
Diagnosis and treatment of pericardial mesothelioma by mediastinal mass resection: a case report
Highlight box
Key findings
• We present a rare case of difficult-to-diagnose PeM in which the diagnosis was clarified by surgery and the patient achieved a long survival
What is known and what is new?
• Pericardial mesothelioma cases are rare, difficult to diagnose, and there are no uniform treatment criteria.
• We have been able to confirm the nature of the disease and improve the quality of life and survival time of patients by combining surgery with pathological examination.
What is the implication, and what should change now?
• The choice of treatment modality was selection biased, and patients refused further treatment due to their financial situation.
Introduction
Mesothelioma is an aggressive tumor characterized by local invasion and a poor prognosis (1). Most patients with mesothelioma have a history of exposure to asbestos, and there has been an upward trend in the incidence of mesothelioma in recent years in some areas (2). Statistically, about 60% of cases occur in the pleura, and about 35% in the peritoneum (3,4); however, Pericardial mesothelioma (PeM) is very rare, according to the latest epidemiological data, the incidence of PeM is 0.049/million/year in men and 0.023/million/year in women, and there is insufficient statistical data available on this disease (5). PeM is believed to originate mainly from pericardial mesenchymal cells (6).
As chest tightness, shortness of breath, cough, and chest pain are the initial symptoms of PeM, and the clinical presentation is usually non-specific, early diagnosis is difficult. Chest X-ray, chest computed tomography (CT), positron emission tomography-CT, pleural effusion, histopathologic analysis with immunohistochemistry must be applied in the diagnosis of this disease. Currently, surgery, chemotherapy, and combination therapy are the main treatments for this disease (7). However, there is no standard treatment for PeM, because of difficulties in diagnosis and delays in treatment, patients have a poor prognosis, with a median survival time from diagnosis to death of 4–6 months, the time duration that has not changed in over 20 years (8). We present a case of PeM with pre-surgical diagnostic difficulties, which was helped by surgery for clinical diagnosis and treatment, and the patient achieved a longer survival, improve the patient’s quality of life, providing clinicians with our experience in treating PeM at an early stage of diagnosis and early treatment. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4719/rc).
Case presentation
A 65-year-old female patient attended Affiliated Hospital of Zunyi Medical University in May 2021, complaining about chest tightness and shortness of breath for the last 2 months, usually after activity, In the last month it was accompanied by edema of the lower limbs. The patient did not have other symptoms, such as cough and expectoration. The patient had not been medically evaluated or treated before entering Affiliated Hospital of Zunyi Medical University.
A physical examination revealed mild edema of both lower limbs. The patient reported that she has been operated on several times for kidney stones. A chest CT-scan after admission revealed partly cystic, partly solid mass in the anterior mediastinum, measuring 13×12×7 cm. The radiologic and clinical differential diagnoses were primarily germ cell tumor and thymoma (Figure 1A,1B). The routine blood and blood biochemical examinations results revealed no abnormalities. Cardiac ultrasound showed the enlargement of the right heart, a widened pulmonary artery, pulmonary hypertension, and severe tricuspid regurgitation. The patient’s heart was severely compressed by a large anterior mediastinal tumor, resulting in cardiac insufficiency.
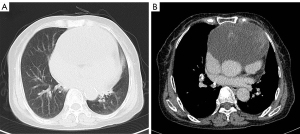
Surgery was indicated. After discussions with the patient and her family, a midline thoracotomy was performed for mediastinal tumor resection. During the operation, the tumor was found to be infiltrating and growing from pericardium towards the myocardium. Carefully free the myocardium from the tumor, ligate the blood supply vessel of the tumor below the innominate artery, investigate the left pulmonary artery trunk where the tumor is ill-defined, remove the tumor after blocking the left pulmonary artery trunk, repair the left pulmonary artery trunk, send the specimen for pathological histological examination, bleed about 800 mL during the operation, give 2 U of red blood cells and 200 mL of plasma intravenously.
The intraoperative frozen section on pathology (Figure 2A-2C) indicated a malignant tumor, favoring an adenocarcinoma. Histology was suggestive of a malignant germ cell tumor of the mediastinum, in the first line a yolk sac tumor. Immunohistochemical analysis revealed positive reactions with keratin (+), calretinin (+++), cytokeratin 5/6 (+++), WT-1 (+++), D2–40 (++), and P53 (+). Proliferation index with Ki-67 was rather low (10%). Reactions with following antibodies were negative: EMA, desmin, AFP, CD117, CD30, Glypican-3, Napsin-A, Oct3/4, PLAP, SALL4, TTF1. Based on morphology and immunohistochemistry results, this tumor was diagnosed as pericardial epithelioid mesothelioma.

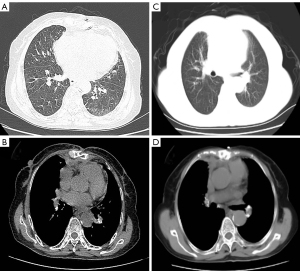
After careful postoperative treatment and care, the patient was able to get out of bed on the 1st day, and the chest drain was successfully removed 3 days later. The patient was discharged on the 19th postoperative day. At the follow-up visit in the 4th (Figure 3A,3B) and 19th month after discharge (Figure 3C,3D), no significant abnormality was found on CT-scan. The patient is currently in a good condition and has not complained of any specific discomfort. The follow-up is ongoing. The patient is very grateful for the medical services provided by our hospital and the great efforts we put into curing her disease. A timeline shows the historical and current information of the case (Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PeM is a highly malignant and extremely rare disease, and its incidence continues to increase in some areas (9). The pathogenesis of PeM remains unclear (5). The first symptoms of PeM are chest pain, dyspnea, and cough (3). Its diagnosis is difficult because of the non-specific clinical manifestations. A preoperative puncture biopsy of pleural effusion and pericardial effusion can be helpful for diagnosis (10); however, the cytopathological sensitivity of MMS has been reported to range from 30% to 75% (11).
In the present case, the intraoperative exploration revealed a tumor growing from the the pericardium, with no obvious boundary between the tumor and the left atrium and left ventricle. Previously, PeM was reported to be associated with a large amount of pericardial fluid (12). The small volume of pericardial fluid and the patient’s inability to lie flat prevented obtaining a biopsy, which made the preoperative diagnosis impossible.
Currently, the clinical treatment plan for mesothelioma, approved in the United States and Europe (13), includes surgical treatment combined with pemetrexed and cisplatin. Treatment plan for PeM are mostly based on pleural mesothelioma, but the prognosis for patients is poor (14). The median survival time of patients has been reported to be about 3.5–9 months, without therapy (8,14). In the recent study by Offin et al., the overall median overall survival (OS) was 25.9 months, and the three patients who received triple therapy (e.g., surgery, adjuvant cisplatin plus pemetrexed and adjuvant radiotherapy with different radiotherapy strategies) had a much longer median survival than those who received systemic therapy only or were lost to follow-up (70.3 vs. 8.2 months, hazard ratio =0.19) (14). In the study by McGehee et al. based on a published review of 103 cases, 88 cases were definitively included in the radiotherapy study, 8 patients received radiotherapy with no significant improvement in survival; 80 patients were included in the chemotherapy study, 44% of whom received chemotherapy, with improved median survival in patients who received chemotherapy (13 vs. 0.5 months, HR 0.31; 95% CI, 0.17–0.57; P=0.001); median survival was 27 months for patients who received surgery compared with those who did not (HR 0.40; 95% CI, 0.17–0.96; P=0.03); median survival was 16 months for patients who received two or three treatments compared with 4.5 months for patients who received a single treatment (HR 0.45; 95% CI, 0.23–0.90; P=0.015) (8).
In the present case, because of the huge tumor mass, compressing heart and causing clinical symptoms in our patient we have chosen surgical treatment as therapeutic and diagnostic option. Our patient was diagnosed as an epithelioid PeM. It is known that the epithelioid histology is associated with better prognosis (reference). After discharge from the hospital, we recommended that the patient continue with chemotherapy. However, the patient refused further treatment for financial reasons. The patient’s post-operative prognosis was good and her survival thus far exceeds previous reports.
According to the latest World Health Organization classification, there are 3 major histopathologic subtypes of mediastinal MM; that is, epithelioid, sarcomatoid, and mixed mediastinal MMS. Our diagnosis of epithelioid mesothelioma was confirmed for the following reasons: (I) no spindle-shaped cells were observed in the pathological images, and the pathological images were consistent with the histological features of epithelial mesothelioma; (II) the most important characteristic of the immunohistochemical differentiation of epithelioid mesothelioma is positive CK. The patient’s immunohistochemical results for CK, WT-I, and CK5/6 were positive, and those for desmin and AFP were negative, which provided further evidence that the tumor was epithelioid mesothelioma, and the scattered P53 (+) and KI-67 (10%) indicated that the tumor was malignant. Thus, the differentiation of mesothelioma was the final test of the pathologist’s inspection level.
Conclusions
To our knowledge, case reports of this type of mass in the literature are extremely rare. Most cases are diagnosed at autopsy (14). PeM is extremely malignant, and the prognosis of patients is poor even after comprehensive treatment. In addition, it is difficult to make a correct diagnosis in the early stages because of the non-specific nature of the symptoms. Research on the diagnosis and treatment of this disease is currently progressing slowly. Hence, if there is any clinical suspicion of PeM, pathological analysis, with obligatory inclusion of immunohistochemistry is required to confirm the diagnosis of the disease.
Several issues arise in relation to the diagnosis and treatment of this patient that require further discussion
Question 1: What types of patients commonly present with giant mediastinal tumors?
Luka Brcic: The term giant mediastinal tumor is actually nowhere strictly defined. We can say that all tumors occurring in mediastinum and bigger than 10 cm are giant mediastinal tumors. They occur in young and old, male and female patients.
Joel W. Neal: Since “giant mediastinal tumors” include a variety of pathologic diagnoses, I will include a blurb below but questions 2 and 3 depend on the path.
Question 2: What are the treatment options for giant mediastinal tumors?
Luka Brcic: Surgery is always the best option for all tumors, if possible. Histologic diagnosis is needed to decide about the most appropriate treatment. In other words, if curative surgery is not possible, than at least biopsy should be obtained to provide material for histologic diagnosis.
Joel W. Neal: Tumors of the anterior mediastinum often are found incidentally on imaging but sometimes as a result of direct mass effect on the heart or chest wall. Classically the differential diagnosis includes the “four T’s”—Thymic epithelial tumors, Thyroid cancer, Teratoma (germ cell tumor), and “Terrible lymphoma”. However, the anterior pericardium may rarely become a nidus for malignant PeM, which is biologically similar to malignant pleural mesothelioma except for the location involved. The posterior mediastinum can also be a primary location of cancers, such as proximal non-small cell lung cancer with lymph node involvement.
Question 3: What is the standard procedure for the treatment of mediastinal tumors of rare pathological types?
Luka Brcic: Like previously said, if possible, surgery is always the best option if possible. Histologic diagnosis is needed for decision about treatment. For mesothelioma there is usually a combination therapy to be planned [chemotherapy (IO?), surgical and radiotherapy].
Joel W. Neal: Treatment depends on the pathologic diagnosis and may include surgical resection, radiotherapy, and systemic therapy.
Acknowledgments
We would like to express our sincere thanks to the patient for generously authorizing us to report her rare case. The authors also appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: The National Natural Science Foundation of China (No. 82160574) and the Guizhou Province Science and Technology Plan (No. Qiankehe Support [2021] General 073) provided funding for this study.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4719/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4719/coif). LB received grants from Takeda, AstraZeneca, BMS and Roche; he also received payment for lectures and participated in advisory boards form Invitae, Eli-Lilly, AstraZeneca, Roche, MSD, Merck, BMS, Pfizer, Novartis, Takeda, Janssen; support for attending meeting from Pfizer. He is Int. Secretary-Austrian Society of Pathology; PPS Membership and Awards Committee; Member of the Mesothelioma Committee of IASLC. JWN reports grants and personal fees from Genentech/Roche, Exelixis, Takeda Pharmaceuticals, and AstraZeneca; grants from Merck, Novartis, Boehringer Ingelheim, Nektar Therapeutics, Adaptimmune, GSK, Janssen, and AbbVie; personal fees from Eli Lilly and Company, Jounce Therapeutics, Calithera Biosciences, Amgen, Iovance Biotherapeutics, Blueprint Pharmaceuticals, Regeneron Pharmaceuticals, Natera, Sanofi/Regeneron, D2G Oncology, Surface Oncology, Turning Point Therapeutics, Mirati Therapeutics, and Gilead; other from CME Matters, Clinical Care Options, Research to Practice, Medscape, Biomedical Learning Institute, MLI Peerview, Prime Oncology, Projects in Knowledge, Rockpointe, MJH Life Sciences, Medical Educator Consortium, and HMP Education. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Savarrakhsh A, Vakilpour A, Davani SZ, et al. Malignant primary pericardial mesothelioma presenting as effusive constrictive pericarditis: a case report study. J Cardiothorac Surg 2021;16:298. [Crossref] [PubMed]
- Geltner C, Errhalt P, Baumgartner B, et al. Management of malignant pleural mesothelioma - part 1: epidemiology, diagnosis, and staging: Consensus of the Austrian Mesothelioma Interest Group (AMIG). Wien Klin Wochenschr 2016;128:611-7. [Crossref] [PubMed]
- Zhang S, Song P, Zhang B. Giant malignant mesothelioma in the upper mediastinum: A case report. Oncol Lett 2013;6:181-4. [Crossref] [PubMed]
- Antman KH. Current concepts: malignant mesothelioma. N Engl J Med 1980;303:200-2. [Crossref] [PubMed]
- Grosso F, Cerbone L, Pasello G. Pericardial Mesothelioma, a Disease for Brave Hearts. J Thorac Oncol 2022;17:1333-4. [Crossref] [PubMed]
- Erdogan E, Demirkazik FB, Gulsun M, et al. Incidental localized (solitary) mediastinal malignant mesothelioma. Br J Radiol 2005;78:858-61. [Crossref] [PubMed]
- Cardinale L, Cortese G, Familiari U, et al. Fibrous tumour of the pleura (SFTP): a proteiform disease. Clinical, histological and atypical radiological patterns selected among our cases. Radiol Med 2009;114:204-15. [Crossref] [PubMed]
- McGehee E, Gerber DE, Reisch J, et al. Treatment and Outcomes of Primary Pericardial Mesothelioma: A Contemporary Review of 103 Published Cases. Clin Lung Cancer 2019;20:e152-7. [Crossref] [PubMed]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [Crossref] [PubMed]
- Scherpereel A, Opitz I, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020;55:1900953. [Crossref] [PubMed]
- Henderson DW, Reid G, Kao SC, et al. Challenges and controversies in the diagnosis of mesothelioma: Part 1. Cytology-only diagnosis, biopsies, immunohistochemistry, discrimination between mesothelioma and reactive mesothelial hyperplasia, and biomarkers. J Clin Pathol 2013;66:847-53. [Crossref] [PubMed]
- Kawakami N, Kawai M, Namkoong H, et al. Cardiac tamponade due to primary malignant pericardial mesothelioma diagnosed with surgical pericardial resection. J Cardiol Cases 2021;24:149-52. [Crossref] [PubMed]
- Zucali PA, Giaccone G. Biology and management of malignant pleural mesothelioma. Eur J Cancer 2006;42:2706-14. [Crossref] [PubMed]
- Offin M, De Silva DL, Sauter JL, et al. Multimodality Therapy in Patients With Primary Pericardial Mesothelioma. J Thorac Oncol 2022;17:1428-32. [Crossref] [PubMed]



