Krughoff K, et al. Targeted focal therapy for the management of organ confined prostate cancer. J Urol 2014;192:749-53. [Crossref] [PubMed]
- Mendez MH, Passoni NM, Pow-Sang J, et al. Comparison of Outcomes Between Preoperatively Potent Men Treated with Focal Versus Whole Gland Cryotherapy in a Matched Population. J Endourol 2015;29:1193-8. [Crossref] [PubMed]
- Phenix CP, Togtema M, Pichardo S, et al. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J Pharm Pharm Sci 2014;17:136-53. [Crossref] [PubMed]
- Aptel F, Lafon C. Therapeutic applications of ultrasound in ophthalmology. Int J Hyperthermia 2012;28:405-18. [Crossref] [PubMed]
- Partin AW, Dmochowski RR, Kavoussi LR, et al. Campbell-Walsh-Wein Urology: "High Intensity Focused Ultrasound for the Treatment of Prostate Cancer". Twelfth ed. Philadelphia PA: Elsevier; 2021. Ch 106.
- Reddy D, Peters M, Shah TT, et al. Cancer Control Outcomes Following Focal Therapy Using High-intensity Focused Ultrasound in 1379 Men with Nonmetastatic Prostate Cancer: A Multi-institute 15-year Experience. Eur Urol 2022;81:407-13. [Crossref] [PubMed]
- Crouzet S, Rebillard X, Chevallier D, et al. Multicentric oncologic outcomes of high-intensity focused ultrasound for localized prostate cancer in 803 patients. Eur Urol 2010;58:559-66. [Crossref] [PubMed]
- Guillaumier S, Peters M, Arya M, et al. A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur Urol 2018;74:422-9. [Crossref] [PubMed]
- Crouzet S, Blana A, Murat FJ, et al. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU Int 2017;119:896-904. [Crossref] [PubMed]
- Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 2007;6:295-300. [Crossref] [PubMed]
- Wendler JJ, Porsch M, Hühne S, et al. Short- and mid-term effects of irreversible electroporation on normal renal tissue: an animal model. Cardiovasc Intervent Radiol 2013;36:512-20. [Crossref] [PubMed]
- van den Bos W, Scheltema MJ, Siriwardana AR, et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int 2018;121:716-24. [Crossref] [PubMed]
- Valerio M, Dickinson L, Ali A, et al. Nanoknife Electroporation Ablation Trial: A Prospective Development Study Investigating Focal Irreversible Electroporation for Localized Prostate Cancer. J Urol 2017;197:647-54. [Crossref] [PubMed]
- Ting F, Tran M, Böhm M, et al. Focal irreversible electroporation for prostate cancer: functional outcomes and short-term oncological control. Prostate Cancer Prostatic Dis 2016;19:46-52. [Crossref] [PubMed]
- Scheltema MJ, van den Bos W, Siriwardana AR, et al. Feasibility and safety of focal irreversible electroporation as salvage treatment for localized radio-recurrent prostate cancer. BJU Int 2017;120:51-8. [Crossref] [PubMed]
- Partin AW, Dmochowski RR, Kavoussi LR, et al. Campbell-Walsh-Wein Urology: "Focal Therapy for Prostate Cancerr". Twelfth ed. Philadelphia PA: Elsevier; 2021. Ch 117.
- Gill IS, Azzouzi AR, Emberton M, et al. Randomized Trial of Partial Gland Ablation with Vascular Targeted Phototherapy versus Active Surveillance for Low Risk Prostate Cancer: Extended Followup and Analyses of Effectiveness. J Urol 2018;200:786-93. [Crossref] [PubMed]
- Azzouzi AR, Vincendeau S, Barret E, et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 2017;18:181-91. [Crossref] [PubMed]
- Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A 2019;116:18590-6. [Crossref] [PubMed]
- Stern JM, Kibanov Solomonov VV, Sazykina E, et al. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int J Toxicol 2016;35:38-46. [Crossref] [PubMed]
- Bomers JGR, Cornel EB, Fütterer JJ, et al. MRI-guided focal laser ablation for prostate cancer followed by radical prostatectomy: correlation of treatment effects with imaging. World J Urol 2017;35:703-711. [Crossref] [PubMed]
- Eggener SE, Yousuf A, Watson S, et al. Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer. J Urol 2016;196:1670-5. [Crossref] [PubMed]
- Lepor H, Llukani E, Sperling D, et al. Complications, recovery, and early functional outcomes and oncologic control following in-bore focal laser ablation of prostate cancer. Eur Urol 2015;68:924-6. [Crossref] [PubMed]
- Natarajan S, Raman S, Priester AM, et al. Focal laser ablation of prostate cancer: phase I clinical trial. J Urol 2016;196:68-75. [Crossref] [PubMed]
- Khoo CC, Miah S, Connor MJ, et al. A systematic review of salvage focal therapies for localised non-metastatic radiorecurrent prostate cancer. Transl Androl Urol 2020;9:1535-45. [Crossref] [PubMed]
- Aluwini S, van Rooij P, Hoogeman M, et al. Stereotactic body radiotherapy with a focal boost to the MRI-visible tumor as monotherapy for low- and intermediate-risk prostate cancer: early results. Radiat Oncol 2013;8:84. [Crossref] [PubMed]
- Baust JG, Bischof JC, Jiang-Hughes S, et al. Re-purposing cryoablation: a combinatorial 'therapy' for the destruction of tissue. Prostate Cancer Prostatic Dis 2015;18:87-95. [Crossref] [PubMed]
- Ehdaie B, Tempany CM, Holland F, et al. MRI-guided focused ultrasound focal therapy for patients with intermediate-risk prostate cancer: a phase 2b, multicentre study. Lancet Oncol 2022;23:910-8. [Crossref] [PubMed]
- Polascik TJ, Amin MB. Surveillance after focal therapy. In Sanchez-Salas R, Desai M (eds): Image-guided therapy in urology. SIU-ICUD Joint Consultations. Melbourne, Australia: Societé Internationale d'Urologie; 2015.
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Ramsay CR, Adewuyi TE, Gray J, et al. Ablative therapy for people with localised prostate cancer: a systematic review and economic evaluation. Health Technol Assess 2015;19:1-490. [Crossref] [PubMed]


