
A retrospective two-center cohort study on the use of routine chest X-ray after peripherally inserted central catheter placement under ultrasound and intracavitary electrocardiography guidance
Highlight box
Key findings
• Routine postprocedural CXR is unnecessary especially when the PICC is catheterized in the right arm, and is not a wise option.
What is known and what is new?
• The placement of peripherally inserted central venous catheters (PICCs) has traditionally relied on measurements and anatomical landmarks. It involves post-placement chest X-rays (CXRs) and occasional repositioning, which incur additional direct and indirect costs, such as delays in care and staff time.
• The overall incidence of intraprocedural and primary catheter misplacement was 7.3% (n=210) and 0.70% (n=20), respectively. There was a high risk of primary catheter misplacement when the left-arm was chosen for placement (OR: 11.163; 95% CI: 3.720–33.495; P<0.001).
What is the implication, and what should change now?
• A routine CXR isn’t necessary after a PICC catheterization, especially if the catheter is in the right arm.
Introduction
Peripherally inserted central venous catheters (PICCs) are critical to the treatment and care of cancer patients, especially in the chemotherapy and nutritional support treatment of this population. With the increasing application in clinical practice, PICCs-related complications are increasingly common. Improper position of the catheter could cause discomfort and pain to the patient and lead to serious consequences, such as that the PICC cannot be used normally (1) or there is an increased risk of deep venous thrombosis incidence (2). The chest X-ray (CXR) is the most frequently used method to verify PICC tip position and was recommended as the gold standard. X-rays have the limitation that the postprocedural confirmation may delay intravenous (IV) therapy for a patient by a significant amount. Further, if incorrect PICC tip position is detected by postprocedural X-ray, repeated catheter manipulation and CXRs are required, resulting in a delay in patient treatment and further time. In addition, there is a possibility for complications, including catheter-related bloodstream infection owing to the integrity of the dressing being interrupted.
The use of ultrasound (3) and intracavitary electrocardiography (IC-ECG) to locate the tip of PICCs (4-7) has been reported to significantly reduce the risk of PICC-related mechanical complications, improve patient’s safety, and reduce the incidence of related venous thrombotic events (VTE). In this setting, the CXR is still widely used by PICC operators in China as the gold standard for evaluating PICC catheter tip position. A major limitation is it having been adopted diffusely in clinical practice only in the last years, and there is a lack of research reporting the incidence of malposition into the azygos vein. Another is the absence of an economic evaluation to demonstrate that routine postprocedural CXR is sufficiently cost-effective.
In this study, we performed a retrospective study in two Chinese cohorts to clarify the incidence of PICC-associated misplacement at hospital, followed by a cost analysis to study the use of postprocedural CXR as a screening test to exclude PICC-associated misplacement. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5417/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Peking University Cancer Hospital & Institute and The Affiliated Qingdao Central Hospital of Qingdao University Ethics Committee (approval ID: 2020KT56, IEC-AF-081-03.0). Individual consent for this retrospective analysis was waived.
Participants
This retrospective study examined the clinical records of all adult patients with PICCs in the Venous Access Center of Peking University Cancer Hospital & Institute and The Affiliated Qingdao Central Hospital of Qingdao University between 1 January 2019 and 30 June 2020.
We included all patients (age ≥18 years) who received PICCs in the Venous Access Center and underwent postprocedural CXR. We excluded patients who (I) had a baseline non-sinus rhythm; (II) had an artificial pacemaker or implantable cardioverter-defibrillator (ICD); (III) did not have a postprocedural CXR check; and (IV) were younger than 18 years of age.
PICC insertion technique and perioperative adjuncts
Personnel qualifications
All patients were inserted uniformly by the nurses of the Venous Access Center, who specialized in the intravenous treatment and had more than 5 years of working experience. All nurses and medical staff were trained in IC-ECG and ultrasound-guided procedures at the beginning of the study.
Catheter types
The catheters used were single lumen 4 French PICC catheters (B. Braun Melsungen AG, 34209 Melsungen, Germany), and a Braun transducer and switch were used for shifting from surface electrocardiogram (ECG) tracing to IC-ECG tracing were used in the study.
Placement sites and vein puncture
The left and right upper limbs were selected for placement according to the patient’s wishes and vascular characteristics. Usually, the right upper limb is preferred. The basilic vein is mainly selected for puncture, followed by the brachial vein and the cephalic vein.
Placement procedures
Before placement, the patient’s veins were assessed by a nurse from the Venous Access Center using the LOGIQ Book ultrasound system (GE Healthcare, Chicago, IL, USA). The ultrasound-guided modified Seldinger technique (MST) was applied according to international guidelines-based practice. After the successful puncture, the catheter was inserted. When the catheter was inserted to the length of 30 cm, a sterile electrical lead was used to correctly connect the catheter guidewire and the IC-ECG lead. The catheter was then slowly inserted while observing the changes of the P-wave on ECG. When the tip of the catheter reached the atrium, the P-wave showed negative deflection. If there was no dynamic change in the P-wave, the ultrasound would determine whether the tip of the catheter was misplaced and connected to other vessels. After biphasic P-waves occurred and the catheter was withdrawn to the highest level of positive P-wave amplitude, the tip of the catheter remained in this place. The catheter was flushed with isotonic saline solution and was sealed by the positive pressure technique. A typical ECG pattern is illustrated in Figure 1.

Outcome measures
After the implantation, the CXR results of all patients were obtained, and body positions were kept consistent. Catheter tip positions were independently recorded by 2 certified radiologists with 5 and 12 years of experience, respectively. We utilized carina as an anatomical landmark from which to measure the PICC tip. The optimal position was estimated to be 1.6–4 cm under the carina in our study (8). The PICC tip position was considered as an inappropriate position if the catheter tip is above the carina (the catheter tip was located in the middle or upper third of the superior vena cava) or deeper more than 5 cm under the tracheal carina, the catheter tip may enter into the right atrium. The primary endpoint was misplacement during PICC insertion and secondary endpoint was misplacement following PICC insertion.
Data collection
The following data were collected: patient age, gender, body mass index (BMI); target vein for PICC, left/right arm, catheter tip position, vessel diameter and depth, arm circumference, number of insertions, and the vein with misplaced PICC on B-scan ultrasonography during the operation.
Statistical analysis
Descriptive data were expressed as median and interquartile range (IQR; i.e., 25th–75th percentile). Risk assessment was divided into odds ratio (OR) with 95% confidence interval (95% CI). Univariate and multivariate logistic regression models were used to evaluate the risk factors related to PICC misplacements. All statistically significant variables (P<0.05) in the univariate analysis were included in the multivariate analysis. Results are reported as odds ratios with associated 95% CIs and P values. All statistical analyses were performed using SPSS 16.0 (IBM Corp., Chicago, IL, USA), and a P value <0.05 was considered statistically significant.
Results
A total of 3,191 PICCs were initially enrolled, among which 255 cases were excluded mainly due to the presence of non-sinus rhythm (n=116). There were 73 cases without postprocedural CXR which were excluded, and no patients in this cohort developed clinical PICC-related complications. The complete screening procedure is displayed in Figure 2.
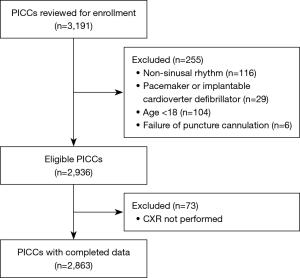
The analysis included 2,863 samples from 2,653 patients with complete records, in which 184 patients received 2 or more PICCs. Patient demographic characteristics are presented in Table 1. The average age of the patients was 55.5 years (IQR: 47–65 years). Males accounted for 49.2%. The majority of PICCs (73.2%) were inserted in the right arm. The basilic vein was the most common site of placement (84%; n=2,404), followed by the brachial vein (14.3%; n=408), and the cephalic vein (1.8%; n=51). There were 91% of the PICCs reached the desired position in the first attempt.
Table 1
| Demographics | Total |
|---|---|
| Age (years) | 55.5 [47–65] |
| Sex, n (%) | |
| Male | 1,410 (49.2) |
| Female | 1,453 (50.8) |
| Body mass index (kg/m2) | 23.8 (21.2–26.0) |
| Insertion arm, n (%) | |
| Left | 766 (26.8) |
| Right | 2,097 (73.2) |
| Insertion vein, n (%) | |
| Basilic | 2,404 (84.0) |
| Brachial | 408 (14.3) |
| Cephalic | 51 (1.8) |
| Vascular diameter (cm) | 0.41 (0.35–0.45) |
| Vascular depth (cm) | 0.90 (0.62–1.11) |
| Arm circumference (cm) | 26.77 (25.00–28.60) |
| No. of delivered attempts, n (%) | |
| 1 | 2,604 (91.0) |
| >1 | 259 (9.0) |
| Misplacement during PICC insertion, n (%) | 210 (7.3) |
| Misplacement following PICC insertion, n (%) | 20 (0.7) |
Data are presented as n (%) and median (interquartile range). PICC, peripherally inserted central venous catheter.
Misplacement
A total of 210 misplaced PICCs (7.3%) were identified by ultrasound and IC-ECG, and all of them were repositioned to the superior vena cava (SVC) by individualized treatment methods. However, there were still 20 PICCs (0.70%) misplaced on CXR, including 19 cases via the basilic vein, 1 case via the brachial vein, 16 cases via the left arm, and 4 cases via the right arm. All of them were misplaced into the azygos vein (Figures 3,4). The most common site of primary malposition was the internal jugular vein in 158 cases (158/210, 75.2%), followed by the axillary vein (27/210, 12.9%), and the cephalic vein (16/210, 7.6%). The vessels and veins with misplaced PICCs are shown in Table 2.


Table 2
| Ectopic vessels | Left arm | Right arm | |||||
|---|---|---|---|---|---|---|---|
| Basilic vein | Brachial vein | Cephalic vein | Basilic vein | Brachial vein | Cephalic vein | ||
| Azygos vein | 16 | 0 | 0 | 3 | 1 | 0 | |
| Subclavian vein | 4 | 0 | 0 | 4 | 1 | 0 | |
| Axillary vein | 4 | 4 | 0 | 11 | 4 | 4 | |
| Jugular vein | 20 | 7 | 0 | 121 | 9 | 1 | |
| Cephalic vein | 4 | 5 | 1 | 5 | 1 | 0 | |
In the univariate analysis, intraprocedural catheter misplacement was associated with arm circumference (OR: 0.952; 95% CI: 0.907–0.999; P=0.044), and the number of insertions (OR: 5.958; 95% CI: 4.849–7.322; P<0.001). The primary catheter misplacement was associated with left/right arm insertion (OR: 11.163; 95% CI: 3.720–33.495; P<0.001), with a significantly higher rate of misplacement via the left arm than the right arm, and there was a correlation with patient age (OR: 1.029; 95% CI: 0.992–1.067; P=0.126) (Table 3).
Table 3
| Predictors | Misplacement during PICC insertion | Misplacement following PICC insertion | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | 0.99 (0.99–1.007) | 0.548 | 1.029 (0.992–1.067) | 0.126 | |
| Sex (male vs. female) | 1.099 (0.829–1.455) | 0.512 | 1.262 (0.521–3.054) | 0.606 | |
| Height | 1.000 (0.983–1.02) | 0.997 | 1.036 (0.981–1.093) | 0.208 | |
| Weight | 0.992 (0.980–1.003) | 0.161 | 1.007 (0.997–1.017) | 0.169 | |
| BMI | 0.968 (0.932–1.006) | 0.101 | 1.020 (0.983–1.058) | 0.300 | |
| Catheter side (left vs. right) | 0.944 (0.684–1.301) | 0.723 | 11.163 (3.720–33.495) | <0.001 | |
| Cannulated vein | 0.905 | NA | |||
| Brachial | Reference | – | – | – | |
| Basilic | 0.961 (0.646–1.429) | 0.843 | – | – | |
| Cephalic | 0.760 (0.224–2.581) | 0.660 | – | – | |
| Vascular diameter | 0.592 (0.100–3.519) | 0.564 | 8.237 (0.580–116.906) | 0.119 | |
| Vascular depth | 0.835 (0.563–1.240) | 0.371 | 0.896 (0.262–3.062) | 0.862 | |
| Arm circumference | 0.952 (0.907–0.999) | 0.044 | 1.106 (0.955–1.281) | 0.177 | |
| No. of delivered attempts | 5.958 (4.849–7.322) | <0.001 | 0.976 (0.587–1.624) | 0.927 | |
PICC, peripherally inserted central venous catheter; OR, odds ratio; CI, confidence interval; BMI, body mass index; NA, not applicable.
In the multivariate analysis, intraprocedural catheter misplacement was associated with the number of insertions (OR: 5.974; 95% CI: 4.857–7.347; P<0.001). Primary catheter misplacement was associated with left/right arm insertion (OR: 10.583; 95% CI: 3.50–32.00; P<0.001), and patients with left-arm insertion were prone to experience misplacement (Table 4).
Table 4
| Predictors | Misplacement during PICC insertion | Misplacement following PICC insertion | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Arm circumference | 1.011 (0.948–1.078) | 0.748 | |||
| No. of delivered attempts | 5.974 (4.857–7.347) | <0.001 | |||
| Age | 1.028 (0.992–1.066) | 0.126 | |||
| Catheter side (left vs. right) | 10.583 (3.50–32.00) | <0.001 | |||
PICC, peripherally inserted central venous catheter; OR, odds ratio; CI, confidence interval.
All patients with post-insertion misplaced PICCs (n=20) had negative or uncertain ECG and underwent secondary placement, and no misplacements were reviewed after the second placement. No patient experienced complications related to PICC misplacement.
The cost of CXR varies depending on the geographic location. In this analysis, we used our hospital’s quoted cost of $12.50 per CXR. The total number of CXRs performed in this study was 2,863, so the total cost was $35,787 (1.5 years), or $23,858 per year. The cost of using CXR to diagnose 1 case of catheter misplacement, namely, PICC repositioning, was $1,789. PICC placement under fluoroscopic control results in nearly 100% proper tip position (9). However, radiation exposure and staff requirements are increased.
Discussion
The results of this study showed that the incidence of PICC misplacement was 7.3% during PICC insertion, which reduced to 0.7% on postprocedural CXR after intraprocedural adjustments and subsequent IC-ECG check. Cost analysis showed that postprocedural CXR was a very expensive screening test when used to eliminate PICC-related mechanical complications. Notably, all primary catheter misplacements occurred in the azygos vein without any serious complications, and patients had negative or uncertain ECG, which can be used to determine the further use of CXR, thus further clarifying the location of the tip of the catheter.
This study demonstrated that the incidence of catheter misplacement was very low and the cost of routine postprocedural CXR was very high. This finding suggests that IC-ECG can better avoid various catheter tip misplacements. Traditionally, an X-ray examination following PICC insertion is necessary to identify whether the catheter is misplaced (10). Previous guidelines recommend postprocedural CXR to determine catheter tip position, and misplaced catheter tips are usually repositioned and examined by another imaging test to reassure optimal position (11). This procedure may delay the use of the catheter. In addition, possible and unnecessary radiation exposure from CXR should be considered (12). In the ECG test group, the PICC tip position can be adjusted in real time by observing the lead II P wave morphology on the monitor. In the traditional approach, CXRs are required to confirm the correct tip position whenever the PICC tip is readjusted. This increases radiation exposure to patients. In addition, CXRs have important limitations, including subjective interpretation of the position of the tip and respiratory movement of the catheter (which may cause the tip to move as much as 4–5 cm in craniocaudal direction) (13,14).
We also determined that placement via the left arm increased the incidence of catheter misplacement, by around 10 times that of right-sided insertion. There have been no previous studies of left- or right-sided insertion for PICC misplacement. A previous study found a significantly increased risk of central venous catheter tip misplacement by left-sided insertion (15). Another study found that there was no significant difference in the incidence of tunneled hemodialysis catheter misplacement to the azygos vein by left- or right-sided insertion (16). The exact reason is unclear. In contrast, on the left side, the route from the left internal jugular vein (LIJV) to the SVC is more complicated as it passes through 2.5 inches of the inclined downward and arcuate, left innominate vein (LIV). This route is s-shaped with 2 bands between the LIJV and LIV and again between LIV and SVC. The LIV has far more tributaries than the right innominate vein (17) and allows more opportunity for guidewire or catheter tip diversion and malposition. This appears to be the mechanism of catheter misplacement into the azygos vein (18).
These findings suggest that routine postprocedural CXR is not necessary for routine placement. However, it still should be considered in case of a high suspicion of catheter misplacement, such as after multiple attempts of insertion, or if the insertion site is not right-sided and there was no dynamic change in the ECG.
This study focused on perioperative PICC placement performed under ultrasound guidance. The results of this study may not be applicable to other clinical settings, as the use of ultrasound significantly reduces the dependency on the practitioner’s experience in vascular cannulation. This study was performed in 2 university hospitals in China, where ultrasound machines are widely used, and the operators have extensive experience with ultrasound-guided cannulation. The applicability of our findings to other clinical settings or to operators with different levels of experience must be considered on an individual basis.
In our study, the primary malposition rate and the incidence of vessels with misplaced catheters were similar to other studies (10). Our primary malposition rate was significantly decreased after intraprocedural adjustments by IC-ECG, and all primary catheter misplacements occurred in the azygos vein with an incidence of 0.7%. This indicates that the IC-ECG method may be inconclusive if the catheter tip is misplaced into the azygos vein and thus may not prevent this event. Even if the placement is successful, inappropriate patient movement or high intracranial pressure during severe nausea, vomiting, hiccups, and constipation may also cause misplacement, termed secondary malposition (19). In the present study, we monitored normal blood return with aspiration catheter after IC-ECG examination during the operation, but found misplacement of the catheter tip on postprocedural CXR. Besides, abnormalities were found on a repeated catheter aspiration and IC-ECG localization. Therefore, whether the catheter tip is misplaced into the azygos vein due to the patient’s body position change after the placement still needs further investigation.
Recently, there has been a significant focus on the “Choosing Wisely” movement in the North American health care system, with the aim of reducing low-value and wasteful tests or procedures (20,21). These unnecessary tests and interventions fail to bring meaningful benefits to patients and can be potentially harmful, requiring additional effort to investigate false positives, which is a significant waste of resources for both patients and health professionals. More than 50 medical societies are involved in this movement and have compiled lists of the most commonly overused tests or procedures in various relevant clinical areas (20). In the present study, our findings suggested that routine CXR after the use of ultrasound- and IC-ECG-guided PICCs is not a wise choice and should be included in the list of unnecessary test and procedures.
However, it is difficult to make changes in clinical practice (22,23), and there is a need for cultural awareness, physician perception, and redesign of patient care processes and related guidelines (24). This can be facilitated by the combination of ultrasound-guided PICC implantation and subsequent ECG localization.
Limitations
A major limitation of this study is the unavoidable information bias due to the retrospective design. To ensure that information bias was minimized, we evaluated each patient’s electronic CXR film and radiologist’s report to verify any mechanical PICC-related complications. We also conducted data verification by a second evaluator. The other limitation is the lack of data on the duration of each PICC placement. Moreover, the cost analysis for this study was based on cost estimates for a single CXR at our institute, which may vary at other institutions.
Conclusions
Our results show that PICC misplacement after ultrasound guidance is rare and postprocedural CXR has a low predictability to determine an intervention for PICC-related mechanical complications. Besides, postprocedural CXR is high-cost. We conclude that once the ECG-guided system is identified to be highly accurate, less expensive, and safer, it will be further understood routine postprocedural CXR is unnecessary and also uneconomical in our setting. The applicability of our results in other clinical settings must be considered on an individual basis.
Acknowledgments
We thank the PICC team nurses of Peking University Cancer Hospital & Institute and The Affiliated Qingdao Central Hospital of Qingdao University who judiciously maintain the PICC database.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5417/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5417/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5417/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Peking University Cancer Hospital & Institute and The Affiliated Qingdao Central Hospital of Qingdao University Ethics Committee (approval ID: 2020KT56, IEC-AF-081-03.0). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mitbander UB, Geer MJ, Taxbro K, et al. Patterns of use and outcomes of peripherally inserted central catheters in hospitalized patients with solid tumors: A multicenter study. Cancer 2022;128:3681-90. [Crossref] [PubMed]
- Taglialatela I, Mariani L, Dotti KF, et al. Central venous catheters-related-thrombosis and risk factors in oncological patients: a retrospective evaluation of recent risk scores. Tumori 2022; Epub ahead of print. [Crossref]
- He B, Zhang A, He S. Therapeutic Effect of Ultrasound-Guided Peripherally Inserted Central Catheter Combined with Predictive Nursing in Patients with Large-Area Severe Burns. Comput Math Methods Med 2022;2022:1019829. [Crossref] [PubMed]
- Pittiruti M, Bilancia A, Ortiz Miluy G, et al. A comparison between two radiological criteria for verifying tip location of central venous catheters. J Vasc Access 2022. [Epub ahead of print]. doi:
10.1177/11297298221126818 . - Liu YJ, Dong L, Lou XP, et al. Evaluating ECG-aided tip localization of peripherally inserted central catheter in patients with cancer. Int J Clin Exp Med 2015;8:14127-9.
- Oliver G, Jones M. ECG-based PICC tip verification system: an evaluation 5 years on. Br J Nurs 2016;25:S4-S10. [Crossref] [PubMed]
- Li A, Jiao J, Zhang Y, et al. A randomized controlled study of bedside electrocardiograph-guided tip location technique & the traditional chest radiography tip location technique for peripherally inserted central venous catheter in cancer patients. Indian J Med Res 2018;147:477-83. [Crossref] [PubMed]
- Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med 2015;163:S1-40. [Crossref] [PubMed]
- Gebauer B, Teichgräber UK, Podrabsky P, et al. Ultrasound- and fluoroscopy-guided implantation of peripherally inserted central venous catheters (PICCs). Rofo 2004;176:386-91. [Crossref] [PubMed]
- Song L, Li H. Malposition of peripherally inserted central catheter: Experience from 3,012 patients with cancer. Exp Ther Med 2013;6:891-3. [Crossref] [PubMed]
- Infusion Nurses Society. Infusion Nursing Standards of Practice. J Infus Nurs 2006;29:S1-92. [Crossref] [PubMed]
- Expert Panel on Thoracic Imaging. ACR Appropriateness Criteria® Routine Chest Radiography. J Thorac Imaging 2016;31:W13-5. [Crossref] [PubMed]
- Connolly B, Amaral J, Walsh S, et al. Influence of arm movement on central tip location of peripherally inserted central catheters (PICCs). Pediatr Radiol 2006;36:845-50. [Crossref] [PubMed]
- Gnannt R, Connolly BL, Parra DA, et al. Variables decreasing tip movement of peripherally inserted central catheters in pediatric patients. Pediatr Radiol 2016;46:1532-8. [Crossref] [PubMed]
- Weber E, Liberek T, Wołyniec W, et al. Catheter tip malposition after percutaneous placement of tunneled hemodialysis catheters. Hemodial Int 2015;19:509-13. [Crossref] [PubMed]
- Li XY, Ye JB, Zhang LG, et al. Misplacement of Tunneled Hemodialysis Catheter into Azygos Vein: Left or Right Jugular Insertion Has Similar Susceptibility. Blood Purif 2019;48:1-9. [Crossref] [PubMed]
- Saremi F, Vojdani E, Vorobiof G, et al. Right to left shunting through communications between the left superior intercostal vein tributaries and the left atrium: a potential cause of paradoxical embolism. Int J Cardiol 2013;167:2867-74. [Crossref] [PubMed]
- Granata A, Figuera M, Castellino S, et al. Azygos arch cannulation by central venous catheters for hemodialysis. J Vasc Access 2006;7:43-5.
- Qiu XX, Guo Y, Fan HB, et al. Incidence, risk factors and clinical outcomes of peripherally inserted central catheter spontaneous dislodgment in oncology patients: a prospective cohort study. Int J Nurs Stud 2014;51:955-63. [Crossref] [PubMed]
- Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med 2014;89:990-5. [Crossref] [PubMed]
- Brody H. Medicine's ethical responsibility for health care reform--the Top Five list. N Engl J Med 2010;362:283-5. [Crossref] [PubMed]
- Parks AL, O'Malley PG. From Choosing Wisely to Practicing Value-More to the Story. JAMA Intern Med 2016;176:1571-2. [Crossref] [PubMed]
- Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med 2015;175:1913-20. [Crossref] [PubMed]
- Parks AL, O'Malley PG. Applying Academic Detailing and Process Change to Promote Choosing Wisely-Reply. JAMA Intern Med 2017;177:283. [Crossref] [PubMed]
(English Language Editor: J. Jones)


