
Ameliorative effect of ginsenoside Rg1 on dextran sulfate sodium-induced colitis: involvement of intestinal barrier remodeling in mice
Highlight box
Key findings
• Ginsenoside Rg1 therapy can significantly ameliorate the severity of DSS-induced colitis in mice. Treatment with ginsenoside Rg1 can repair intestinal barrier structure by reducing colonic pro-inflammatory cytokine TNF-α and IFN-γ levels and increasing the anti-inflammatory cytokine IL-4 level, eliminate intestinal inflammation and further regulate mucosal immune function in DSS-induced colitis mice.
What is known and what is new?
• Ginsenoside Rg1 can ameliorate the DSS-induced colitis in mice.
• Ginsenoside Rg1 can repair intestinal barrier structure by reducing colonic pro-inflammatory cytokine TNF-α and IFN-γ levels and increasing the anti-inflammatory cytokine IL-4 level, eliminate intestinal inflammation and further regulate mucosal immune function.
What is the implication, and what should change now?
• It implicated that ginsenoside Rg1 may provide a promising and novel approach to the treatment of IBD. We can offer new treatments for IBD.
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, involves chronic inflammation of the gastrointestinal tract, and is the most common clinical refractory disease. The pathogenesis of IBD has been widely studied. Chronic inflammation from a pathologic activation of the intestinal immune system is understood to be central to the pathogenesis (1). Moreover, loss of intestinal barrier integrity or an increased permeability of the intestinal epithelial cells (IECs) has been documented to play a fundamental role in the pathogenesis of IBD; the increased permeability of the IECs is pivotal to the inflammatory process and may be the primary defect in IBD (2).
Over the past decade, the pharmaceutical treatment of IBD has evolved alongside the increased understanding of the underlying mechanism of IBD. However, the lack of safety and efficacy of standard therapies is a major challenge for IBD treatment. Currently the aim of treatment for IBD is to suppress inflammation and restore intestinal barrier properties (3). An international consensus panel recently also proposed mucosal healing as an important therapeutic goal for Crohn’s disease and ulcerative colitis (4). Many substances and therapies have been under investigation (3).
Natural products derived from plants and herbs have been considered an attractive approach for IBD treatment due to their low toxicity and high patient compliance (5). Ginseng is the root of Panax ginseng, which belongs to the Araliaceae family.. Its main pharmacologically active ingredients are protopanaxadiol-type ginsenosides Rb1 and Rb2, and protopanaxatriol-type ginsenosides Re and Rg1, of which ginsenoside Rg1 is one of the most abundant and important components of pharmaceutical actions (6,7). To date, a wide range of roles have been reported for ginsenoside Rg1, including neuroprotection, immune-regulatory activity, progenitor cell proliferation, detumescence, analgesia, and anti-inflammation (8). Yousuf et al. find the immuno-modulatory and gut microbiota-reshaping effects of ginsenoside Rg1 were evaluated. Ginsenoside Rg1 metabolites of Lachnospiraceae bacterium enhanced the proliferation of CD4+ T cells and T regulatory (Treg) cells. This study suggested that Rg1 strengthens immunity with regulating the homeostasis of intestinal microbiota in mice (9).
It has been reported that ginsenoside Rg1 can inhibit the production of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and nitric oxide, and that it ameliorates the inflammatory response in colitis model mice (7,10,11). However, it is not yet known whether ginsenoside Rg1 affects the intestinal mucosal epithelial barrier. The present study was designed to investigate the effect of ginsenoside Rg1 on intestinal barrier disruption in dextran sulfate sodium (DSS)-induced colitis mice, the most popular model that mimics human ulcerative colitis due to its simplicity, high degree of uniformity, and reproducibility. This study may shed light on the therapeutic potential of ginsenoside Rg1 in human colitis. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5467/rc).
Methods
Animals
Specific-pathogen-free male BALB/c mice (6–8 weeks old) were purchased from Shanghai SLAC Laboratory Animal Co. (Shanghai, China), and were group-housed in stainless steel cages, 5 mice per cage, in a temperature-controlled room (22±2 ℃) with 55%±10% humidity and a 12-hour light/dark cycle. Food and water were available ad libitum. All procedures involving animals were approved by the Ethics Committee on Animal Experiments of Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine (No. 201704) and were in compliance with national guidelines for the care and use of animals. A protocol was prepared before the study without registration. All efforts were made to minimize animal suffering.
Induction of DSS colitis
DSS with a molecular weight of 40 kilodaltons (Pharmacia Industries, Rockville, MD, USA) was dissolved in tap water in a final concentration of 5% (wt/vol) and provided to experimental group mice for 7 days, as described by Yoon et al. (12). A fresh DSS solution was prepared daily. Control group mice received normal drinking water only. Mice with colitis were supplied with 6% sucrose in drinking water to prevent dehydration (Table 1).
Table 1
| Group | N | Colitis (yes/no) | Dose |
|---|---|---|---|
| Normal | 10 | No | – |
| Colitis | 10 | Yes | – |
| Control | 10 | Yes | – |
| Rg1 LD | 10 | Yes | 50 mg/kg/d |
| Rg1 MD | 10 | Yes | 100 mg/kg/d |
| Rg1 HD | 10 | Yes | 200 mg/kg/d |
| 5-ASA | 10 | Yes | 200 mg/kg/d |
5-ASA, 5-aminosalicylic acid; LD, low dose; MD, middle dose; HD, high dose.
Drug treatment
After administering 5% DSS for 7 days, the pharmacological treatments were initiated. Colitis model mice were divided into three groups (n=10 in each group) and were administered by gavage with ginsenoside Rg1 (Chengdu Scholar Bio-Tech, Chengdu, China) at dosages of 50 mg/kg (low-dose group), 100 mg/kg (middle-dose group), and 200 mg/kg (high-dose group), suspended in 0.5% sodium carboxymethyl cellulose (CMC; Kayon, Shanghai, China), respectively, for 7 days, as described by Fan et al. (13). 5-aminosalicylic acid (5-ASA) group (n=10) of mice was treated with 5-ASA (Jiamusi pharmaceutical Co., Heilongjiang, China) by gavage at doses of 200 mg/kg, suspended in 0.5% CMC, as described by Zhou et al. (14). Control group mice received 0.5% CMC by gavage (Table 1).
Characterization of inflammation
The disease activity index (DAI) was calculated using a clinical scoring system for DSS colitis, described by Gong et al. (15), including body weight loss (0, none; 1, decreased 1–5%; 2, decreased 5–10%; 3, decreased 11–15%; 4, decreased >15%), stool consistency (0, normal; 2, loose stool; 4, watery diarrhea); and bloody stools (0, normal; 2, slight bleeding; 4, gross bleeding). At the end of experiments, mice were sacrificed by decapitation, and the colonic tissue was obtained for further analysis of tissue morphology, cytokines, as well as ZO-1protein expression.
Assay of cytokines
Colonic tissue was homogenized in phosphate-buffered saline (PBS) solution containing protease inhibitor, and centrifuged at 1,000 ×g for 15 minutes at 4 ℃. The supernatant was collected for analysis of TNF-α, interferon-γ (IFN-γ) and interleukin-4 (IL-4) using an enzyme-linked immunosorbent assay (ELISA) commercial kit (Epitope Diagnostics, Inc., San Diego, CA, USA) in accordance with manufacturer’s protocols. Protein concentrations were quantified using a micro-bicinchoninic acid (BCA) protein assay (Solarbio, Beijing, China).
Histological evaluation and electron microscopy observation of the ultrastructure of the colonic mucosa
Distal segments of the colon were cut into 1 cm portions and fixed, and 5 µm-thick histological sections were mounted on gelatinized glass slides, dewaxed in xylene, dehydrated using graded concentrations of alcohol, and finally stained with hematoxylin and eosin (H&E).
Samples were immediately fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 2 hours at 22 ℃, then rinsed for 18 hours at 4 ℃ with 0.05 M Tris buffer (pH 7.6) and washed 3 times for 5 minutes each time. The ultrastructure of the colonic mucosa was observed by H-6600 transmission electron microscopy (Philips Tecnai-12, Amsterdam, Netherlands).
Immunohistochemical analysis
Paraffin sections were baked at 60 ℃ for 1 hour, then conventional xylene dewaxed to water, washed twice with distilled water, and then heated in a water bath for 15 minutes in citrate buffer solution for antigen repair. After being naturally brought to room temperature, rabbit serum working solution was added and incubated at room temperature for 10 minutes. Rabbit anti-mouse ZO-1 (CD117) polyclonal antibody (1:100) was added to the slides. The slides were incubated overnight at 4 ℃, washed with PBS 3 times the next day, for 3 minutes each time. Then, IgG II antibody was added, incubated at room temperature for 15 minutes, and washed 3 times with PBS, for 3 minutes each time. Horseradish enzyme-labeled streptomyces ovalbumin working solution (S-A/HRP) was added, incubated at room temperature for 15 minutes, and washed 3 times with PBS, for 3 minutes each time. Then, fresh DAB color solution was used to dye the sections for 3 minutes, after which the color was stopped. After washing fully with distilled water, the sections were re-stained with hematoxylin for 1 minute. The slides were then washed in water, dehydrated, and sealed in transparent and neutral tree film. The immunohistochemical staining results were observed under the microscope and photographed. The medical image analysis (MIA) system was used to detect the area density of positive cells (area density = positive cell area/positive cell area + negative cell area) in each high field of vision (200 times), and the area density of positive cells was analyzed by immunohistochemical staining. Image Pro Plus 6.0 was used to convert the surface density of positive cells into optical density (OD) for statistical analysis.
Western blotting analysis
After sacrificing the mice, 200 mg of intestinal tissues were collected and homogenized in cell lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.1% dodecyl sodium sulfate (SDS), 1% Nonidet P-40, 0.5% sodium deoxycholate, and 100 mg/mL phenylmethylsulfonyl). The homogenate was then centrifuged at 12,000 g for 5 minutes at 4 ℃, and the supernatant was collected. Proteins were quantified with micro-BCA protein assay (Solarbio, China), separated with 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline (TBS) for 2 hours at room temperature, and then were incubated with primary antisera (rabbit anti-mouse ZO-1 1:200 and anti-mouse tubulin 1:5,000, respectively; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 ℃ overnight, followed by incubation with a secondary antibody (goat anti-rabbit IgG-HRP 1:3,000, Santa Cruz Biotech) at 4 ℃ overnight. After a final wash in TBS + tween (TBST), chemiluminescent signals were detected on an autoradiographic film using an enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL, USA). Finally, band intensities were analyzed using gel electrophoresis and the imaging analysis system (PowerPac 200; Bio-Rad, Hercules, CA, USA). The results were expressed as the ratio between the relative intensity of the target protein and that of the internal standard tubulin.
Statistical analysis
All results were expressed as the mean ± SEM. Statistical analysis was performed by 2-way analysis of variance (ANOVA) and least significant difference-t (LSD-t) test, with SigmaStat statistical software (SPSS 11.5, IBM Corp., Chicago, IL, USA). A P value <0.05 was considered significant.
Results
Ginsenoside Rg1 ameliorated the severity of DSS-induced colitis
The DAI score, an indicator of the severity of intestinal inflammation, was first used to observe the severity of DSS-induced colitis. Mice treated with DSS for 7 days developed marked symptoms of acute colitis, with a DAI score of 7.3±1.06. Ginsenoside Rg1 treatment for 7 days dose-dependently reduced the DAI score in mice with colitis (Figure 1A), and there were significant differences between middle-dose or high-dose treated mice and control mice, P<0.05. Notably, as a first-line therapeutic agent for controlling inflammation in ulcerative colitis (16), our experimental results showed that the efficacy of high-dose of ginsenoside Rg1 is comparable to the 5-ASA treatment (Figure 1A).
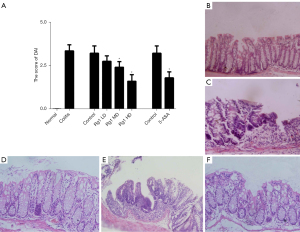
The morphology of distal segments of the colon was next observed with H&E straining under the optical microscope. Compared with normal mice (Figure 1B), mice with DSS-induced colitis exhibited a marked erosion of the lamina propria mucosae, disappearance of the glandular epithelium, and infiltration of inflammatory cells (Figure 1C). However, the administration of ginsenoside Rg1, particularly at a high-dose, significantly improved histopathologic features of colon tissue (Figure 1D). The erosion of the glandular epithelium disappeared, and the infiltration of inflammatory cells and other abnormalities tended to be less severe than that observed in control mice (Figure 1E), which were similar effects to those of the 5-ASA treatment (Figure 1F). These results indicated that ginsenoside Rg1, similar to 5-ASA, can significantly ameliorate the severity of DSS-induced colitis in a dose-dependent manner.
Ginsenoside Rg1 improved intestinal barrier disruption
Intercellular junctions in epithelial cells play a pivotal role in regulating mucosal barrier properties, which consist of adhesive structures known as tight junctions (TJ) and adherens junctions (AJ) (17). TJ proteins play an important role in maintaining the gut barrier (2). Therefore, we observed the effect of ginsenoside Rg1 on the ultrastructure of the colonic mucosa for morphological analysis of TJ using electron microscopy. Compared to that of normal mice (Figure 2A), the intestinal mucosa in mice with colitis exhibited a decrease in the number of microvilli, widened the intercellular space between epithelial cells, shorted the structure of TJ, and obscured or disappeared the dotted crystal structures (Figure 2B). However, the intestinal epithelium structure in mice treated with high-dose ginsenoside Rg1 appeared to have been repaired, and showed regularly aligned microvilli and distinct junction complexes (Figure 2C), compared to that of control mice (Figure 2D). The intestinal epithelium repaired by high-dose ginsenoside Rg1 was superior to that of 5-ASA-treated mice (Figure 2E).

Cytoplasmatic plaque proteins ZO-1 play a crucial role in maintaining the structure of TJ and the epithelial barrier function (18). We also observed the effect of ginsenoside Rg1 on the expression of ZO-1 protein by immunohistochemistry and western blot analysis. As shown in Figures 2F,2G, mice with DSS-induced colitis exhibited significant down-regulation of ZO-1 protein compared with the normal mice (P<0.05). High- and middle-dose ginsenoside Rg1 treatment for 7 days significantly up-regulated the expression of ZO-1 protein in mice with DSS-induced colitis (P<0.05), which were higher than that of 5-ASA treatment. These results suggested that ginsenoside Rg1 can repair intestinal barrier disruption in DSS-induced colitis mice.
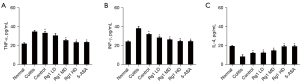
Ginsenoside Rg1 decreased colonic TNF-α and IFN-γ levels, while increased colonic IL-4 levels
Chronic inflammation is central to the pathogenesis of ulcerative colitis. In particular, TNF-α and IFN-γ can affect intestinal barrier function (19). To understand the mechanism of the effect of ginsenoside Rg1, we further analyzed the change of colonic TNF-α and IFN-γ levels before and after the treatment. Mice with DSS-induced colitis exhibited significantly higher levels of colonic TNF-α and IFN-γ than normal mice (Figure 3A,3B) (P<0.05), while the level of colonic IL-4 is decreased (Figure 3C). All ginsenoside Rg1-treated mice showed a dose-dependent reduction in TNF-α and IFN-γ levels (Figure 3A,3B) and increase in IL-4. In particular, high-dose ginsenoside Rg1 versus the control significantly reduced colonic TNF-α and IFN-γ levels (P<0.05), while the level of IL-4 significantly increases (P<0.05), which is consistent with that of 5-ASA treatment (Figure 3A-3C). These results indicated that the effect of ginsenoside Rg1 may be mediated by reducing colonic proinflammatory cytokines levels, at least those of TNF-α and IFN-γ, increasing the release of anti-inflammatory cytokine IL-4.
Discussion
Currently, IBD treatment mainly focuses on unspecific medications exerting anti-inflammatory or suppressive effects on the intestinal immune system (3), although mucosal healing is considered an important therapeutic approach in the management of IBD. Unfortunately, many of the medications have failed in clinical studies or had beneficial effects in subgroups of patients only (20). Although manipulation of intestinal permeability is a promising therapeutic approach, less is known about agents routinely used in IBD treatment in terms of restoring epithelial integrity and permeability (21). Some fatty acids, amino acids, oligoelements, and probiotics, when supplemented in IBD animal models, can decrease inflammation and restore mucosal permeability, but their therapeutic efficacy in IBD remains debatable (19). Je et al. found that ID-JPL934 down-regulates the expression of pro-inflammatory cytokines (including TNF-α, interleukin IL-1β, and IL-6), inhibits mucosal and submucosal immune cell infiltration, severe crypt damage, and goblet and epithelial cell loss, thereby improving enteritis symptoms (22). Kim et al. found that Moringa (Moringa oleifera Lam.) seed extract (MSE) rich in Moringa isothiocyanate-1 (MIC-1) (its specific biological activity) has a therapeutic effect on ulcerative colitis (UC), and its mechanism is related to the regulation of Nrf2 signaling pathway (23). In addition, more and more studies have been conducted on the effects of intestinal immunity and microflora on IBD. SCFA is an important fuel for intestinal epithelial cells and can strengthen intestinal barrier function. Recent studies have shown that SCFAs, especially butyrate, also have important immunomodulatory functions. SCFAs work by signaling through cell surface G-protein-coupled receptors (GPCRs), such as GPR41, GPR43, and GPR109A, which trigger an immune cascade (24). Liu et al. designed and prepared colon-targeted core-shell hydrogel microspheres, which could gather in colon tissues and regulate intestinal immune microenvironment to treat IBD. Probiotics are currently used as a treatment regimen for patients with IBD (25). Degirolamo et al. proposed that the use of VSL#3 enhances excretion of faecal BA and synthesis of BA in the liver, and the FXR-FGF15-BA-intestinal microbiota-intestinal epithelial barrier signaling pathway may be the mechanism of action (26).
The treatment of colitis with traditional Chinese medicine has a history of more than 100 years. In recent years, some scholars have done relevant research on the treatment of colitis by traditional Chinese medicine. Studies have shown that Moringa seed extract has anti-inflammatory and antioxidant activities, which can be used to prevent and treat UC (27). Moringa seed extract QYSXD can improve colitis /RIP3/NLRP3 pathway related proteins and reverse mitochondrial dysfunction to control inflammation by inhibiting the expression of RIP1 (28). In addition, a study has shown that Xianglian pills can inhibit inflammation and improve the intestinal epithelial barrier, and Quyu Shengxin Decoction can inhibit enteritis by inhibiting the expression of proteins related to the RIP1/RIP3/NLRP3 signaling pathway (29).
In the present study, we demonstrated that ginsenoside Rg1 can ameliorate the severity of colitis, reduce intestinal inflammation, and particularly also improve intestinal barrier disruption in DSS-induced colitis mice, as shown by ZO-1 protein expression and ultrastructure of the intestinal mucosa. To the best of our knowledge, this study is the first to demonstrate that ginsenoside Rg1 can repair intestinal mucosal barrier disruption. DSS-induced colitis is a well-established experimental model of intestinal inflammation, which has histological, physiologic, and biochemical features that mimic human disease (30). In the present study, DSS-treated mice exhibited predominant symptoms of colitis that were referred to as the DAI score, and high levels of pro-inflammatory cytokines and epithelial barrier dysfunction. Ginsenoside Rg1 administration (100 and 200 mg/kg) significantly ameliorated the symptoms of colitis, as demonstrated by DAI scores, and its efficacy is comparable to that of 5-ASA. 5-ASA is widely used as a first-line therapeutic agent for inducing remission and preventing relapse of ulcerative colitis through specifically reducing intestinal inflammation (16).
Intercellular junctions in the gut barrier consist of TJ and AJ (31). The TJ of epithelial cells are the most important component of the junction complex and constitute a network of continuous and anastomosing filaments between adjacent epithelial cells, which regulate the mucosal barrier capability (32). Altered TJ structure and epithelial permeability have been observed in IBD (33). In the present study, ultrastructure observation of the colonic mucosa demonstrated TJ structure destruction in DSS-induced colitis, and ginsenoside Rg1 administration, particularly when administered at a high dose (200 mg/kg), can repair the structure of intestinal epithelium and TJ in mice with colitis.
Little is known about the mechanisms by which ginsenoside Rg1 repairs intestinal barrier structure. TJ consist of an array of membrane-spanning proteins such as claudins and cytoplasmatic plaque proteins ZO-1 and ZO-2, which bind to the cytoskeleton (20,34). ZO-1 plays a crucial regulative role in maintaining the intestinal permeability barrier (18). Several studies have demonstrated that inflammatory cytokines induce the removal of TJ proteins from the membrane, resulting in a reduction in barrier function (21) and that IFN-γ and TNF-α induce selective endocytosis of the TJ proteins occludin in the intestinal epithelium (19,20), and decrease protein levels of ZO-1 in epithelial cells and the level of anti-inflammatory cytokine IL-4 (35-37).
Impaired intestinal barrier function leads to intestinal microbial translocation, promotes the production of pro-inflammatory cytokines, and further promotes the high activation of mucosal immune system, thus leading to inflammation in IBD patients (38,39). Previous works have shown the anti-inflammatory effect of ginsenoside Rg1 in mice with DSS-induced colitis (7). In our work, the anti-inflammatory effect of ginsenoside Rg1 also was observed. Ginsenoside Rg1 dose-dependently reduced colonic TNF-α, IFN-γ levels and increased IL-4, and particularly at the high dose, it significantly regulated colonic cytokine levels in mice with DSS-induced colitis. Its anti-inflammatory pathway has been demonstrated to inhibit NF-κB activation, phosphorylation of transforming growth factor beta-activated kinase 1, the binding of lipopolysaccharide (LPS) to toll-like receptor 4 on macrophages, pro-inflammatory cytokines expression and release, and so on (7,10). The mechanism of ginsenoside Rg1 repairing intestinal barrier structure may be associated with its effect of reducing pro-inflammatory cytokines and increasing anti-inflammatory cytokines. Indeed, in the present study, ginsenoside Rg1 administration, particularly high dose, significantly up-regulated the expression of ZO-1 protein in DSS-induced colitis mice. However, as not all anti-inflammatory therapeutics can repair intestinal barrier disruption, there may be other mechanisms by which ginsenoside Rg1 repairs intestinal barrier disruption.
Conclusions
This study demonstrated that ginsenoside Rg1 therapy can significantly ameliorate the severity of DSS-induced colitis in mice, and its efficacy is comparable to that of 5-ASA. Treatment with ginsenoside Rg1 can repair intestinal barrier structure by reducing colonic pro-inflammatory cytokine TNF-α and IFN-γ levels and increasing the anti-inflammatory cytokine IL-4 level, eliminate intestinal inflammation and further regulate mucosal immune function in DSS-induced colitis mice. We propose that ginsenoside Rg1 may provide a promising and novel approach to the treatment of IBD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5467/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5467/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5467/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures involving animals were approved by the Ethics Committee on Animal Experiments of Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine (No. 201704) and were in compliance with national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oh SJ, Seo Y, Ahn JS, et al. Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells. Mar Drugs 2019;17:622. [Crossref] [PubMed]
- Jergens AE, Parvinroo S, Kopper J, et al. Rules of Engagement: Epithelial-Microbe Interactions and Inflammatory Bowel Disease. Front Med (Lausanne) 2021;8:669913. [Crossref] [PubMed]
- Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther 2011;5:185-210. [Crossref] [PubMed]
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570-83. [Crossref] [PubMed]
- Triantafyllidi A, Xanthos T, Papalois A, et al. Herbal and plant therapy in patients with inflammatory bowel disease. Ann Gastroenterol 2015;28:210-20.
- Kim DH. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res 2012;36:1-15. [Crossref] [PubMed]
- Jin J, Zhong Y, Long J, et al. Ginsenoside Rg1 relieves experimental colitis by regulating balanced differentiation of Tfh/Treg cells. Int Immunopharmacol 2021;100:108133. [Crossref] [PubMed]
- Xie CL, Wang WW, Xue XD, et al. A systematic review and meta-analysis of Ginsenoside-Rg1 (G-Rg1) in experimental ischemic stroke. Sci Rep 2015;5:7790. [Crossref] [PubMed]
- Yousuf S, Liu H, Yingshu Z, et al. Ginsenoside Rg1 modulates intestinal microbiota and supports re-generation of immune cells in dexamethasone-treated mice. Acta Microbiol Immunol Hung 2022;69:259-69. [Crossref] [PubMed]
- Lee SY, Jeong JJ, Eun SH, et al. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol 2015;762:333-43. [Crossref] [PubMed]
- Zhu G, Wang H, Wang T, et al. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int Immunopharmacol 2017;50:1-5. [Crossref] [PubMed]
- Yoon SW, Lee CH, Kim JY, et al. Lactobacillus casei secreting alpha-MSH induces the therapeutic effect on DSS-induced acute colitis in Balb/c Mice. J Microbiol Biotechnol 2008;18:1975-83.
- Fan Y, Xia J, Jia D, et al. Mechanism of ginsenoside Rg1 renal protection in a mouse model of d-galactose-induced subacute damage. Pharm Biol 2016;54:1815-21. [Crossref] [PubMed]
- Zhou YH, Yu JP, Liu YF, et al. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm 2006;2006:92642. [Crossref] [PubMed]
- Gong Y, Li H, Li Y. Effects of Bacillus subtilis on Epithelial Tight Junctions of Mice with Inflammatory Bowel Disease. J Interferon Cytokine Res 2016;36:75-85. [Crossref] [PubMed]
- Hauso Ø, Martinsen TC, Waldum H. 5-Aminosalicylic acid, a specific drug for ulcerative colitis. Scand J Gastroenterol 2015;50:933-41. [Crossref] [PubMed]
- Naydenov NG, Feygin A, Wang D, et al. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci Rep 2016;6:24161. [Crossref] [PubMed]
- Rodgers LS, Beam MT, Anderson JM, et al. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J Cell Sci 2013;126:1565-75. [Crossref] [PubMed]
- Wang Y, Ye H, Qiao L, et al. Intestinal Anti-Inflammatory Effects of Selenized Ulva pertusa Polysaccharides in a Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease Model. J Med Food 2021;24:236-47. [Crossref] [PubMed]
- Katsanos KH, Papadakis KA. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver 2017;11:455-63. [Crossref] [PubMed]
- Michielan A, D'Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm 2015;2015:628157. [Crossref] [PubMed]
- Je IG, Lee DG, Jeong DG, et al. The Probiotic, ID-JPL934, Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice Through Inhibition of Proinflammatory Cytokines Expression. J Med Food 2018;21:858-65. [Crossref] [PubMed]
- Kim Y, Wu AG, Jaja-Chimedza A, et al. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One 2017;12:e0184709. [Crossref] [PubMed]
- Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol 2019;10:277. Erratum in: Front Immunol 2019;10:1486. [Crossref] [PubMed]
- Liu H, Cai Z, Wang F, et al. Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora. Adv Sci (Weinh) 2021;8:e2101619. [Crossref] [PubMed]
- Degirolamo C, Rainaldi S, Bovenga F, et al. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep 2014;7:12-8. [Crossref] [PubMed]
- Kim Y, Wu AG, Jaja-Chimedza A, et al. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One 2017;12:e0184709. [Crossref] [PubMed]
- Wang B, Gong Z, Zhan J, et al. Xianglian Pill Suppresses Inflammation and Protects Intestinal Epithelial Barrier by Promoting Autophagy in DSS Induced Ulcerative Colitis Mice. Front Pharmacol 2021;11:594847. [Crossref] [PubMed]
- Wu C, Yang H, Han C, et al. Quyu Shengxin Decoction Alleviates DSS-Induced Ulcerative Colitis in Mice by Suppressing RIP1/RIP3/NLRP3 Signalling. Evid Based Complement Alternat Med 2021;2021:6682233. [Crossref] [PubMed]
- Chassaing B, Aitken JD, Malleshappa M, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 2014;104:15.25.1-15.25.14.
- Bush KT, Keller SH, Nigam SK. Genesis and reversal of the ischemic phenotype in epithelial cells. J Clin Invest 2000;106:621-6. [Crossref] [PubMed]
- Das P, Goswami P, Das TK, et al. Comparative tight junction protein expressions in colonic Crohn's disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch 2012;460:261-70. [Crossref] [PubMed]
- Cichon C, Sabharwal H, Rüter C, et al. MicroRNAs regulate tight junction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers 2014;2:e944446. [Crossref] [PubMed]
- Otani T, Nguyen TP, Tokuda S, et al. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J Cell Biol 2019;218:3372-96. [Crossref] [PubMed]
- Bowie RV, Donatello S, Lyes C, et al. Lipid rafts are disrupted in mildly inflamed intestinal microenvironments without overt disruption of the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 2012;302:G781-93. [Crossref] [PubMed]
- Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 1999;276:G1279-88. [Crossref] [PubMed]
- Ho SW, El-Nezami H, Corke H, et al. L-citrulline enriched fermented milk with Lactobacillus helveticus attenuates dextran sulfate sodium (DSS) induced colitis in mice. J Nutr Biochem 2022;99:108858. [Crossref] [PubMed]
- Amoroso C, Perillo F, Strati F, et al. The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation. Cells 2020;9:1234. [Crossref] [PubMed]
- Wu Y, Jha R, Li A, et al. Probiotics (Lactobacillus plantarum HNU082) Supplementation Relieves Ulcerative Colitis by Affecting Intestinal Barrier Functions, Immunity-Related Gene Expression, Gut Microbiota, and Metabolic Pathways in Mice. Microbiol Spectr 2022;10:e0165122. [Crossref] [PubMed]
(English Language Editor: J. Jones)


