
Efficacy and safety of nimotuzumab combined with chemoradiotherapy in the treatment of locally advanced cervical cancer
Highlight box
Key findings
• Nimotuzumab combined with chemoradiotherapy (CCRT) is well tolerated and more effective than CCRT alone in treating locally advanced cervical cancer (LACC).
What is known and what is new?
• LACC patients who receive CCRT alone have a high recurrence rate with about 60% of 5-year overall survival;
• This study used Nimotuzumab combined with CCRT in the treatment of LACC, significantly improve the clinical benefits of LACC patients without the increase of adverse events.
What is the implication, and what should change now?
• Nimotuzumab combined with CCRT in the treatment of LACC improve patients’ short-term and long-term efficacy. This treatment should be further widely used in clinic.
Introduction
Cervical cancer is the most common malignant tumor of the female reproductive system. It is estimated that about 80% of patients are at a locally advanced stage [International Federation of Gynecologists and Obstetricians (FIGO) stage IB2–IVA] at initial diagnosis (1,2). Most patients with locally advanced cervical cancer (LACC) lose the opportunity for surgery, while the primary therapy is platinum-based concurrent chemoradiotherapy (CCRT) (3). Patients who receive CCRT have a 5-year overall survival (OS) of 59.8% and a short-term recurrence rate of about 40% (4-6). Improving the local control rate and prolonging survival is the focus of clinical research in LACC. Epidermal growth factor receptor (EGFR) is overexpressed in cervical cancer tissues and associated with poor prognosis (7,8). Compared with chemotherapy, nimotuzumab, as an anti-EGFR targeted therapy, can more accurately inhibit tumor progression, and reduce damage to normal cells, and thus reduce the occurrence of adverse events (AEs). As China’s first humanized anti-EGFR monoclonal antibody, nimotuzumab has been shown to significantly improve the 3-year OS for nasopharyngeal carcinoma in combination with CCRT (9). Furthermore, the same trend of increasing efficacy can also be seen in other clinical studies of LACC (9-11), but these studies have some limitations such as single arm study or small sample size, which needs more clinical studies to prove this conclusion. In our study, we obtained the efficacy and safety data of 120 patients with LACC who received CCRT with or without nimotuzumab, observed and compared the efficacy and safety of the two groups of patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5739/rc).
Methods
Patients
We designed a retrospective comparative cohort study and analyzed 120 patients who had LACC and were treated by CCRT with or without nimotuzumab in The Affiliated Hospital of Qingdao University between March 2017 and December 2019. Patients were assigned to the nimotuzumab plus chemoradiotherapy (N + CCRT) group (65 cases) or the CCRT group (55 cases). The inclusion criteria were as follows: (I) cervical cancer (squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma) confirmed by biopsy; (II) stage IB2–IVA (12); (III) Eastern Cooperative Oncology Group Physical performance status (ECOG PS) score <3. Patients were excluded for the following reasons: (I) they were receiving other anti-EGFR-targeted therapy; (II) they were pregnant or breastfeeding, or (III) they had severe chronic disease. We collected the medical records data from our database, which included age, pathological type, stage, treatment, efficacy, and AEs. Efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), the long-term efficacy were OS and progression-free survival (PFS), and the short-term efficacy were complete response rate (CRR), objective response rate (ORR).AEs were assessed according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0), mainly include fever, fatigue, gastrointestinal reactions, bone marrow suppression, radiation enteritis, radiation cystitis and hepatic impairment. There was no statistical difference in demographic and clinical characteristics between the two groups at baseline (P>0.05, Table 1). The study was performed in accordance with the Declaration of Helsinki (as revised in 2013), and all methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols of this study were approved by the Ethics Review Committee of The Affiliated Hospital of Qingdao University (No. QYFY-WZLL-27211). Informed consent was obtained from all subjects or their legal guardians.
Table 1
| Characteristics | N + CCRT group (N=65), n (%) |
CCRT group (N=55), n (%) |
P value |
|---|---|---|---|
| Age, years, mean ± SD, years | 54.66±9.89 | 56.35±8.18 | 0.317 |
| Histology | 0.735 | ||
| Squamous cell carcinoma | 57 (87.69) | 47 (85.45) | |
| Adenocarcinoma | 7 (10.77) | 7 (12.73) | |
| Adenosquamous | 1 (1.54) | 1 (1.82) | |
| ECOG PS score | 0.811 | ||
| 0 | 17 (26.15) | 12 (21.82) | |
| 1 | 45 (69.23) | 41 (74.55) | |
| 2 | 3 (4.62) | 2 (3.64) | |
| FIGO stage | 0.710 | ||
| IB | 4 (6.15) | 4 (7.27) | |
| II | 29 (44.62) | 24 (43.64) | |
| III | 29 (44.62) | 21 (38.18) | |
| IVA | 3 (4.62) | 6 (10.91) | |
| Differentiation | 0.699 | ||
| Low | 21 (32.31) | 19 (34.55) | |
| Medium | 40 (61.54) | 29 (52.73) | |
| High | 4 (6.15) | 7 (12.73) |
N + CCRT, nimotuzumab plus chemoradiotherapy; CCRT, concurrent chemoradiotherapy; SD, standard deviation; ECOG PS, Eastern Cooperative Oncology Group Physical performance status; FIGO, International Federation of Gynecologists and Obstetricians.
Treatment
A total of 120 patients received CCRT after 1–2 cycles of chemotherapy followed by 0–2 cycles of consolidation chemotherapy. We added nimotuzumab to patients with EGFR positive expression. The N + CCRT group received nimotuzumab combined with chemotherapy (400 mg once every 3 weeks, 2–4 cycles; CCRT: 200 mg weekly, 6–7 cycles).
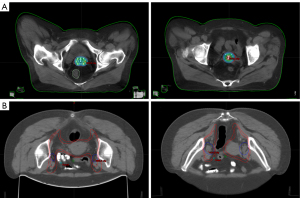
The chemotherapy regimen was paclitaxel + cisplatin (TP). Radiotherapy (RT) included external beam radiation therapy (EBRT) and intracavitary brachytherapy (ICBT). The target areas of RT were designed by pelvic enhanced computed tomography (CT) images based on the American Radiation Therapy Oncology Collaborative Group (RTOG) standards. The gross target volume (GTV) and gross target volume of lymph nodes (GTV-n) were defined as tumors and metastatic lymph nodes, respectively. The high-risk clinical target volume (CTV-hr) included the GTV, parametrium, cervix, partial or total vagina, and GTV-n. The low-risk clinical target volume (CTV-lr) included the CTV-hr and other lymph node drainage areas for preventive irradiation. The dose of CTV-hr was 54–56 Gy/26–28 fraction (f), and the dose of CTV-lr was 45–47 Gy. Patients with lower 1/3 vaginal involvement received preventive irradiation to the inguinal lymph nodes at the same dose as CTV-lr. EBRT used intensity-modulated radiation therapy (IMRT) with a source of 6 mV X-rays; ICBT was initiated after approximately 15 EBRTs of Ir-192. The equivalent dose in 2 Gy/f (EQD2) was 80–90 Gy. The treatment flow diagram of the two groups is shown in Figure 1, and the target areas of RT are shown in Figure 2.

Symptomatic treatment was given to patients with liver dysfunction, severe gastrointestinal reactions, bone marrow suppression, and other adverse reactions during chemotherapy, and then the dose of chemotherapy was maintained or reduced. Chemotherapy was withdrawn if patients could not tolerate the AEs from treatment.
Follow-up
Patients were followed up at least every three months for the first three years and every six months afterward. Medical history records, physical examination, pelvic CT or magnetic resonance imaging (MRI), and gynecologic color ultrasound were performed at each follow-up. Patients who did not undergo further consultation were followed up by telephone. The last follow-up was updated on June 1, 2022.
Statistical analysis
SPSS 21.0 (IBM, USA) was used for the statistical analysis. Measured data were expressed as mean ± standard deviation. T-tests were used for comparisons between groups. The quantitative index was converted into frequency and percentage. The chi-square test was used for comparisons between the two groups. Survival curves were plotted using the Kaplan-Meier method, and a two-sided log-rank test for equality was used at a 5% significance level.
Results
Patient characteristics
A total of 120 patients were included in this study, with 65 assigned to the N + CCRT group and 55 to the CCRT group.
Completion of treatment
In the N + CCRT group, 64 patients completed RT, and one completed EBRT but rejected ICBT. In the CCRT group, two patients failed to complete RT due to AEs (one with radiation enterocolitis and the other with grade 4 bone marrow suppression, Table 2). The median dose of EQD2 was 84.31 and 84.42 Gy in the N + CCRT and CCRT groups, respectively. There was no significant difference between the two groups in the EBRT and ICBT doses, RT cycles, or chemotherapy cycles (P>0.05).
Table 2
| Characteristics | N + CCRT group (N=65), mean ± SD |
CCRT group (N=55), mean ± SD |
P value |
|---|---|---|---|
| Cycles of chemotherapy | 3.91±1.62 | 4.05±1.55 | 0.614 |
| EBRT | |||
| Dose of CTV-hr, Gy | 55.12±0.74 | 55.07±0.88 | 0.734 |
| ICBT | |||
| Total dose, Gy | 22.25±3.25 | 22.22±3.54 | 0.964 |
| EQD2, Gy | 28.83±4.26 | 28.97±4.69 | 0.860 |
| Total EQD2, Gy | 83.95±4.22 | 84.04±4.70 | 0.908 |
| Radiotherapy, days | 48.20±6.44 | 49.67±10.90 | 0.361 |
N + CCRT, nimotuzumab plus chemoradiotherapy; CCRT, concurrent chemoradiotherapy; SD, standard deviation; EBRT, external beam radiation therapy; CTV-hr, high-risk clinical target volume; ICBT, intracavitary brachytherapy; EQD2, equivalent dose in 2 Gy/fraction.
Short-term efficacy
The CRR of the N + CCRT group was 86.15% (56/65), significantly higher than that of the CCRT group [70.91% (39/55), P=0.040]. The ORR was 92.31% (60/65) vs. 87.27% (48/55) in the two groups, respectively (P=0.360). The results are shown in Table 3.
Table 3
| Response | N + CCRT group (N=65), n (%) |
CCRT group (N=55), n (%) |
P value |
|---|---|---|---|
| CR | 56 (86.15) | 39 (70.91) | 0.040 |
| PR | 4 (6.15) | 9 (16.36) | 0.073 |
| ORR | 60 (92.31) | 48 (87.27) | 0.360 |
| SD | 0 (0.00) | 2 (3.64) | 0.121 |
| PD | 5 (7.69) | 5 (9.09) | 0.782 |
N + CCRT, nimotuzumab plus chemoradiotherapy; CCRT, concurrent chemoradiotherapy; CR, complete response; PR, partial response; ORR, objective response rate (CR + PR); SD, stable disease; PD, progressive disease.
Long-term efficacy
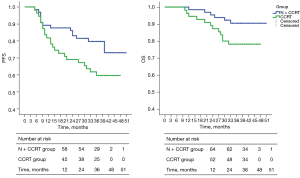
The median duration of follow-up for the whole cohort was 37 months (10–51 months). The 1-, 2-, and 3-year follow-up rates in the N + CCRT group were 100.00%, 100.00%, and 81.54%, respectively, and were 100.00%, 100.00%, and 89.09%, respectively, in the CCRT group.
The 1-, 2-, and 3-year cumulative survival rates of the N + CCRT group vs. the CCRT group were 98.46% vs. 94.55%, 95.38% vs. 87.27%, and 90.50% vs. 78.18% (log-rank test), respectively. There was no significant difference between the two groups (P=0.056).
The 1-, 2-, and 3-year cumulative PFS rates of the two groups were 89.23% vs. 81.82%, 83.08% vs. 69.09%, and 79.73% vs. 59.69% (log-rank test), respectively, and were significantly higher in the N + CCRT group than in the CCRT group (P=0.039). The results are shown in Figure 3.
We also compared patients with 1-, 2-, and 3-year follow-ups. In the N + CCRT group, the percentage of patients who survived in the first, second, and third years was 98.46% (64/65), 95.38% (62/65), and 88.68% (47/53), respectively. The percentage of patients who were progression-free in the first, second, and third years was 89.23% (58/65), 83.08% (54/65), and 75.47% (40/53), respectively. In the CCRT group, the percentage of patients who survived in the first, second, and third years was 94.55% (52/55), 87.27% (48/55), and 75.51% (37/49), and the percentage of patients who were progression-free was 81.82% (45/55), 69.09% (38/55) and 55.10% (27/49), respectively. The results are shown in Figure 4.
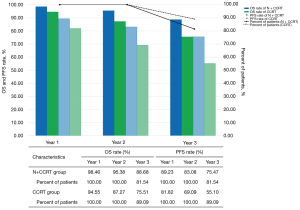
Patients who did not reach the 3-year follow-up were excluded when calculating the proportions of 3-year overall and PFS.
AEs
The most common AEs were bone marrow suppression (95.38% vs. 92.73%) and nausea (86.15% vs. 90.91%). No patient died due to AEs. There was no significant difference in the incidence of adverse reactions between the two groups (P>0.05). The AEs are shown in Table 4.
Table 4
| Adverse events | N + CCRT group (N=65), n (%) |
CCRT group (N=55), n (%) |
P |
|---|---|---|---|
| Fever | 5 (7.69) | 3 (5.45) | 0.624 |
| Fatigue | 6 (9.23) | 6 (10.90) | 0.760 |
| Gastrointestinal reactions | 0.627 | ||
| Stage 0 | 9 (13.85) | 5 (9.09) | |
| Stage 1 | 21 (32.31) | 14 (25.45) | |
| Stage 2 | 23 (35.38) | 24 (43.64) | |
| Stage 3 | 12 (18.46) | 12 (21.82) | |
| Stage 4 | 0 (0.00) | 0 (0.00) | |
| Bone marrow suppression | 0.780 | ||
| Stage 0 | 3 (4.62) | 4 (7.27) | |
| Stage 1 | 9 (13.85) | 7 (12.73) | |
| Stage 2 | 19 (29.23) | 16 (29.09) | |
| Stage 3 | 22 (33.85) | 14 (25.45) | |
| Stage 4 | 12 (18.46) | 14 (25.45) | |
| Hemoglobin, mean ± SD | |||
| Before treatment | 117.83±22.76 | 121.15±19.73 | 0.400 |
| During treatment | 96.17±17.33 | 95.65±15.33 | 0.865 |
| After treatment | 112.92±15.73 | 113.27±16.33 | 0.905 |
| Radiation enteritis | 0.735 | ||
| Stage 0 | 32 (49.23) | 24 (43.64) | |
| Stage 1–2 | 30 (46.15) | 27 (49.09) | |
| Stage 3 | 3 (4.62) | 4 (7.27) | |
| Radiation cystitis | 0.901 | ||
| No occurrence | 49 (75.38) | 42 (76.36) | |
| Occurrence | 16 (24.62) | 13 (23.64) | |
| Hepatic impairment | 8 (12.31) | 7 (12.73) | 0.945 |
N + CCRT, nimotuzumab plus chemoradiotherapy; CCRT, concurrent chemoradiotherapy; SD, standard deviation.
Discussion
Nowadays, platinum-based CCRT remains the primary treatment for LACC patients, with paclitaxel and cisplatin as the most commonly used chemotherapeutic agents. EBRT is the current standard technique for RT. The combination of IMRT and ICBT can improve the efficacy and safety of pelvic RT (13-15). However, it has been reported that recurrence or metastasis occurs in 35–40% of LACC patients after chemoradiotherapy, with a 5-year OS of only 70% (5,16). The current study concluded that consolidation chemotherapy after CCRT not only improved PFS and OS in LACC patients (17) but also increased the incidence of grade 3 and 4 bone marrow suppression (P<0.05) (18). RTOG 0417 was a clinical trial of bevacizumab (an anti-angiogenic agent) combined with CCRT as the primary treatment for LACC patients. The median follow-up time was 3.8 years. The 3-year OS was 81.3%, and the 3-year disease-free survival (DFS) was 68.7%, which was not statistically different from the outcome data from the RTOG 9001 trial (19,20); Tewari et al. (21) found that in cervical cancer, a bevacizumab-containing regimen had a significantly higher risk of hypertension (25.0% vs. 2.0%, P<0.001), genitourinary fistula (6.0% vs. 0.0%, P=0.002) and thrombosis (8.0% vs. 1.0%, P=0.001) than chemotherapy alone. Finding more effective and safe therapies is the current research direction for LACC treatment. For example, in immunotherapy and targeted therapy research, PD-1/PD-L1 immunotherapy in combination with chemotherapy has shown superiority as a first- and second-line treatment of recurrent and metastatic cervical cancer (22-24). A phase 2 clinical study applying the immune checkpoint inhibitor pembrolizumab combined with CCRT as the first-line treatment for LACC patients (25) is ongoing, and the results are awaited with interest.
EGFR is a transmembrane glycoprotein of the ErbB family that regulates various cellular functions. EGFR overexpression is widely found in various malignancies, including head and neck cancer, breast cancer, and cervical cancer (26,27), and can accelerate tumor progression through multiple pathways (28). Nimotuzumab is a humanized EGFR monoclonal antibody that inhibits the proliferation of tumor cells and promotes apoptosis by binding to EGFR. The combination of nimotuzumab and CCRT can improve short- and long-term efficacy in various solid tumors, including nasopharyngeal carcinoma, head and neck squamous, and pancreatic cancer.
Fei et al. (29) found that nimotuzumab plus CCRT for locally advanced nasopharyngeal carcinoma significantly improved 3-year OS compared with CCRT alone (98.0% vs. 91.0%, P=0.032). Rodríguez et al. (26) added nimotuzumab during RT for head and neck squamous carcinoma and significantly increased the CRR (59.5% vs. 34.2%, P=0.028). The application of nimotuzumab in combination with gemcitabine for locally advanced or metastatic pancreatic cancer significantly improved OS (10.9 vs. 8.5 months, P=0.025) and PFS (4.2 vs. 3.6 months, P=0.013), compared with gemcitabine monotherapy (30).
There are still no large-scale clinical studies and few studies of nimotuzumab in cervical cancer. A randomized controlled trial compared the efficacy and safety of nimotuzumab plus CCRT vs. CCRT alone in LACC patients. The experimental group exhibited a significantly higher ORR (87.0% vs. 67.4%, P=0.045) and 3-year PFS (73.9% vs. 50.0%, P=0.042). No significant increase was observed in AEs (31). Also, Chen et al. (10) retrospectively analyzed the case data of LACC patients treated with the same therapy. The result showed that the CRR (78.3% vs. 50.0%, P=0.035) and median PFS (not reached vs. 27 months, P=0.037) were higher in patients treated with nimotuzumab plus CCRT. Nimotuzumab-related AEs mainly included mild fever, chills, gastrointestinal reactions, decreased blood pressure, fatigue, headache, anemia, and rash, but the incidence was low.
In our study, the CRR was significantly higher in LACC patients who received nimotuzumab plus CCRT than those who received CCRT alone (P=0.040). The PFS difference was also statistically significant (P=0.039). For short-term efficacy, ORR in the N + CCRT group was similar to that in the CCRT group, but the CRR was significantly higher, suggesting that the combination of nimotuzumab and CCRT may lead to a shift in efficacy from partial response (PR) to CR. Although we observed a high ORR in both groups, there was no statistical difference. The most likely reason was the small sample size (120 cases). For long-term efficacy, neither PFS nor OS was reached. The 3-year PFS of the N + CCRT group was significantly higher than the CCRT group (P=0.039). The 3-year OS was higher in the N + CCRT group but was not statistically different from the CCRT group (P>0.05). Moreover, patients who reached the 3-year follow-up had an increasing progression-free trend in the N + CCRT group. These results indicated that adding nimotuzumab to CCRT potentially increased the PFS and OS. Further follow-up should be conducted to observe the long-term benefit for LACC patients. Nimotuzumab-related AEs mainly included fever and fatigue, with comparable rates in the two groups. Nimotuzumab combined with CCRT did not increase the adverse reactions of patients.
Our study had three main limitations: (I) it was a single-center retrospective study; (II) the study cohort was small; and (III) there was insufficient follow-up time to assess long-term efficacy. A prospective study with a larger sample size focusing on the long-term outcomes of LACC patients is needed.
Conclusions
Nimotuzumab combined with CCRT is well tolerated and effective in treating LACC and demonstrates higher CR and PFS rates than CCRT alone.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5739/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5739/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5739/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in accordance with the Declaration of Helsinki (as revised in 2013), and all methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols of this study were approved by the Ethics Review Committee of The Affiliated Hospital of Qingdao University (No. QYFY-WZLL-27211). Informed consent was obtained from all subjects or their legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Long X, Guo M, Zhou Q. Radiotherapy for locally advanced cervical cancer Chinese Journal of Practical Gynecology and Obstetrics 2018;34:1193-9.
- Naga Ch P, Gurram L, Chopra S, et al. The management of locally advanced cervical cancer. Curr Opin Oncol 2018;30:323-9. [Crossref] [PubMed]
- Gennigens C, De Cuypere M, Hermesse J, et al. Optimal treatment in locally advanced cervical cancer. Expert Rev Anticancer Ther 2021;21:657-71. [Crossref] [PubMed]
- Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 2019;393:169-82. [Crossref] [PubMed]
- Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. [Crossref] [PubMed]
- Kim GE, Kim YB, Cho NH, et al. Synchronous coexpression of epidermal growth factor receptor and cyclooxygenase-2 in carcinomas of the uterine cervix: a potential predictor of poor survival. Clin Cancer Res 2004;10:1366-74. [Crossref] [PubMed]
- Oh MJ, Choi JH, Kim IH, et al. Detection of epidermal growth factor receptor in the serum of patients with cervical carcinoma. Clin Cancer Res 2000;6:4760-3.
- Cetina L, Crombet T, Jiménez-Lima R, et al. A pilot study of nimotuzumab plus single agent chemotherapy as second- or third-line treatment or more in patients with recurrent, persistent or metastatic cervical cancer. Cancer Biol Ther 2015;16:684-9.
- Chen W, Li T, Wang J, et al. Clinical study of nimotuzumab combined with concurrent radiochemotherapy for treatment of locally advanced cervical cancer. Cancer Manag Res 2019;11:8157-65. [Crossref] [PubMed]
- Chen YF, Tang WB, Pan XX, et al. Safety and efficacy of nimotuzumab combined with chemoradiotherapy in Chinese patients with locally advanced cervical cancer. Onco Targets Ther 2017;10:4113-9. [Crossref] [PubMed]
- Grigsby PW, Massad LS, Mutch DG, et al. FIGO 2018 staging criteria for cervical cancer: Impact on stage migration and survival. Gynecol Oncol 2020;157:639-43. [Crossref] [PubMed]
- Small W Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer 2017;123:2404-12. [Crossref] [PubMed]
- Peiffert D, Hannoun-Lévi JM, Oldrini S, et al. End of the commercialisation of (192)Ir wires in France: proposals of the groupe de Curiethérapie de la SFRO. Cancer Radiother 2014;18:441-6. [Crossref] [PubMed]
- Qian S, Ye L, Tian YH, et al. Californium-252 neutron brachytherapy combined with external pelvic radiotherapy plus concurrent chemotherapy for cervical cancer: a retrospective clinical study. Chin J Cancer 2017;36:24. [Crossref] [PubMed]
- Xu Y, Huang YD, Zeng GL. Assessment of the serum tumor markers and lesion cancer cell proliferation after Nimotuzumab combined with cisplatin and radiotherapy treatment of middle-advanced cervical cancer. Journal of Hainan Medical University 2017;23:1255-8.
- Mileshkin LR, Moore KN, Barnes E, et al. Adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone: The randomized phase III OUTBACK Trial (ANZGOG 0902, RTOG 1174, NRG 0274). J Clin Oncol 2021; [Crossref]
- Kou L, Zhang T, Peng S, et al. Adjuvant chemotherapy after concurrent chemoradiation therapy for locally advanced cervical cancer. J Clin Oncol 2020;38:abstr 6031.
- Schefter T, Winter K, Kwon JS, et al. RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int J Radiat Oncol Biol Phys 2014;88:101-5. [Crossref] [PubMed]
- Wolfson AH, Winter K, Crook W, et al. Are increased tumor aneuploidy and heightened cell proliferation along with heterogeneity associated with patient outcome for carcinomas of the uterine cervix? A combined analysis of subjects treated in RTOG 9001 and a single-institution trial. Int J Radiat Oncol Biol Phys 2008;70:111-7. [Crossref] [PubMed]
- Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370:734-43. [Crossref] [PubMed]
- Mauricio D, Zeybek B, Tymon-Rosario J, et al. Immunotherapy in Cervical Cancer. Curr Oncol Rep 2021;23:61. [Crossref] [PubMed]
- Kumar L, Harish P, Malik PS, et al. Chemotherapy and targeted therapy in the management of cervical cancer. Curr Probl Cancer 2018;42:120-8. [Crossref] [PubMed]
- Liu Y, Wu L, Tong R, et al. PD-1/PD-L1 Inhibitors in Cervical Cancer. Front Pharmacol 2019;10:65. [Crossref] [PubMed]
- Duska LR, Scalici JM, Temkin SM, et al. Results of an early safety analysis of a study of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer. Cancer 2020;126:4948-56. [Crossref] [PubMed]
- Rodríguez MO, Rivero TC, del Castillo Bahi R, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther 2010;9:343-9. [Crossref] [PubMed]
- Liu ZG, Zhao Y, Tang J, et al. Nimotuzumab combined with concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective analysis. Oncotarget 2016;7:24429-35. [Crossref] [PubMed]
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002;110:669-72. [Crossref] [PubMed]
- Fei Z, Xu T, Li M, et al. Effectiveness and cost-effectiveness analysis of nimotuzumab for the radiotherapy of locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol 2020;15:230. [Crossref] [PubMed]
- Qin S, Bai Y, Wang Z, et al. Nimotuzumab combined with gemcitabine versus gemcitabine in K-RAS wild-type locally advanced or metastatic pancreatic cancer: A prospective, randomized-controlled, double-blinded, multicenter, and phase III clinical trial. J Clin Oncol 2022; [Crossref]
- Cao Y, Deng L, Lian S, et al. Research on the efficacy of cisplatin and nimotuzumab combined with concurrent chemoradiotherapy on locally advanced cervical cancer. J BUON 2019;24:2013-9.
(English Language Editor: D. Fitzgerald)


