Detection of HER2 polymorphism and expression using circulating DNA and RNA as a tool in lung adenocarcinoma patients: a case control study
Introduction
Globally, every year million patients are diagnosed with lung cancer, making it most common type of cancer worldwide (1). Non-small cell lung cancer (NSCLC) accounts nearly 80% of all lung cancers and became the major cause of mortality (2,3). Patients detected at late stage of disease limits the treatment option for NSCLC patients and most of the cancer patients diagnosed at the metastatic stage (2). HER2 aberrations are more prevalent in adenocarcinoma patients (4) and it was observed that HER2 is associated with tumorigenesis, metastases of disease and worse clinical prognosis in patients. Ligand binding causes receptor dimerization and passes signal by auto-phosphorylation of HER2 tyrosine kinase domain and activates the target proteins, such as mTOR, Src, STAT, MAPK (5). HER2 gene amplification and overexpression occurs in lung cancer and contributed to worse prognosis (6-8). Functional implications of HER2 polymorphisms might result in increased autophosphorylation and tyrosine kinase activity (9,10), single nucleotide polymorphisms (SNPs) are the most common genetic variation, and that may contribute to an individual’s cancer susceptibility (11,12). It was analysed that HER2 is highly expressed in several solid tumours and increased HER2 gene expression has been associated with poor prognosis of patients (13,14). HER2 gene activation, overexpression and its potential prognostic relevance in NSCLC is still under evaluation (15,16). The over-expression of HER2 has found to be associated with disease aggressiveness, increased mortality and higher relapse ratio (17,18), and over-expression of HER2 also an indicative of increased metastatic behaviour cancer cells (7). Main features of cancer cells are the apoptosis resistance (19) and HER2 over-expression defines apoptosis suppression in breast cancer patients (20). Apoptosis suppression, over-expression of HER2 has been linked to disrupt both, intrinsic and extrinsic apoptotic pathways and it is also required to maintain HER2 expression for HER2 mediated suppression of apoptosis (21). The potential clinical relevance of HER2 gene expression in NSCLC is still under evaluation (16), however, the current role of HER2 gene amplification in the resistance to tyrosine kinase inhibitor is reported in 12–13% of patients (22). Thus the present study aimed to analyze the clinical usefulness of circulating DNA and RNA in detection of HER2 promoter polymorphism and HER2 mRNA expression in Lung adenocarcinoma patients is first study from India in which HER2 (-3444C/T) gene promoter polymorphism were analysed with its expression.
Methods
Study population and sample collection
This study was conducted in a cohort of 100 histopathologically confirm newly diagnosed lung adenocarcinoma patients and 100 age and sex matched healthy controls. Study was approved by institutional ethics committee of Maulana Azad Medical College & Associated Hospitals and All India Institute of Medical Sciences New Delhi. After informed consent, patients’ 3 mL blood sample was collected from each subject before any treatment and serum was separated and stored at –80 °C until circulating DNA, RNA extraction from cases as well as from healthy controls, patients and healthy controls serum sample processed and stored in similar manner. Patients included in study were followed from the 2013 to 2015 for overall survival analysis.
Circulating DNA, RNA isolation and cDNA synthesis
Circulating DNA was extracted using commercially available kit (Epigentech, USA) following manufacturer’s protocol and circulating RNA was extracted by Trizol reagent according to the manufacturer’s protocol (AMRESCO, USA) of lung adenocarcinoma cases as well as from healthy control serum stored at –80 °C. Quality of DNA and RNA was checked by Nanodrop and cDNA was synthesized by using 100 ng total RNA following manufacturers protocol (Verso, Thermo Scientific, USA). Briefly, 100 ng of total RNA, 5X cDNA synthesis buffer, dNTPs (5 mM each), RT enhancer, Verso enzyme mix and random hexamers (400 ng/μL) in the total volume of 20 μL incubated for 60 min at 42 °C.
Genotyping and quantitative real time polymerase chain reaction (PCR)
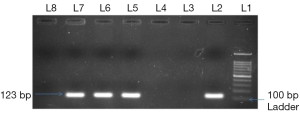
The -3444C/T (rs2643194) promoter polymorphism of HER2 gene was genotyped using allele specific (AS) PCR using circulating DNA with forward wild type primer 5'-ATGGC GTCCACAGTAGCTTTC-3'; forward mutant type primer 5'-ATGGCGTCCA CAGTAGCTTTT-3'; and common reverse primer 5'-CTTAGAGGCCATCGGGATGTTA-3'. PCR was performed in 25 μL reaction volume containing 3 μL of 100 ng template circulating DNA, 0.25 μL 25 pmol each primer, 10 μL of mastermix containing 10 mM dNTPs, 20 mM MgCl2, 5 U/μL Taq polymerase with 10× Taq Buffer (Fermantas) and 25 μL reaction volume was maintained by adding nuclease-free ddH2O followed by programme 10 min of initial denaturation at 95 °C and 40 cycles at 95 °C for 40 s, 60 °C for 40 s and 72 °C for 40 s with a final 10 min extension step at 72 °C and PCR product of 123 bp was visualized on 2% agarose gel containing ethidium bromide (Figure 1). HER2 mRNA expression was studied by QRT-PCR (SYBR Green I technology) with β-actin gene as internal control. The primer sequences for HER2 mRNA expression were forward primer 5'-AGTACCTGGGTCTGGACGTG-3', reverse primer 5'-CTGGGAACTCAAGCAGGAAG-3' (23), for β-actin were forward primer 5'-CGACAACGGCTCCGGCATGTGC-3', reverse primer 5'-GTCACCGGAGTCCATCACGATGC-3'. The expression of HER2 and β-actin was performed by PCR programme for 40 cycles, denaturation at 94 °C for 40 s, annealing at 60 °C for 40 s, extension at 72 °C for 40 s and reaction volume was 20 μL. A final extension step at 72 °C for 5 min to complete the reaction and melting curve analysis was performed between the range 40 to 90 °C to ensure the specific amplification. A control without cDNA was included in each experiment as non template control and all reaction were performed in duplicate. The relative quantification method (2−∆∆CT) was used to analyse the circulating HER2 mRNA expression level by using β-actin as internal control and final results were expressed as mean fold change in circulating HER2 mRNA expression in lung adenocarcinoma patients as compared to control.
Statistical analysis
Differences in select demographic variables and HER2 genotype frequencies between the cases and controls were evaluated by using the Chi-square test. The associations between HER2 variant genotypes and risk of lung adenocarcinoma were estimated by computing the odds ratios (ORs) and risk ratio (RR) with 95% confidence intervals (CIs). Allele frequencies between the cases and controls were evaluated using Hardy-Weinberg equilibrium test. Mann Whitney and Kruskal Wallis test were used to analyze the association of gene expression with different variables included in study. The Kaplan-Meier method was used to calculate the survival of lung adenocarcinoma patients. A P value <0.05 was considered indicative of a statistically significant difference. All statistical analyses were performed using the SPSS 16 and Graph Pad version 6.0.
Results
Demographics
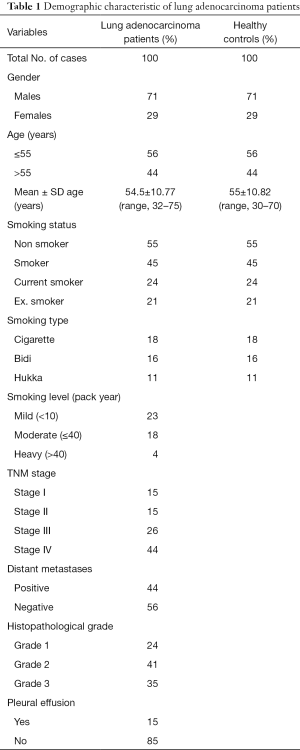
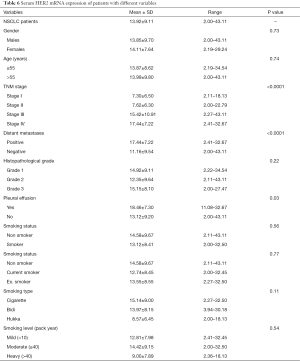
All demographic features of the subjects are depicted (Table 1). In brief, total of 100 lung adenocarcinoma patients were analyzed and healthy controls were age, sex and history of smoking status and type was also matched. This study included both males (71%) and females (29%) and mean age of 54.37 years. A total of 44% patients were in stage IV and 15%, 15%, 26% patients in stage I, II and III respectively while 44% patients had distant metastases. Patients with different pathological grade, grade 1 (well differentiated) includes 24%, grade 2 (moderately differentiated) includes 41% and grade 3 (poorly differentiated) includes 35% cases. We included smoker 45% as well as non smoker 55% with different smoking type as cigarette, bidi, and hukka, 18% cases smoked cigarette, 16% cases smoked bidi and 11% cases smoked hukka.
Full table
Case-control genotype distribution
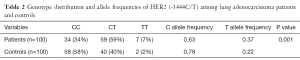
The genotype and allele distribution of HER2 (-3444C/T) in cases and controls are summarised in Table 2.
Full table
We observed a statistically significant difference in the frequency of HER2 CC, CT, and CT genotype among lung adenocarcinoma cases vs. healthy controls (P=0.001). The frequency of T allele (fT) was found to be higher in lung adenocarcinoma cases (0.33) whereas, the higher frequency of C allele (fC) was observed among healthy controls (0.78).
HER2 (-3444C/T) polymorphism and lung adenocarcinoma risk
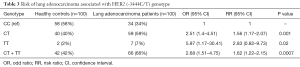
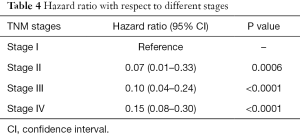
The degree of association between the HER2 (-3444C/T) genotype and risk of lung adenocarcinoma was estimated by calculating OR, RR with 95% CIs and hazard ratio with different stages was also depicted in Tables 3,4 respectively. Compared to the CC genotype, OR 2.51 (1.4–4.51), 5.97 (1.17–30.41) and RR 1.56 (1.17–2.07), 2.83 (0.82–9.73) for heterozygous CT and homozygous TT genotypes were estimated, suggesting possible dominant effect on lung adenocarcinoma risk in Indian population.
Full table
Full table
HER2 (-3444C/T) genotypes and HER2 expression
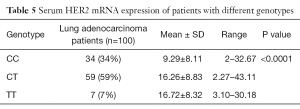
HER2 genotype was found to be significantly associated with expression of HER2 mRNA depicted in Table 5.
Full table
Lung adenocarcinoma cases with CC genotype showed 9.29 fold increased mRNA expression while cases with heterozygous CT and homozygous TT genotype showed 16.26, 16.72 fold increased mRNA expression (P<0.0001).
HER2 mRNA expression and lung adenocarcinoma patients
We analysed circulating HER2 mRNA expression with several variables of lung adenocarcinoma cases in this study and observed increased gene expression was 13.92 fold in NSCLC (adenocarcinoma) patients. Patients in stage I showed 7.3 fold increased HER2 mRNA expression, stage II showed 7.62 fold increased gene expression while in stage III showed 15.42 fold increased HER2 mRNA expression and stage IV showed 17.44 fold increased gene expression which is found to be significantly associated (P<0.0001). Patients with distant metastases had 17.44 fold increased HER2 mRNA expression while patients without metastases had 11.16 fold increased HER2 mRNA expression also showed significant differences (P<0.0001). Patients who had pleural effusion showed 18.46 fold increased HER2 mRNA expression while patients without pleural effusion showed 13.12 fold increased HER2 mRNA expression was also found to be significantly associated (P=0.03) data showed in Table 6.
Full table
Patient’s overall survival with respect to HER2 (-3444C/T) genotype and HER2 expression
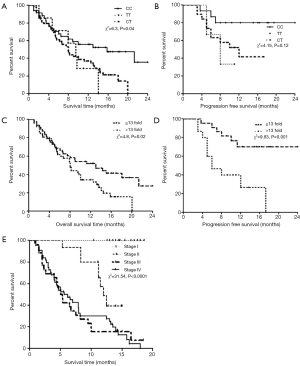
Survival analysis of 100 lung adenocarcinoma patients included in study was analysed by Kaplan-Meier method, and it was observed that the mean follow-up time of the patients was 8.79 months (median, 8.1 months). There were 70 (70%) lung adenocarcinoma-related death events in the patients with a mean follow-up time of 6.6 months (median, 5.3 months), and for the 30 (30%) patients who survived, the mean follow-up time was approximately 13.88 months (median, 14.15 months). Patients with CC, TT, CT (P=0.02) and CT + TT (P=0.008) genotype showed 15.8, 7.9, 9.5 and 7.9 months of overall median survival time and found to be significantly associated, respectively (Figure 2A,B). Patients with >13- and ≤13-fold increased HER mRNA expression showed 7.9 and 11.5 months of overall median survival time was observed and found to be significantly associated (P=0.01) (Figure 2C). It was also observed that patients with >13 fold increased mRNA expression showed 6 months of progression free survival while patients with <13 fold increased showed 12.62 months (Figure 2D). Patients in stage III (5.1 months) and IV (5.6 months) showed reduced overall survival while stage II and I showed 12 and 14.88 months of overall survival respectively (Figure 2E).
Discussion
Single nucleotide alterations are the most common form of genetic variation in human genome, and may contribute individual’s cancer susceptibility (11,12). Several studies demonstrated that polymorphisms in gene alters the gene expression are associated with the risk of lung cancer (24-26). HER2 is a oncogene belonging to the EGFR family of genes, play major role in proliferation, growth, cellular differentiation and apoptosis (27,28) controlled by any of the ErbB-receptor family genes (7). It was found that HER2 overexpression has been associated with disease aggressiveness, increased mortality and higher relapse ratio (17,18). Present study investigated the role of circulating DNA and RNA to evaluate the HER2 gene promoter polymorphism and HER2 mRNA expression in Lung adenocarcinoma patients and statistically significant difference was observed in genotype of HER2 (-3444C/T) in lung adenocarcinoma cases vs. healthy controls. It was observed that heterozygous HER2-3444CT and homozygous TT genotype had more than 2 and 5 fold increased risk of developing lung adenocarcinoma than CC genotype respectively and more death events was observed with homozygous TT and heterozygous CT genotype compare to homozygous CC genotype. To best of our knowledge, this is the first report in genetic association of HER2 (-3444C/T) polymorphism with the lung adenocarcinoma risk and prognosis, suggesting possible role of HER2 gene in the pathogenesis of malignancy. Jo et al. in 2008 (29) found that the HER2 (-3444C/T) polymorphism is associated with the risk of lung cancer in Korean population. Han et al. in 2005 (30) also investigated that the 5'-untranslated HER2 (rs2643195-3444C/T) gene polymorphism was found to be associated with HER2 protein expression and disease outcome in breast cancer and has functional impact on cancer aggressiveness. On the other hand, it was also found that HER2 overexpression is an independent prognostic factor in patients survival outcome and became important target in lung cancer, using an EGFR tyrosine kinase inhibitor along with HER2 dimerization inhibitors (7,8,31). This study also explored the circulating HER2 mRNA expression in lung adenocarcinoma patients and it was found to be significantly associated with TNM stages, metastatic behaviour, pleural effusion and overall survival. The major findings in the study was higher circulating HER2 mRNA gene expression observed in patients with stage III and stage IV in comparison of stage I and stage II and found to be significantly associated. Patients with distant metastases showed 1.56 times higher circulating HER2 mRNA expression in contrast of patients without any distant metastases. It was also observed that the lung adenocarcinoma patients had 1.4 times higher HER2 mRNA expression in patients with pleural effusion vs. patients without pleural effusion. HER2 mRNA expression had significant impact on patients’ overall survival, patients with >13 fold increased gene expression was found to be significantly associated with reduced lung adenocarcinoma patients overall survival. In favour of present statement, Schneider et al. found 4–32-fold higher HER2 gene expression in NSCLC cell lines while 8–32 fold higher HER2 gene expression levels were observed in adenocarcinomas cell lines (A549, H322, H522, and H596) (32). Prognostic relevance of HER2 gene overexpression found to be associated with poor patients’ survival in NSCLC patients (33) and increased HER2 expression levels also have been reported in a number of malignant tumor types, and its gene amplification induces gene overexpression (34). HER2 gene overexpression regulates invasive growth of cancer in association with the cadherin-catenin complex (35) and found to be associated with increased migration capacity of tumor cells (36). HER2 overexpression has been reported in NSCLC and particularly in adenocarcinoma patients (37) and overexpression is associated with poor clinical prognosis and worse patients’ survival (38). It has been also suggested that HER2 overexpression is associated with metastatses behaviour of cells and poor prognosis of patients (39). Alterations of the HER2 proto-oncogene have been defined in the carcinogenesis and prognosis of many cancers, mainly breast cancer and HER2 overexpression leads to apoptosis suppression in breast cancer cells (20,40). The overexpression of HER2 has been found to be associated with disease aggressiveness, increased mortality and higher relapse ratio (17). High expression of HER2 had been shown to be associated with poor prognosis in breast cancer (41) endometrial cancer (42) and ovarian cancer (43).
Conclusions
In conclusion, our work provides evidence that circulating DNA and RNA may be a potential prognostic tool in management and monitoring of Lung adenocarcinoma patients. Promoter polymorphism of HER2 (-3444C/T) gene had significant impact on higher HER2 mRNA expression could be a predictive factor for patients’ worse overall survival, metastatic behaviour of lung adenocarcinoma patients. Understanding the molecular biology of HER2 gene will help in progress of molecular-based treatment of cancer patients having HER2 overexpression. The findings of present study need to be validated further independent and prospective studies on larger population and further investigations would be necessary to clarify the efficacy of HER2 as molecular prognostic tool in lung adenocarcinoma patients.
Acknowledgements
The authors thank to all the study subjects and All India Institute of Medical Sciences, New Delhi, for assistance in recruiting the subjects.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee of Maulana Azad Medical College & Associated Hospitals and All India Institute of Medical Sciences New Delhi and written informed consent was obtained from all patients.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Mayo C, Bertran-Alamillo J, Molina-Vila MÁ, et al. Pharmacogenetics of EGFR in lung cancer: perspectives and clinical applications. Pharmacogenomics 2012;13:789-802. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Liu L, Shao X, Gao W, et al. The role of human epidermal growth factor receptor 2 as a prognostic factor in lung cancer: a meta-analysis of published data. J Thorac Oncol 2010;5:1922-32. [Crossref] [PubMed]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341-54. [Crossref] [PubMed]
- Bae NC, Chae MH, Lee MH, et al. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genet Cytogenet 2007;173:107-13. [Crossref] [PubMed]
- Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 2000;19:6115-21. [Crossref] [PubMed]
- Micke P, Hengstler JG, Ros R, et al. c-erbB-2 expression in small-cell lung cancer is associated with poor prognosis. Int J Cancer 2001;92:474-9. [Crossref] [PubMed]
- Benusiglio PR, Lesueur F, Luccarini C, et al. Common ERBB2 polymorphisms and risk of breast cancer in a white British population: a case-control study. Breast Cancer Res 2005;7:R204-9. [Crossref] [PubMed]
- Puputti M, Sihto H, Isola J, et al. Allelic imbalance of HER2 variant in sporadic breast and ovarian cancer. Cancer Genet Cytogenet 2006;167:32-8. [Crossref] [PubMed]
- Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004;119:591-602. [Crossref] [PubMed]
- Erichsen HC, Chanock SJ. SNPs in cancer research and treatment. Br J Cancer 2004;90:747-51. [Crossref] [PubMed]
- Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist 2002;7 Suppl 4:31-9. [Crossref] [PubMed]
- Brabender J, Danenberg KD, Metzger R, et al. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin Cancer Res 2001;7:1850-5. [PubMed]
- Hirsch FR, Franklin WA, Veve R, et al. HER2/neu expression in malignant lung tumors. Semin Oncol 2002;29:51-8. [Crossref] [PubMed]
- Cagle PT, Chirieac LR. Advances in treatment of lung cancer with targeted therapy. Arch Pathol Lab Med 2012;136:504-9. [Crossref] [PubMed]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51. [Crossref] [PubMed]
- Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res 2006;12:6326-30. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Yu D, Jing T, Liu B, et al. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell 1998;2:581-91. [Crossref] [PubMed]
- Carpenter RL, Lo HW. Regulation of Apoptosis by HER2 in Breast Cancer. J Carcinog Mutagen 2013;2013.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Mahesh K. Differences in Gene Expression Profiles between Human Breast Tissue and Peripheral Blood Samples for Breast Cancer Detection. J Cancer Sci Ther 2012;4:379-85.
- Kiyohara C, Otsu A, Shirakawa T, et al. Genetic polymorphisms and lung cancer susceptibility: a review. Lung Cancer 2002;37:241-56. [Crossref] [PubMed]
- Spinola M, Meyer P, Kammerer S, et al. Association of the PDCD5 locus with lung cancer risk and prognosis in smokers. J Clin Oncol 2006;24:1672-8. [Crossref] [PubMed]
- Kim JH, Kim H, Lee KY, et al. Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet 2006;15:1181-6. [Crossref] [PubMed]
- Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159-67. [Crossref] [PubMed]
- Arman K, Ergün S, Temiz E, et al. The interrelationship between HER2 and CASP3/8 with apoptosis in different cancer cell lines. Mol Biol Rep 2014;41:8031-6. [Crossref] [PubMed]
- Jo UH, Han SG, Seo JH, et al. The genetic polymorphisms of HER-2 and the risk of lung cancer in a Korean population. BMC Cancer 2008;8:359. [Crossref] [PubMed]
- Han W, Kang D, Lee JE, et al. A haplotype analysis of HER-2 gene polymorphisms: association with breast cancer risk, HER-2 protein expression in the tumor, and disease recurrence in Korea. Clin Cancer Res 2005;11:4775-8. [Crossref] [PubMed]
- Canoz O, Ozkan M, Arsav V, et al. The role of c-erbB-2 expression on the survival of patients with small-cell lung cancer. Lung 2006;184:267-72. [Crossref] [PubMed]
- Schneider PM, Hung MC, Chiocca SM, et al. Differential expression of the c-erbB-2 gene in human small cell and non-small cell lung cancer. Cancer Res 1989;49:4968-71. [PubMed]
- Hirsch FR, Varella-Garcia M, Bunn PA Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 2003;21:3798-807. [Crossref] [PubMed]
- Koeppen HK, Wright BD, Burt AD, et al. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology 2001;38:96-104. [Crossref] [PubMed]
- Ochiai A, Akimoto S, Kanai Y, et al. c-erbB-2 gene product associates with catenins in human cancer cells. Biochem Biophys Res Commun 1994;205:73-8. [Crossref] [PubMed]
- Bernstein JJ, Goldberg WJ, Laws ER Jr. Migration of fresh human malignant astrocytoma cells into hydrated gel wafers in vitro. J Neurooncol 1994;18:151-61. [Crossref] [PubMed]
- Kern JA, Slebos RJ, Top B, et al. C-erbB-2 expression and codon 12 K-ras mutations both predict shortened survival for patients with pulmonary adenocarcinomas. J Clin Invest 1994;93:516-20. [Crossref] [PubMed]
- Scheurle D, Jahanzeb M, Aronsohn RS, et al. HER-2/neu expression in archival non-small cell lung carcinomas using FDA-approved Hercep test. Anticancer Res 2000;20:2091-6. [PubMed]
- Harpole DH Jr, Herndon JE 2nd, Wolfe WG, et al. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res 1995;55:51-6. [PubMed]
- Shintani S, Nakahara Y, Li C, et al. HER2/neu expression in oral squamous cell carcinoma. Asian J Oral Maxillofac Surg 2004;16:172-6. [Crossref]
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [Crossref] [PubMed]
- Morrison C, Zanagnolo V, Ramirez N, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol 2006;24:2376-85. [Crossref] [PubMed]
- Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 2000;19:6102-14. [Crossref] [PubMed]


