
Application of oligoclonal bands and other cerebrospinal fluid variables in multiple sclerosis and other neuroimmunological diseases: a narrative review
Introduction
The presence of cerebrospinal fluid-specific oligoclonal bands (CSF-OCBs) reflects intrathecal immunoglobulin G (IgG) synthesis, which has been considered important in the diagnosis of multiple sclerosis (MS). In this review, we summarized the history of development of oligoclonal bands (OCBs), and interpreted the clinical implications of different types of OCBs in neuroimmune and other diseases of central nervous system (CNS), especially in MS, extending the scope of clinical application of OCB as a diagnostic indicator. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-3073/rc).
Methods
The search engine PubMed (https://www.ncbi.nlm.nih.gov/pmc/) was used to research the keywords: “blood brain barrier”, “blood brain barrier permeability”, “detection methods”, “multiple sclerosis” and “oligoclonal bands”. A narrative review was conducted to review findings of published literatures in English from 1937 to 2021 including case reports, case series, cohort studies, reviews, experiment studies and so on. We used a table to present detailed search strategy (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 2021.6.1 |
| Databases and other sources searched | PubMed |
| Search terms used | “Blood brain barrier” [MeSH], “blood brain barrier permeability” [MeSH], “detection methods” [MeSH], “multiple sclerosis” [MeSH], “oligoclonal bands” [MeSH] |
| Timeframe | 1937–2021 |
| Inclusion and exclusion criteria | Focus was placed on original papers and reviews in English about the history of oligoclonal bands, biomarker of multiple sclerosis, blood-brain barrier permeability and intrathecal synthesis of immunoglobulin. We excluded articles without information about oligoclonal bands |
| Selection process | The literature searches were conducted by Haiqiang Jin, Qianshuo Lu, and Feng Gao |
The history of oligoclonal bands
In 1937, the Swedish scientist Tiselius (1) established electrophoresis technology, and for the first time it was proved that the serum was composed of albumin (Alb) and α, β, and γ globulins. Tiselius won the 1948 Nobel Prize in Chemistry for this work. In 1942, Kabat et al. (2) performed electrophoresis of cerebrospinal fluid (CSF) and reported increased gamma globulins in multiple sclerosis (MS). Afterwards, in 1950, they reported that 80% of MS patients had elevated γ globulin levels in the CSF (3). Many researchers thereafter successively reported increased γ globulins in the CSF of MS patients, with a prevalence ranging from 57–83% (4,5).
In 1948, Kabat performed quantitative immunochemical precipitation of albumin and IgG on CSF analysis (6). During the 1950s and 1970s, with the development of electrophoresis technology, CSF protein electrophoresis technique also improved rapidly. Lowenthal et al. (7) found some specific bands in the γ globulin zone of the CSF of multiple sclerosis (MS) and subacute sclerosing panencephalitis (SSPE) patients, which could not be detected in serum. In 1967, Link et al. (8,9) confirmed that these bands have the property of IgG when he isolated the immunoglobulins from the CSF of MS patients. Meanwhile, Tourtellotte et al. (10,11) found that there was a positive correlation between the IgG level in the demyelinating plaques and CSF of MS patients. And the concentration of γ globulin increased apparently in MS brains than normal brains. Therefore, they put forward the theory of local synthesis of IgG in the brain of MS patients. Subsequently, Felgenhauer (12) demonstrated the absence of high molecular weight haptoglobin oligomers in normal CSF. Laterre et al. (13) detected and analyzed the electrophoretic pattern of CSF γ globulins in MS and some other CNS inflammatory diseases, and confirmed the diagnostic value of this pattern of discrete bands within the γ globulins, which had been designated as “oligoclonal aspect”. Finally, these specific bands were named as oligoclonal bands (OCB) (14). The term OCB was coined based on the assumption that, in inflammatory neurological illnesses like MS, a highly restricted number of B-cell clones were triggered in the CNS and CSF compartments and transformed into Ig-secreting plasma cells, each clone producing Ig of highly restricted mobility on electrophoresis.
Detection techniques
Conventional electrophoresis techniques using agarose gel or polyacrylamide gel as a separation medium. There are two techniques: agarose gel electrophoresis/Coomassie blue staining and polyacrylamide gel electrophoresis (PAGE)/silver ammonia staining. Both techniques can only separate albumin and α1, α2, β and γ globulin and some OCBs depending on the difference in molecular weight. The resolution of OCBs detection is limited.
With the development of electrophoresis technology, isoelectric focusing electrophoresis (IFE) can further improve the resolution of protein separation according to the difference of isoelectric point (15). Currently, it is widely used in the detection of OCB. More specifically, IFE is based on an equal state of the isoelectric point of the protein molecule itself and the pH of the amphoteric dielectric of the separation medium of PAGE, hence, the protein separation is more sensitive and diverse. Furthermore, immunofixation or western blotting are added using enzyme-labeled anti-IgG (or IgA/IgM) antibodies to identify components and subclass of antibody of the bands (16). IgG band is much more common compared with IgA and IgM bands. Moreover, polyclonal background staining was largely reduced with anti-immunoglobulin staining (16).
For both conventional electrophoresis techniques and IFE, the oligoclonal bands represent two or more bands on the electrophoresis because the oligoclonal antibodies are thought to be derived from several B cell lineages. The IFE method with specificity on immunoglobulin IgG is generally recommended for its higher sensitivity and specificity, and is currently recommended as the "gold standard" for OCB detection (17). Furthermore, double staining of freeκandλlight chains expand the identification of immunoglobulin constituents, which is helpful in the diagnosis of MS (18).
OCBs interpretation and clinical implications
In the 1950s and 1960s, positive OCBs were defined as the presence of more than two bands in the gamma globulin region in the conventional protein electrophoresis. However, with the IFE, it is emphasized that the bands should appear within the range of pH 3.0–10.0. For more focused detection, the range of pH 6.0–9.0 is recommended (19).
There are five typical OCB patterns for CSF and serum by IFE and immunofixation assays, as shown in Figure 1.
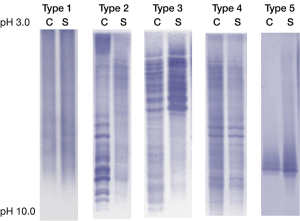
Type 1: OCB was not detected in CSF and serum. Polyclonal bands might exist, but they are not stained by immunofixation or western blotting assays. Type 1 pattern is always reported as both negative in CSF and serum. This pattern is seen in normal individuals or various neurological disorders without immune reactions within the CNS.
Type 2: OCB appears in the CSF but not in the serum. This pattern is reported as CSF specific OCB and is commonly seen in MS and is a biomarker to support the diagnosis of MS. However, this pattern is not unique to MS. It can be seen in autoimmune encephalitis, connective tissue disease, optic neuromyelitis and some specific infection, including tuberculosis, syphilis, human immunodeficiency virus (HIV) and viral encephalitis (20-23). This pattern indicates the intrathecal synthesis of immunoglobulin. In MS, once this pattern appears, it tends to persist despite of traditional treatment, with exception of after potent immunomodulating agents such as Natalizumab (24). Therefore, this pattern might indicate persisting immunoglobulin synthesis in CNS.
Type 3: Multiple bands appear both in the CSF and in the serum. However, some bands in CSF are different from those in the paired serum. This pattern can also be reported as CSF specific OCBs, however, “more in CSF” might be a detailed description of this pattern and mark the difference from pattern II clearly. This pattern is seen in MS and infections of CNS, such as cerebral cysticercosis or hydatid infection. It is also seen in acute disseminated encephalomyelitis (ADEM), acute myelitis, encephalitis and connective tissue disease (21-23). Although intrathecal immunoglobulin synthesis is also indicated in this pattern, the persistence of synthesis is less known.
Type 4: Same multiple bands appear both in the CSF and in the serum, which presents as “mirror identical”. This pattern is reported as identical OCBs in CSF and serum. Increased BBB or blood-nerve barrier (BNB) permeability is considered as the cause of “mirror identical” (25). Other potential mechanisms for this pattern need to be analyzed in combination with IgG index and IgG synthesis rate.
Type 5: This pattern is not seen with conventional detection method, instead, it appears with the isofocus electrophoresis + immunolabeling technique. The bands are very dense and confluent in the CSF and serum like “twins”, which is called “monoclonal bands”. It usually occurs in patients with plasma cell diseases, such as polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes (POEMS) syndrome and monoclonal gammaglobulinemia (MGUS). Patients with this OCB pattern were recommended to perform bone marrow aspiration biopsy, serum M protein, kappa light chain and lambda light chain, as well as urine Bence-Jones protein. This pattern itself does not suggest that synthesis within the central nervous system. However, the exact mechanism remains unclear, which may need the comprehensive evaluation of the BBB permeability, IgG index and IgG synthesis rate (26).
In summary, Type 1 often indicates normal immune response within CNS. Type 2 indicates an intrathecal synthetic immunoglobulin, perhaps due to persistent limited B cell clone response. Type 3 indicates an immune response against specific infectious agents or an autoimmune process that activates certain types of B cells to produce antibodies. Type 4 indicates impaired BBB. The Type 5 indicates a plasma cell disease instead of intrathecal immunoglobulin synthesis (27-30).
Clinical significance of OCB
OCB in MS
CSF specific OCB is considered to be manifestation of chronic immune activation in CNS. Numerous results have shown that CSF specific OCB is supportive of MS with high sensitivity and specificity, especially in clinically isolated syndrome (CIS) patients. A meta-analysis reported that, when using CSF specific OCB to predict the conversion from CIS to MS, the sensitivity was 0.84, the specificity was 0.54, the positive predictive value was 0.64 and the negative predictive value was 0.77 (31). CIS patients with CSF specific OCB are more likely to relapse and convert to MS than those without OCB (32,33). Therefore, OCB is considered as a good biomarker for predicting the conversion from CIS to MS. In 2017 McDonald criteria, OCB was included as a diagnostic biomarker in judging dissemination in time due to its prognostic significance of relapse (34,35). The 2017 revision allows earlier diagnosis of MS and initiation of disease-modifying therapy in CIS patients with positive OCB (36).
However, the application of OCB in MS diagnosis also has some problems. First, the high sensitivity and specificity of OCB in diagnosing MS is derived from typical CIS patients, which excluded most of other diseases with positive OCB. In other words, the specificity will decrease significantly when doctors want to differentiate MS from other CNS inflammatory diseases via OCB merely (37). Secondly, reliable OCB detection requires standard detection method and experienced interpretation (36). Therefore, it is important is to make differential diagnosis between MS and other immunological or infectious neurological diseases by clinical features first, because the initial diagnosis will influence the subsequent diagnostic thinking and explanation of the detected OCBs.
Early study showed that there was no obvious difference in demographic and clinical features, including sex predominance, mean age of onset, proportion of cases with a primary progressive course and rate of magnetic resonance imaging (MRI) positivity, between MS patients with and without OCB (38). However, recently, it is reported that MS patients with OCB may develop much more cortical lesions than patients without OCB after disease duration of almost 10 years, possibly resulting from B cells response and proinflammatory CSF profile (39). It suggested that OCB might be a potential biomarker to predict disease prognosis. So, whether OCB status is predictive of disease progression remains inconclusive.
Differences in OCB prevalence in MS between Eastern and Western countries
In the recently published manuscript, Kim et al. reported 88.6% Korean patients with MS were positive for CSF-OCBs, which suggests the prevalence of CSF-OCBs is not different between Korean and Western patients with MS (40). Similarly, a recent study from Japan reported a high prevalence (74%) of CSF-OCBs in 83 patients with MS after careful exclusion of NMOSD, myelin oligodendrocyte glycoprotein associated disease (MOGAD), and other MS-mimics (41). Furthermore, the detection method may also influence the positive rate of OCBs in Chinese MS. So the large-scale studies with multiple centers in Eastern countries are needed to further study the positive rate of OCBs in Asian patients with MS.
There are many studies have proved that environmental factors can affect the incidence of OCB and are directly related to latitude. The prevalence of MS is positively related to latitude. A research suggested that this may be due to different latitudes of ultraviolet radiation and vitamin D intake, because ultraviolet/vitamin D plays a key regulatory role in the immune system (42). A database analysis of a large number of MS patients showed that the presence of OCB was positively correlated with latitude, which meant that the farther away from the equator, the higher the probability of OCB positive (37). However, Italy and China are at the same latitude, there was 79% positive rate of OCBs in Italian patients with CIS, which was higher than that of China (43). The reason for this difference is unclear, but it may reflect changing cultural habits, including attitudes to sunbathing and sunscreen, which in turn affect vitamin D levels in the population (44).
OCB in neuromyelitis optica spectrum disease (NMOSD) and myelin oligodendrocyte glycoprotein (MOG) antibody-associated disorders
Aquaporins 4 (AQP4) autoantibody is a specific biomarker of NMOSD (45) and MOG-IgG antibody is a biomarker of MOG-associated encephalomyelitis (MOG-EM) (46). Oligoclonal bands are found in up to 95% of patients with relapsing-remitting MS (RRMS), but only in 10–25% of patients with NMOSD (47). Jarius et al. (48) reported that CFS specific OCB was found in 29/177 samples from AQP4-IgG positive NMOSD patients of mostly Caucasian origin. Among the 29 samples, 19 cases were type 2 OCB, indicating the intrathecal IgG synthesis. More interesting, Jarius et al. (48) observed that OCB could disappear in 6 NMOSD patients during the course of disease, which was rarely reported in MS. So, the presence of OCB in the CSF might be transitional in NMOSD. Wang et al., found that NMOSD patients with or without spinal cord atrophy (SCA) have similar proportion of positive OCBs (49). Chen et al demonstrated that a combination of the onset age and IgG index could serve as an alternative to CSF-OCB for differentiating between RRMS and NMOSD in Chinese patients (50). Obviously, the proportion of positive OCB in NMOSD was greatly lower than MS. In the face of a relative scarcity of biomarkers differentiating NMOSD from MS, OCB remain one of the most helpful parameters. Similar to NMOSD, only 13% of MOG-IgG antibody-associated syndromes had OCB and it disappeared in 2 out of 6 patients (51). So, comprehensively collecting and assessing the data from a candidate MS patient is important before a probable diagnosis is drawn.
OCB in other inflammatory diseases
Based on the understanding of the nature of OCB, we could speculate that any disorder that can trigger B cells response and subsequent intrathecal IgG synthesis within subarachnoid space may have positive CSF OCB. Actually, in addition to MS, NMOSD and MOG antibody-associated disorders, other infectious and autoimmunological diseases, such as neurosyphilis, herpes virus encephalitis, Guilin-Barre syndrome, lupus encephalopathy, neurosarcoidosis, Behcet’s disease, CNS vasculitis and paraneoplastic disorders have also been reported to have positive OCB (37). Some, but rarely all of the CSF OCB observed in CNS infections may contain antibodies directed against the etiologic agent. During follow up of such patients, the CSF OCB could persist for more than 2 years although the disease is in a stable state. In most of them, a continuous decrease in the number of OCBs over time was observed. In the minority patients, the persistent CSF OCB after a viral CNS infection may reflect a latent infection in the CNS or unspecific immune cell stimulation resulting in continuous antibody synthesis within the CNS (52,53). So, the results of OCBs only indicate the inflammation of CNS and should be evaluated with the clinical context. Explanation of OCBs should be interpreted with caution, to avoid both the misdiagnosis and overdiagnosis of MS.
The evaluation of BBB permeability and intrathecal immunoglobulin synthesis
Detection of albumin, IgG, IgA, IgM is equally important, which reflects the BBB permeability and intrathecal immunoglobulin synthesis. Immunoturbidimetry is recommended to detect these proteins because of higher sensitivity and specificity. As a result, CSF/serum albumin ratio, IgG-Index, IgA-Index, IgM-Index, intrathecal IgG synthesis (IgG-syn) rate, IgA-syn rate and IgM-syn rate can be calculated.
Analysis of BBB permeability
BBB are composed of many structures that prevent some macromolecules, such as proteins, from entering the CSF from the blood. However, in CNS diseases, increased CSF immunoglobulin level can be observed. It is due to the impaired BBB permeability that allows immunoglobulin to flow into CSF from the blood, or intrathecal synthesis of immunoglobulin, or combination of both (54-56).
During the inflammation process of CNS and peripheral nervous system (PNS), B cells migrate to nerve system and produce immunoglobulin. The newly synthesized immunoglobulin filled into the immunoglobulin pool of CSF, which also includes the immunoglobulin derived from serum (57,58). Since the integrity of the BBB and the flow of serum determine the total protein content of CSF, the integrity of BBB can be assessed by detecting the total protein of CSF (59). CSF/serum albumin ratio, also known as CSF/serum albumin quotient (QAlb), is the basis of BBB permeability analysis. Normal BBB permeability in adults was usually defined as a QAlb ≤6.5 if age <40 years and QAlb ≤8.0 if age ≥40 years (54). Uher et al. (60) demonstrated that increased QAlb in patients after first clinical event suggestive of multiple sclerosis is associated with development of brain atrophy and greater disability 48 months later. QAlb can not only reflect the BBB permeability, but also can reflect the severity of ongoing inflammation or damage in the CNS. Akaishi et al. (61) found that in MS patients, the multiple sclerosis severity score showed a higher correlation with QAlb; in NMOSD patients, QAlb elevated in both the acute phase and chronic phase, and such elevation was larger in the acute phase than in the chronic phase.
Analysis of intrathecal immunoglobulin synthesis
To prove the intrathecal immunoglobulin synthesis, extra immunoglobulins, except for that caused by the impairment of BBB permeability, should be acquired. There are several methods to adjust the BBB permeability. The simplest is the IgG-Index, which is calculated as (CSF IgG/serum IgG)/(CSF Alb/serum Alb). However, this index only takes the apparent IgG values into account. A more complicated formula was introduced to overcome the simple proportional calculation by adjusting the molecular weight and CSF production per day. The IgG synthesis rate is calculated with an empirical formula as {[(CSF IgG − serum IgG)/K1] − [(CSF Alb − serum Alb)/K2] × (serum IgG/serum Alb) × 0.43} × 5. K1 and K2 indicate the average normal serum: CSF ratios for IgG and albumin, respectively; 0.43 is the molecular weight ratio of albumin:IgG; 5 indicates that more than 5 dL CSF is produced per day for an adult (62). This formula gives more exact evaluation than IgG index. Importantly, the above parameter should be refined based on reference value of its own laboratory (63). In the 2017 McDonald criteria of MS, the Panel’s discussion of CSF recognized the importance of using appropriate and standardized technology in detecting intrathecal synthesis (34). The qualitative demonstration of two or more CSF-specific oligoclonal bands more reliably indicates intrathecal antibody synthesis than other tests do, such as the IgG index (32-34). In the diagnosis of MS, positive results on these other tests should be interpreted with caution when testing for OCBs is negative or not done.
Detection of other CSF immunologic biomarkers for MS
Over the past few years, there have been many studies exploring the role of CSF free immunoglobulin light chains (FLCs) in the diagnosis of MS (64-66). The presence of OCB in CSF is an important feature of MS, but for patients who meet the diagnostic criteria of MS without detectable IgG OCB, whether they have MS is still a problem. One explanation is that IFE with WB is not 100% sensitive to OCB detection. Goffette et al. (64) found that in patients with typical clinical signs and imaging evidence suggestive of MS but without CSF IgG OCBs, oligoclonal free kappa light chains could be detected by an immunoaffinity mediated immunoblotting technique. Therefore, the presence of oligoclonal free kappa light chains in CSF might be an important clue for MS diagnosis.
There are two types of FLC in humans, κFLC and λFLC. It is generally accepted that the concentration of κFLC increases in CSF of MS patients (65). However, the data about λFLC is limited. Makshakov et al. (66) reported that the levels of κFLC and λFLC elevated in MS patients compared with non-inflammatory neurologic diseases. However the relative increase of λFLC was less than κFLC. Moreover, DeCarli et al. (67) detected the CSF κFLC and λFLC in patients with MS or acute CNS infections. They observed that compared to patients with CNS infectious diseases, MS patients had higher level of κFLC but similar level of λFLC. The disproportionate increase of the concentration of κFLC and λFLC in CSF is a common phenomenon in CIS and MS with clinical manifestations. They also found a significant correlation between κFLC and total IgG in patients with MS, and between λFLC and total IgG in CNS infections. Therefore, κFLC is thought to have greater value in the diagnosis of MS than λFLC, and elevated λFLC is considered a potential marker of infection.
In addition to FLC, oligoclonal IgM is also an important biomarker for MS, especially for its prognosis. Villar et al. (68) found that RRMS patients with positive CSF oligoclonal IgM bands had a greater proportion of conversion to secondary progressive multiple sclerosis (SPMS) and higher level of expanded disability state scale (EDSS) score than those who without oligoclonal IgM. García-Barragán et al. (69) reported that MS patients with OCB IgM showed better response to immunotherapy than patients without OCB IgM.
Conclusions
In conclusion, OCB has important ancillary diagnostic value in MS and other neuroinflammatory diseases, but it is not disease specific. Therefore, its diagnosis value should be determined with the disease clinical phenotype. Types 2 and 3 OCB are helpful for the determination of intrathecal synthesis. Currently, the relationship between OCB pattern and disease phenotype as well as whether it will disappear or persistently exist in specific neuroinflammatory diseases still need further study. The negative OCB does not rule out MS. Other indicators including IgM, IgA, kappa (κ) and lambda (λ) are also helpful in suggesting the possibility of MS, and their value in differentiating MS from other neuroinflammatory diseases needs to be further studied. The combination of permeability of BBB and intrathecal synthesis rate of IgG may contribute to the differentiation among different neuroinflammatory diseases.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 82071306).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hai-Feng Li and Xiangjun Chen) for the series “Laboratory Investigations in Neuroimmunological Diseases and Their Clinical Significance” published in Annals of Translational Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-3073/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-3073/coif). The series “Laboratory Investigations in Neuroimmunological Diseases and Their Clinical Significance” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tiselius A. A New Apparatus for Electrophoretic Analysis of Colloidal Mixtures. Trans Faraday Soc 1937;33:524-31. [Crossref]
- Kabat EA, Landow H, Moore DH. Electrophoretic Patterns of Concentrated Cerebrospinal Fluid. Experimental Biology & Medicine 1942;49:260-3. [Crossref]
- Kabat EA, Freedman DA. A study of the crystalline albumin, gamma globulin and total protein in the cerebrospinal fluid of 100 cases of multiple sclerosis and in other diseases. Am J Med Sci 1950;219:55-64. [Crossref] [PubMed]
- Tourtellotte WW. Cerebrospinal fluid immunoglobulins and the central nervous system as an immunological organ particularly in multiple sclerosis and subacute sclerosing panencephalitis. Res Publ Assoc Res Nerv Ment Dis 1971;49:112-55. [PubMed]
- Ivers RR, McKenzie BF, McGuckin WF, et al. Spinal-fluid gamma globulin in multiple sclerosis and other neurologic diseases. Electrophoretic patterns in 606 patients. JAMA 1961;176:515-9. [Crossref] [PubMed]
- Kabat EA, Glusman M, Knaub V. Quantitative estimation of the albumin and gamma globulin in normal and pathologic cerebrospinal fluid by immunochemical methods. Am J Med 1948;4:653-62. [Crossref] [PubMed]
- Lowenthal A, Vansande M, Karcher D. The differential diagnosis of neurological diseases by fractionating electrophoretically the CSF gamma-globulins. J Neurochem 1960;6:51-6. [Crossref] [PubMed]
- Link H. Immunoglobulin G and low molecular weight proteins in human cerebrospinal fluid. Chemical and immunological characterisation with special reference to multiple sclerosis. Acta Neurol Scand 1967;43:1-136. [PubMed]
- Link H. Qualitative changes in immunoglobulin G in multiple sclerosis-cerebrospinal fluid. Acta Neurol Scand 1967;43:180-1. [Crossref] [PubMed]
- Tourtellotte WW, Parker JA. Multiple sclerosis: correlation between immunoglobulin-G in cerebrospinal fluid and brain. Science 1966;154:1044-5. [Crossref] [PubMed]
- Tourtellotte WW, Parker JA. Multiple sclerosis: brain immunoglobulin-G and albumin. Nature 1967;214:683-6. [Crossref] [PubMed]
- Felgenhauer K. Quantitation and specific detection methods after disc electrophoresis of serum proteins. Clin Chim Acta 1970;27:305-12. [Crossref] [PubMed]
- Laterre EC, Callewaert A, Heremans JF, et al. Electrophoretic morphology of gamma globulins in cerebrospinal fluid of multiple sclerosis and other diseases of the nervous system. Neurology 1970;20:982-90. [Crossref] [PubMed]
- Link H. Oligoclonal immunoglobulin G in multiple sclerosis brains. J Neurol Sci 1972;16:103-14. [Crossref] [PubMed]
- Bjellqvist B, Ek K, Righetti PG, et al. Isoelectric focusing in immobilized pH gradients: principle, methodology and some applications. J Biochem Biophys Methods 1982;6:317-39. [Crossref] [PubMed]
- Mehta PD, Patrick BA, Wisniewski HM. Isoelectric focusing and immunofixation of cerebrospinal fluid and serum in multiple sclerosis. J Clin Lab Immunol 1981;6:17-22. [PubMed]
- Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 2005;62:865-70. [Crossref] [PubMed]
- Sindic CJ, Laterre EC. Oligoclonal free kappa and lambda bands in the cerebrospinal fluid of patients with multiple sclerosis and other neurological diseases. An immunoaffinity-mediated capillary blot study. J Neuroimmunol 1991;33:63-72. [Crossref] [PubMed]
- Kjellin KG, Vesterberg O. Isoelectric focusing of CSF proteins in neurological diseases. J Neurol Sci 1974;23:199-213. [Crossref] [PubMed]
- Stangel M, Fredrikson S, Meinl E, et al. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol 2013;9:267-76. [Crossref] [PubMed]
- Pars K, Pul R, Schwenkenbecher P, et al. Cerebrospinal Fluid Findings in Neurological Diseases Associated with Sjögren's Syndrome. Eur Neurol 2017;77:91-102. [Crossref] [PubMed]
- Ernerudh J, Olsson T, Lindström F, et al. Cerebrospinal fluid immunoglobulin abnormalities in systemic lupus erythematosus. J Neurol Neurosurg Psychiatry 1985;48:807-13. [Crossref] [PubMed]
- Bourahoui A, De Seze J, Guttierez R, et al. CSF isoelectrofocusing in a large cohort of MS and other neurological diseases. Eur J Neurol 2004;11:525-9. [Crossref] [PubMed]
- von Glehn F, Farias AS, de Oliveira AC, et al. Disappearance of cerebrospinal fluid oligoclonal bands after natalizumab treatment of multiple sclerosis patients. Mult Scler 2012;18:1038-41. [Crossref] [PubMed]
- Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol 2011;686:149-73. [Crossref] [PubMed]
- Jo JC, Yoon DH, Kim S, et al. Clinical significance of the appearance of abnormal protein band in patients with multiple myeloma. Ann Hematol 2014;93:463-9. [Crossref] [PubMed]
- Ferraro D, Franciotta D, Bedin R, et al. A multicenter study on the diagnostic significance of a single cerebrospinal fluid IgG band. J Neurol 2017;264:973-8. [Crossref] [PubMed]
- Chu AB, Sever JL, Madden DL, et al. Oligoclonal IgG bands in cerebrospinal fluid in various neurological diseases. Ann Neurol 1983;13:434-9. [Crossref] [PubMed]
- Riddoch D, Thompson RA. Immunoglobulin levels in the cerebrospinal fluid. Br Med J 1970;1:396-9. [Crossref] [PubMed]
- Davies G, Keir G, Thompson EJ, et al. The clinical significance of an intrathecal monoclonal immunoglobulin band: a follow-up study. Neurology 2003;60:1163-6. [Crossref] [PubMed]
- Dobson R, Ramagopalan S, Davis A, et al. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 2013;84:909-14. [Crossref] [PubMed]
- Schwenkenbecher P, Sarikidi A, Bönig L, et al. Clinically Isolated Syndrome According to McDonald 2010: Intrathecal IgG Synthesis Still Predictive for Conversion to Multiple Sclerosis. Int J Mol Sci 2017;18:2061. [Crossref] [PubMed]
- Boscá I, Magraner MJ, Coret F, et al. The risk of relapse after a clinically isolated syndrome is related to the pattern of oligoclonal bands. J Neuroimmunol 2010;226:143-6. [Crossref] [PubMed]
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162-73. [Crossref] [PubMed]
- Deisenhammer F, Zetterberg H, Fitzner B, et al. The Cerebrospinal Fluid in Multiple Sclerosis. Front Immunol 2019;10:726. [Crossref] [PubMed]
- Hartung HP, Graf J, Aktas O, et al. Diagnosis of multiple sclerosis: revisions of the McDonald criteria 2017 - continuity and change. Curr Opin Neurol 2019;32:327-37. [Crossref] [PubMed]
- Petzold A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol 2013;262:1-10. [Crossref] [PubMed]
- Imrell K, Landtblom AM, Hillert J, et al. Multiple sclerosis with and without CSF bands: clinically indistinguishable but immunogenetically distinct. Neurology 2006;67:1062-4. [Crossref] [PubMed]
- Farina G, Magliozzi R, Pitteri M, et al. Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: a combined CSF and MRI study. J Neuroinflammation 2017;14:40. [Crossref] [PubMed]
- Kim KH, Kim SH, Park NY, et al. Reappraisal of CSF-specific oligoclonal bands in Asia. Mult Scler 2022;28:665-8. [Crossref] [PubMed]
- Akaishi T, Takahashi T, Misu T, et al. Difference in the Source of Anti-AQP4-IgG and Anti-MOG-IgG Antibodies in CSF in Patients With Neuromyelitis Optica Spectrum Disorder. Neurology 2021;97:e1-e12. [Crossref] [PubMed]
- Persson L, Longhi S, Enarsson J, et al. Elevated antibody reactivity to measles virus NCORE protein among patients with multiple sclerosis and their healthy siblings with intrathecal oligoclonal immunoglobulin G production. J Clin Virol 2014;61:107-12. [Crossref] [PubMed]
- Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016;6:28484. [Crossref] [PubMed]
- Lechner-Scott J, Spencer B, de Malmanche T, et al. The frequency of CSF oligoclonal banding in multiple sclerosis increases with latitude. Mult Scler 2012;18:974-82. [Crossref] [PubMed]
- Mader S, Brimberg L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019;8:90. [Crossref] [PubMed]
- Waters P, Woodhall M, O'Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015;2:e89. [Crossref] [PubMed]
- Marignier R, Bernard-Valnet R, Giraudon P, et al. Aquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entity. Neurology 2013;80:2194-200. [Crossref] [PubMed]
- Jarius S, Paul F, Franciotta D, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci 2011;306:82-90. [Crossref] [PubMed]
- Wang Y, Wu A, Chen X, et al. Comparison of clinical characteristics between neuromyelitis optica spectrum disorders with and without spinal cord atrophy. BMC Neurol 2014;14:246. [Crossref] [PubMed]
- Chen B, Tian DS, Bu BT. A Comparison of IgG Index and Oligoclonal Band in the Cerebrospinal Fluid for Differentiating between RRMS and NMOSD. Brain Sci 2021;12:69. [Crossref] [PubMed]
- Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016;13:280. [Crossref] [PubMed]
- Fryden A, Link H, Norrby E. Cerebrospinal fluid and serum immunoglobulins and antibody titers in mumps meningitis and aseptic meningitis of other etiology. Infect Immun 1978;21:852-61. [Crossref] [PubMed]
- Link H, Huang YM. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J Neuroimmunol 2006;180:17-28. [Crossref] [PubMed]
- Lo Sasso B, Agnello L, Bivona G, et al. Cerebrospinal Fluid Analysis in Multiple Sclerosis Diagnosis: An Update. Medicina (Kaunas) 2019;55:245. [Crossref] [PubMed]
- Reiber H, Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta 1987;163:319-28. [Crossref] [PubMed]
- Fischer C, Arneth B, Koehler J, et al. Kappa free light chains in cerebrospinal fluid as markers of intrathecal immunoglobulin synthesis. Clin Chem 2004;50:1809-13. [Crossref] [PubMed]
- Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta 2001;310:173-86. [Crossref] [PubMed]
- Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001;184:101-22. [Crossref] [PubMed]
- Ebers GC. The CSF Proteins: A Biochemical Approach. Canadian Journal of Neurological Sciences 1990;17:260. [Crossref]
- Uher T, Horakova D, Tyblova M, et al. Increased albumin quotient (QAlb) in patients after first clinical event suggestive of multiple sclerosis is associated with development of brain atrophy and greater disability 48 months later. Mult Scler 2016;22:770-81. [Crossref] [PubMed]
- Akaishi T, Narikawa K, Suzuki Y, et al. Importance of the quotient of albumin, quotient of immunoglobulin G and Reibergram in inflammatory neurological disorders with disease-specific patterns of blood–brain barrier permeability. Neurology and Clinical Neuroscience 2015;3:94-100. [Crossref]
- Bonnan M. Intrathecal IgG synthesis: a resistant and valuable target for future multiple sclerosis treatments. Mult Scler Int 2015;2015:296184. [Crossref] [PubMed]
- Tourtellotte WW, Potvin AR, Fleming JO, et al. Multiple sclerosis: measurement and validation of central nervous system IgG synthesis rate. Neurology 1980;30:240-4. [Crossref] [PubMed]
- Goffette S, Schluep M, Henry H, et al. Detection of oligoclonal free kappa chains in the absence of oligoclonal IgG in the CSF of patients with suspected multiple sclerosis. J Neurol Neurosurg Psychiatry 2004;75:308-10. [Crossref] [PubMed]
- Ramsden DB. Multiple sclerosis: assay of free immunoglobulin light chains. Ann Clin Biochem 2017;54:5-13. [Crossref] [PubMed]
- Makshakov G, Nazarov V, Kochetova O, et al. Diagnostic and Prognostic Value of the Cerebrospinal Fluid Concentration of Immunoglobulin Free Light Chains in Clinically Isolated Syndrome with Conversion to Multiple Sclerosis. PLoS One 2015;10:e0143375. [Crossref] [PubMed]
- DeCarli C, Menegus MA, Rudick RA. Free light chains in multiple sclerosis and infections of the CNS. Neurology 1987;37:1334-8. [Crossref] [PubMed]
- Villar LM, Masjuan J, González-Porqué P, et al. Intrathecal IgM synthesis is a prognostic factor in multiple sclerosis. Ann Neurol 2003;53:222-6. [Crossref] [PubMed]
- García-Barragán N, Villar LM, Espiño M, et al. Multiple sclerosis patients with anti-lipid oligoclonal IgM show early favourable response to immunomodulatory treatment. Eur J Neurol 2009;16:380-5. [Crossref] [PubMed]


