
Efficacy and safety of original EGFR-TKI combined with bevacizumab in advanced lung adenocarcinoma patients harboring EGFR-mutation experiencing gradual progression after EGFR-TKI treatment: a single-arm study
Highlight box
Key findings
• Continuous original TKI combined with bevacizumab in EGFR mutation gradual progression NSCLC showed the trend of longer PFS and good tolerance of adverse reactions compared to that of continuation of original EGFR-TKI treatment.
What is known and what is new?
• Continuing the original EGFR-TKI is the current recommended treatment for gradual progression NSCLC patients by the guidelines. Many other strategies are also currently being explored.
• EGFR-TKIs and antiangiogenic drugs are commonly used in advanced NSCLC. Our study yielded some evidence in support of the benefit of combination strategies and to find a new feasible therapeutic option for this group.
What is the implication, and what should change now?
• Our study suggests that the combination strategies of EGFR-TKI with bevacizumab may be a new treatment option for EGFR positive gradual progression NSCLC. Results from the small sample need to be further confirmed in real-world setting.
Introduction
Lung cancer is one of the most common malignancies and is the leading cause of cancer deaths worldwide (1). Non-small cell lung cancer (NSCLC) has the highest incidence and accounts for over 80% of lung cancer cases. As the early symptoms are not obvious, the majority of lung cancer patients are diagnosed at the advanced stages (2). With the discovery of driver mutations in lung cancer, the personalized treatment of advanced NSCLC has made rapid progress over the last decade. Epidermal growth factor receptor (EGFR) mutation is the most common gene mutation in NSCLC patients. About 50% of Chinese NSCLC patients have EGFR mutation. EGFR tyrosine kinase inhibitor (TKI) could inhibit tumor growth and promote tumor cell apoptosis by inhibiting EGFR phosphorylation (3). A series of studies have supported that EGFR-TKIs can provide a favorable treatment outcome in EGFR mutation-positive NSCLC patients with a response rate as high as 80% and around 10–14 months of progression-free survival (PFS) (4-6). At present, EGFR-TKI is the standard first-line treatment for metastatic NSCLC with EGFR-positive, and is recommended by many guidelines, such as those of the National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO).
However, most patients with EGFR-mutation will face the challenge of EGFR-TKI acquired resistance (7) after an average of 10–14 months of first- or second-generation EGFR-TKI treatment. According to the nature of progression after drug resistance, patients are generally divided into 3types: rapid progression, gradual progression, and local progression (8). Gradual progression refers to disease control for ≥6 months, a slight increase in the harmful impact of the tumor on the body compared with previous assessments, and a symptom score of ≤1. In a study, median PFS of the 3 modes were 9.3, 12.9, and 9.2 months, respectively, and the median overall survival (OS) times were 17.1, 39.4, and 23.1 months, respectively (8). Currently, continuing with EGFR-TKI treatment is generally recommended by the guidelines for gradual progression patients; however, many other strategies are also currently being explored. Combination strategies have shown significant tumor response in NSCLC and may also benefit this group. Neovascularization is a fundamental activity in tumor growth, metastasis, and dissemination.
Vascular endothelial growth factor (VEGF) family plays a key role in tumor angiogenesis (9). Through binding to transmembrane tyrosine kinases receptors, VEGF stimulates downstream signal transduction and promotes proliferation, mitosis, differentiation, and migration of endothelial cells to form new vascular cavities (10).
Preclinical studies have confirmed that EGFR and vascular endothelial growth factor receptor (VEGFR) pathways have synergistic effects in tumor development. Anti-angiogenic drugs can promote the normalization of tumor blood vessels and improve the local tumor microenvironment, so that targeted drugs can play a better role (11). Antiangiogenic drugs have been actively investigated in lung cancer. Bevacizumab, a recombinant monoclonal antibody against VEGF, is the first antiangiogenic drug to be approved by Food and Drug Administration (FDA). Based on many promising studies, bevacizumab has been widely accepted as a standard first line treatment with chemotherapy for many advanced metastatic tumors including lung cancer and colorectal cancer (12-14). To date, few studies have explored whether patients with gradual progression after first-line TKI could benefit more from combination therapy than from EGFR-TKI alone. Thus, our study aimed to evaluate the efficacy and the safety profiles of original EGFR-TKIs combined with bevacizumab in advanced NSCLC patients who had gradual progression after EGFR-TKIs treatment in our medical center in a real-world setting. We present the following article in accordance with the TREND reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6101/rc).
Methods
Study design and patient information
From June 2019 to December 2021, 35 NSCLC patients harboring EGFR mutation and experiencing gradual progression of disease after EGFR-TKI treatment who received original EGFR-TKI combined with bevacizumab were enrolled at the Department of Medical Oncology, Chongqing University Cancer Hospital, China. All the patients had been confirmed by cytology or histology. The study protocol was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Chongqing University Cancer Hospital (No. CZLS2019243-A). All participants provided informed consent prior to treatment. The inclusion criteria were as follows: pathologically diagnosed NSCLC; achieved a ≥3-month disease control after original EGFR-TKI treatment; EGFR mutations was reperformed and EGFR-positive redetected by polymerase chain reaction (PCR)-based direct sequencing method or next-generation sequencing (NGS); The objective tumor response was evaluated every 6–8 weeks; at least 1 radiologically measurable lesion did not receive local treatments such as radiotherapy. The radiographic response to EGFR-TKI treatment was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The main exclusion criteria were patients with uncontrolled blood pressure with medication (>140/90 mmHg) and with those with bleeding tendency and receiving thrombolytics or anticoagulants. The patient’s general information collection included gender, age, and pathological types, smoking status, EGFR mutations, Eastern Cooperative Oncology Group (ECOG) scores and prior treatment, and so on. Gradual progression patients were treated with original EGFR-TKIs combined with bevacizumab until rapid progression of the disease or intolerable side effects. EGFR-TKIs at a daily dose and bevacizumab 15 mg/m2 every 3 weeks constituted 1 treatment cycle. Computed tomography (CT) scans of the lungs and other metastatic sites reviewed after 2 cycles of treatment. Other exams included routine blood and biochemical tests. Follow-up time was until rapid progression of the disease or the end of the study.
Responses and toxicity assessments
The size of measurable lesions was determined by CT scan every 2 treatment cycles. The tumor response was evaluated according to RECIST1.1 criteria. The evaluation of tumor efficacy included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The disease control rate (DCR) was defined as the percentage of CR, PR, and SD. In addition, toxicities were assessed by the National Cancer Institute Common Toxicity Criteria Adverse Events version 4.0 (NCICTCAE 4.0) (15).
Follow-ups
PFS1 was defined as the period from the beginning of EGFR-TKI treatment to the rapid progression of the disease. PFS2 was defined as the period from the beginning of EGFR-TKI combined with bevacizumab treatment to the rapid progression of the disease.
Statistical analysis
Data analysis was performed using SPSS version 19.0 (IBM Corp., Chicago, IL, USA). Survival curves were estimated by the Kaplan-Meier method and compared by means of the log-rank test. Patients’ characteristics before the combined treatment were analyzed in univariate and multivariate Cox proportional hazard regression model. Pearson χ2 or the Fisher exact test was used to compare the qualitative data. Differences with a 2-sided P value of 0.05 or less were considered statistically significant.
Results
Patient characteristics
A total of 35 patients with EGFR-mutation advanced NSCLC were enrolled; 14 were male and 21 were female with a median age of 62 years (range, 33–79 years). The histopathology types were all adenocarcinoma. All cases had received EGFR-TKIs treatment including gefitinib, erlotinib, icotinib, and osimertinib before the combined treatment. A total of 17 of 35 (48.6%) patients had deletion of exon 19, whereas 16 patients (45.7%) had L858R in exon 21, and 2 patients had EGFR rare mutation (5.7%). Among them, 6 patients had EGFR T790M mutation (17.1%) and 1 case (2.9%) had EGFR rare mutations. Further, 12 patients had ECOG performance status (PS) scores of 0 and 23 patients had of 1. Reasons for gradual progression included asymptomatic progression of brain lesions in 11 cases (31.4%), malignant pleural effusion in 6 cases (17.1%), and other causes in 18 cases (51.4%). The patients’ characteristics are summarized in Table 1. Follow-ups were conducted up to 25 January 2022.
Table 1
| Characteristic | Values, n (%) |
|---|---|
| Gender | |
| Male | 14 (40.0) |
| Female | 21 (60.0) |
| Age (years) | |
| Median | 62 |
| Range | 33–79 |
| Histology | |
| Adenocarcinoma | 35 (100.0) |
| Others | 0 (0.0) |
| Stage | |
| IIIb/IIIc | 0 (0.0) |
| IV | 35 (100.0) |
| Smoking status | |
| Yes | 9 (25.7) |
| No | 26 (74.3) |
| EGFR mutation status | |
| 19 del | 17 (48.6) |
| L858R | 16 (45.7) |
| Rare mutation | 2 (5.7) |
| Combined mutation | |
| Yes | |
| T790M | 6 (17.1) |
| Rare mutation | 1 (2.9) |
| No | 28 (80.0) |
| ECOG | |
| 0 | 12 (34.3) |
| 1 | 23 (65.7) |
| Site of metastasis at beginning | |
| Brain | |
| Yes | 16 (45.7) |
| No | 19 (54.3) |
| Liver | |
| Yes | 5 (14.3) |
| No | 30 (85.7) |
| Pleura | |
| Yes | 9 (25.7) |
| No | 26 (74.3) |
| Numbers of metastatic organs | |
| ≤2 | 16 (45.7) |
| >2 | 19 (54.3) |
| Site of gradual progression | |
| Brain | 11 (31.4) |
| Pleura | 6 (17.1) |
| Others | 18 (51.4) |
| Previous EGFR-TKI | |
| First/second generation TKI | 31 (88.6) |
| Third generation TKI | 4 (11.4) |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Response and survival
Median follow-up period for the analysis of OS was 13 months (range, 5–42.5 months). A total of 33 patients could participate the efficacy evaluation. As determined by RECIST criteria, there were 31 SD, 2 PD, and no CR or PR, which resulted in a DCR of 93.94%. The results of response are summarized in Table 2. Median PFS1 and PFS2 for all patients were 20.5 months [95% confidence interval (CI): 16.1–24.9 months] and 8 months (95% CI: 6.3–9.7 months), respectively (Figure 1A,1B). Median OS was immature.
Table 2
| Response | N (%) |
|---|---|
| CR | 0 (0.00) |
| PR | 0 (0.00) |
| SD | 31 (93.94) |
| PD | 2 (6.06) |
| DCR (CR + PR + SD) | 31 (93.94) |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate.
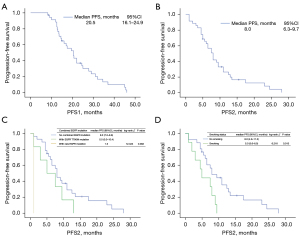
Clinicopathologic factors associated with prognosis
The median PFS2 for patients with EGFR T790M and EGFR rare mutation and without combined mutation was 5.0 months, 1 month, and 8 months, respectively, and the difference was statistically significant (P=0.002) (Figure 1C).
Univariate log-rank test revealed that the median PFS2 differed significantly between patients with smoking and no smoking (8.0 vs. 5.0 months, P=0.013) (Figure 1D).
Patients with less than two metastatic organs, without brain metastasis, and without malignant pleural effusion showed a trend of longer PFS2 (figure not shown). Multivariate analysis demonstrated that smoking status [hazard ratio (HR) =3.692, 95% CI: 1.450–9.404, P=0.006], combined EGFR T790M mutation/rare mutation (HR =2.480, 95% CI: 1.073–5.729, P=0.034) and malignant pleural effusion (HR =3.707, 95% CI: 1.460–9.414, P=0.006) were the independent prognostic factors of PFS2. The ECOG score, EGFR TKI type, and EGFR status of the patients were not predictors of PFS2 (see Table 3).
Table 3
| Factors | PFS2 | ||
|---|---|---|---|
| HR | P value | 95% CI | |
| Gender | 0.345 | 0.471 | 0.099–2.247 |
| Smoking history | 3.692 | 0.006 | 1.450–9.404 |
| Combined mutation | 2.480 | 0.034 | 1.073–5.729 |
| Malignant pleural effusion | 3.707 | 0.006 | 1.460–9.414 |
| Types of EGFR mutation | 0.883 | 0.825 | 0.293–2.657 |
| ECOG PS performance | 0.800 | 0.676 | 0.280–2.281 |
| Numbers of metastatic organ | 0.690 | 0.518 | 0.224–2.215 |
| Brain metastasis | 1.697 | 0.338 | 0.575–5.009 |
| Liver metastasis | 1.741 | 0.449 | 0.414–7.317 |
PFS, progression-free survival; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Adverse events
All patients were assessed for toxicity. Bevacizumab was used from 1 to 25 cycles, with an average of 8.4 cycles. Two patients withdrew from the study due to grade 3 increased aminotransferase after 1 cycle. The most common treatment-related adverse events greater than grade 3 were hypertension (23.7%), proteinuria (8.3%), and increased alanine aminotransferase (ALT; 4.1%) and aspartate aminotransferase (AST; 2.9%). The symptoms related to treatment-related adverse events of grade 3 were quickly reduced and recovered after timely symptomatic treatment. There were no interstitial pneumonias and no treatment-related deaths in any of the cases.
Discussion
EGFR is one of the most common driver genes in NSCLC. The emergence of EGFR-TKI has significantly improved the survival and quality of life of the patients with EGFR-positive advanced NSCLC. Unfortunately, despite the significant response rate, PFS, and OS achieved with TKIs in EGFR mutant NSCLC, most patients treated with first-line EGFR-TKI will experience disease progression after a median of 10 to 14 months. Currently, the guidelines recommend continuing treatment with EGFR-TKIs for asymptomatic gradual progression EGFR-mutant lung cancer. The preclinical research suggested that some tumor cells in tumor tissues which had acquired resistance to TKIs were still effective for EGFR-TKI therap. Therefore, patients with EGFR-TKI-acquired resistance may partly benefit from continuously using TKIs. Retrospective clinical observations have also observed that discontinuation of erlotinib or gefitinib before initiation of study treatment in patients with EGFR-mutant and acquired resistance to TKIs was associated with a significant risk of accelerated progression and the median time to disease flare after TKI discontinuation was 8 days (range, 3–21 days) (16). Increased maximum standardized uptake value (SUVmax) and tumor size had been found in 18-fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography (18F-FDG PET/CT) and CT scans in patients who developed acquired resistance results in symptomatic progression and had stopped erlotinib or gefitinib (17). Therefore, patients with EGFR-TKI resistance, especially those without T790M resistance mutation, should not stop TKIs treatment immediately. Continuing the original TKI treatment after progression may prevent tumor progression from accelerating in a short term and maintain the quality of life. Some clinical trials have also supported this result. For example, Asami et al. (18) reported that a longer clinical benefit can be obtained for EGFR-mutation patients with Iressa failure but continuous Iressa treatment. The ASPIRATION study also explored the efficacy and safety of first-line erlotinib after gradual progression. The median PFS of patients who continued erlotinib after progression was extended to 14.1 months on the basis of 11.0 months, and a PFS of 3.1 months was obtained (19).
Some other studies have explored combination therapy as the treatment mode in gradual progression after first-line EGFR-TKI (20-22). The IMPRESS trial confirmed, compared to chemotherapy, that patients with EGFR-mutated at the time of RECIST progression benefitted from EGFR-TKI combined with chemotherapy (20). A single-center, prospective clinical study initiated by Chang et al. (22) compared different treatment methods for patients with gradual progression but without EGFR-T790M mutation after first-line TKI treatment. They also found that EGFR-TKI combined with chemotherapy had better PFS and OS benefits than single TKI continuous treatment first, and then chemotherapy after clear progression for gradual progression patients after initial TKIs (22). These findings suggest that for patients with gradual progression after first-line TKI, early combination therapy may bring benefits than of EGFR-TKI alone. Anti-angiogenic drugs are also commonly used in combination therapy. As the most important factor influencing neovascularization, increased VEGF messenger RNA (mRNA) expression can be detected in many types of tumor. Clinical evidence from ECOG4599 (12) and AVAIL (13) had shown, compared with standard chemotherapy alone, advanced NSCLC patients have higher response rates and prolonged time to progression when treated with bevacizumab combined with chemotherapy. The RCT-JO25567 study (23) and the NEJ026 study from Japan (24) both demonstrated that the first-line combination of TKI and bevacizumab in non-squamous NSCLC showed significant PFS benefit and good tolerability despite not prolonging the OS of patients. A real-world study also yielded similar results (25). The BELIEF, CTONG1509, and others trials had further verified the higher PFS in patients with TKIs combined with bevacizumab (26,27). The addition of erlotinib to bevacizumab maintenance after first-line chemotherapy in the ATLAS trial also significantly improved PFS (4.76 vs. 3.75 months) and the risk of disease progression was reduced by 28% (28). Preclinical study also confirmed combined blockade of the VEGFR and EGFR pathways are useful for reversing primary or acquired resistance to EGFR TKIs (29). These studies suggest that the combination of bevacizumab with EGFR-TKI could enhance the efficacy and have the potential to overcome TKI resistance. A combined regimen could provide a therapeutic option for NSCLC gradual progression patients after first-line EGFR-TKI resistance. However, considering the side effects and the long-term tolerance of patients, most clinicians and patients prefer to choose first-line single EGFR-TKI therapy and then consider a combination regimen after progression in a real-world setting. The ASPIRATION (28) model also reports that the timing of switching to second-line therapy in EGFR-mutated lung cancer patients is a very personal decision. More than half of the patients with RECIST progression continued to use erlotinib with a median duration of 3 months before changing the protocol. The subjective awareness of patients and physicians plays an important role in the choice of subsequent treatment options in the real world. Moreover, not all first-line EFGR-TKI patients are T790M mutation resistant, and it is theoretically feasible for patients to continue using the original EGFR-TKI. Therefore, for the patients with gradual asymptomatic progression who are not willing to adjust the protocol to second-line TKI immediately, considering decreased benefit of single TKI, the combined mode of anti-vascular drug and TKI could improve the benefit. The data of our study suggested that gradual progression advanced NSCLC patients could acquire longer efficacy from combination strategies of EGFR-TKI with bevacizumab. The DCR of all patients was 93.94% and the overall median PFS was 20.5 months, including 11.5 months for PFS1 and 8 months for PFS2. This data was superior to the current data for second-line chemotherapy and the continuation of the original TKI alone. Moreover, some common adverse reactions of combination therapy which were mainly bevacizumab induced such as hypertension could be well controlled by symptomatic treatment and well tolerated in strictly selected patients.
Some studies have observed that the sensitivity to EGFR-TKIs could be influenced by co-occurring rare partner mutation. Complex mutations account for 5–15% of EGFR mutations in NSCLC (30,31). Patients who harbor the complex mutations which include a resistance mutation such as T790M were reported to have poor clinical responses (32). Due to our data, patients with complex EGFR mutation such as T790M or rare mutations seemed to achieve poor PFS2. Those suggested EGFR mutations in lung cancer are extremely complicated. Patients who are EGFR less sensitive and resistant mutations should select more aggressive treatment or more appropriate and effective TKIs early. Our data also suggested that patients who had pleural effusion did not benefit from combination strategies. Our data exhibited that some clinical-pathological features such as the number of metastatic lesions, brain metastases, and ECOG score did not correlate with PFS in patients who accepted combined therapy. However, for patients with asymptomatic brain metastases who are unwilling to cooperate with long-term radiotherapy, TKIs combined with bevacizumab can delay the progression and avoid prolonged hospital stays at the same time. Since bevacizumab also has the effect of relieving brain edema and a direct anti-tumor effect, which may partially make up for the disadvantage that the first and second generation TKIs has a low brain penetration rate.
Even though we could see some PFS benefit from EGFR-TKIs combined with bevacizumab treatment in gradual progression NSCLC patients after first-line EGFR-TKI treatment, there are many questions which remained to be answered. What are the appropriate gradual progression cases for EGFR-TKI combined with bevacizumab treatment, and when is the best time to use combined therapy? To answer these questions, more clinical studies are needed. The results from this small sample-sized study also need to be further confirmed in larger sample size, prospective studies which are closer to a real-world setting which could provide certain treatment information for doctors’ clinical options.
Conclusions
In summary, our study proposed that EGFR-TKIs combined with bevacizumab treatment in gradual progression NSCLC patients after first-line EGFR-TKI treatment could slightly prolong PFS in comparison with continuing the original TKI treatment after progression. Further investigation is needed to identify the value of combined treatment in EGFR mutation gradual progression NSCLC.
Acknowledgments
Funding: This study was supported by a grant from High-level Medical Personnel Training of Chongqing Health Commission, China (No. 2019GDRC009) and a grant from Integrated Innovation and Application of Key Technologies for Precise Prevention and Treatment of Primary Lung Cancer (No. 2019ZX002).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6101/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6101/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6101/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Chongqing University Cancer Hospital (No. CZLS2019243-A). All participants provided informed consent prior to treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535-46. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60. [Crossref] [PubMed]
- Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33-9. [Crossref] [PubMed]
- Vijayvergia N, Mehra R. Clinical challenges in targeting anaplastic lymphoma kinase in advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2014;74:437-46. [Crossref] [PubMed]
- Dvorak HF. Tumor Stroma, Tumor Blood Vessels, and Antiangiogenesis Therapy. Cancer J 2015;21:237-43. [Crossref] [PubMed]
- Li F, Zhu T, Cao B, et al. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer 2017;84:184-92. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Common Toxicity Criteria Adverse Events version 4.0 (NCICTC 4.0) Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298-303. [Crossref] [PubMed]
- Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res 2007;13:5150-5. [Crossref] [PubMed]
- Asami K, Okuma T, Hirashima T, et al. Continued treatment with gefitinib beyond progressive disease benefits patients with activating EGFR mutations. Lung Cancer 2013;79:276-82. [Crossref] [PubMed]
- Park K, Yu CJ, Kim SW, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol 2016;2:305-12. [Crossref] [PubMed]
- Maruyama R, Wataya H, Seto T, et al. Treatment after the failure of gefitinib in patients with advanced or recurrent non-small cell lung cancer. Anticancer Res 2009;29:4217-21. [PubMed]
- Mok TSK, Kim SW, Wu YL, et al. Gefitinib Plus Chemotherapy Versus Chemotherapy in Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer Resistant to First-Line Gefitinib (IMPRESS): Overall Survival and Biomarker Analyses. J Clin Oncol 2017;35:4027-34. [Crossref] [PubMed]
- Chang Q, Xu J, Qiang H, et al. EGFR Tyrosine Kinase Inhibitor (TKI) Combined With Concurrent or Sequential Chemotherapy for Patients With Advanced Lung Cancer and Gradual Progression After First-Line EGFR-TKI Therapy: A Randomized Controlled Study. Clin Lung Cancer 2021;22:e395-404. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Kawashima Y, Fukuhara T, Saito H, et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med 2022;10:72-82. [Crossref] [PubMed]
- Zeng L, Xiao L, Jiang W, et al. Investigation of efficacy and acquired resistance for EGFR-TKI plus bevacizumab as first-line treatment in patients with EGFR sensitive mutant non-small cell lung cancer in a Real world population. Lung Cancer 2020;141:82-8. [Crossref] [PubMed]
- Zhou Q, Xu CR, Cheng Y, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell 2021;39:1279-1291.e3. [Crossref] [PubMed]
- Rosell R, Dafni U, Felip E, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med 2017;5:435-44. Erratum in: Lancet Respir Med 2018;6:e57. [Crossref] [PubMed]
- Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2013;31:3926-34. [Crossref] [PubMed]
- Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009;15:3484-94. [Crossref] [PubMed]
- Peng L, Song ZG, Jiao SC. Efficacy analysis of tyrosine kinase inhibitors on rare non-small cell lung cancer patients harboring complex EGFR mutations. Sci Rep 2014;4:6104. [Crossref] [PubMed]
- Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:45-51. [Crossref] [PubMed]
- Zhang B, Wang S, Qian J, et al. Complex epidermal growth factor receptor mutations and their responses to tyrosine kinase inhibitors in previously untreated advanced lung adenocarcinomas. Cancer 2018;124:2399-406. [Crossref] [PubMed]
(English Language Editor: J. Jones)


