
Association between ondansetron use and mortality of patients on mechanical ventilation in the intensive care unit: a retrospective cohort study
Highlight box
Key findings
• Ondansetron usage was significantly associated with a lower mortality risk of ventilated patients in the ICU.
What is known and what is new?
• Ondansetron, a 5-HT3 receptor antagonist, is a widely used antiemetic drug and a highly safe medication even for pregnant women.
• This retrospective cohort study was conducted to assess the effects of ondansetron on mortality of patients on ventilation in the ICU.
What is the implication, and what should change now?
• The MIMIC-IV database was reviewed to identify patients on mechanical ventilation in the ICU. Our results suggest that the use of 5-HT3 receptor antagonists may be a new potential adjuvant treatment strategy for patients on mechanical ventilation in the ICU.
Introduction
The mortality rate of patients on mechanical ventilation in the intensive care unit (ICU) is high (1,2). Some risk factors had been demonstrated to associate with mortality in patients on mechanical ventilation in the ICU, such as gender, age, laboratory findings and comorbidities including hypertension, diabetes, heart disease, chronic obstructive pulmonary disease, chronic kidney disease and malignant neoplasm (3,4).
The 5-hydroxytryptamine (5-HT) receptors have been demonstrated as involved in cigarette smoke-induced airway inflammation, mucus hypersecretion, and airway remodeling in a murine model of chronic obstructive pulmonary disease (5,6), and also involved in the occurrence of sepsis (7) and acute respiratory distress syndrome (8), which are critical illnesses often requiring ventilator-assisted respiration in the ICU. Furthermore, inhibition of lung-derived serotonin attenuates pulmonary hypertension in mice (9). Basic studies have shown that 5-HT receptor antagonists can protect the lungs. For example, they attenuated ischemia-reperfusion injury after lung preservation in a rabbit model (10), and also reduced myofibroblast differentiation and connective tissue deposits in fibrosing interstitial lung diseases (11).
Ondansetron, a 5-HT type 3 (5-HT3) receptor antagonist, is a widely used antiemetic and a highly safe medication even for pregnant women (12-15). Neuroepithelial bodies in the mammalian lung express functional 5-HT3 receptors (16), and recently 5-HT3 receptor antagonists were demonstrated to have a broad therapeutic window (17). Importantly, they protected the lungs from polymicrobial sepsis-induced acute lung injury in mice (18), and regulate cell proliferation, migration, and invasion in lung cancer (19-21) and even reducing the death risk (7). Whether 5-HT3 receptor antagonists ondansetron benefits patients with mechanical ventilation in clinical is unclear. Therefore, we conducted a retrospective cohort study to assess the effects of ondansetron on reducing the mortality rate of patients on ventilation in the ICU, which would help to find new potential adjunctive therapeutic strategy for patients on mechanical ventilation. We present the following article in accordance with the STROBE reporting checklist (22) (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6256/rc).
Methods
From the Medical Information Mart for Intensive Care-IV (MIMIC-IV) (version 0.4) database (23,24), we enrolled a cohort of patients on mechanical ventilation administered ondansetron or not. The MIMIC-IV is a real-world and publicly available clinical database at the Beth Israel Deaconess Medical Center. We obtained approval to use the database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study design and study population
This is a retrospective cohort study. All patients aged >16 years on mechanical ventilation in the ICU were included. Patients receiving ondansetron only after induction of mechanical ventilation or during out-of-ICU stay were excluded. Only the data of their first ICU stay were included for patients admitted to the ICU more than once.
Definitions and outcomes
Ventilator-associated pneumonia (VAP) was defined as pneumonia developing in patients on mechanical ventilation for at least 48 h and 48 h after extubation. VAP data were extracted from the “Diagnosis” section in the MIMIC-IV database. Ondansetron use was defined as any kind of ondansetron administration regardless of the dose before the induction of mechanical ventilation during the ICU stay. The primary outcome was in-hospital death. Secondary outcomes were the VAP incidence, 60-day mortality rate, ventilator duration, and vasopressor duration.
Covariates
Demographic characteristics, as well as clinical, and medical history data for the first 24 h after the ICU admission were extracted. The simplified acute physiology score (SAPS) II (25), sequential organ failure assessment (SOFA) score (26), and Glasgow coma scale (GCS) score (27) were calculated. The ICU diagnosis, treatment measures, and antibiotic drugs administered throughout the entire ICU stay were also extracted (Table 1). Vasopressors used included dobutamine, vasopressin, phenylephrine, epinephrine, dopamine, and norepinephrine. Disease diagnosis was extracted from the “Diagnosis” section in the MIMIC-IV database. As for the antibiotics, carbapenems (meropenem), glycopeptide (vancomycin), β-lactams (ceftriaxone, cefotaxime, and cefepime), and aminoglycosides (gentamicin and amikacin) were extracted for our analysis. These covariates included as much basic information and clinical characteristics of patients as possible and were designed according to other related studies (3,4).
Table 1
| Characteristics | Total (n=18,566) | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|---|
| Non-ondansetron (n=12,831) | Ondansetron (n=5,735) | SMD | Non-ondansetron (n=4,739) | Ondansetron (n=4,739) | SMD | |||
| Demographic data | ||||||||
| Age (years), mean (SD) | 67.3 (56.4–77.5) | 67.6 (56.1–78.6) | 66.8 (56.9–75.4) | 0.035 | 65.41 (15.74) | 65.27 (14.68) | 0.009 | |
| Sex (male), n (%) | 11,542 (62.2) | 8,069 (62.9) | 3,473 (60.6) | 0.048 | 2,864 (60.4) | 2,871 (60.6) | 0.003 | |
| Ethnicity (non-White), n (%) | 6,731 (36.3) | 4,950 (38.6) | 1,781 (31.1) | 0.158 | 3,210 (67.7) | 3,209 (67.7) | 0.001 | |
| Married, n (%) | 8,646 (46.6) | 5,627 (43.9) | 3,019 (52.6) | 0.177 | 2,390 (50.4) | 2,409 (50.8) | 0.008 | |
| Insurance (Medicare), n (%) | 9,370 (50.5) | 6,720 (52.4) | 2,650 (46.2) | 0.124 | 2,259 (47.7) | 2,260 (47.7) | <0.001 | |
| Admission type (emergency), n (%) | 8,996 (48.5) | 7,177 (55.9) | 1,819 (31.7) | 0.503 | 1,823 (38.5) | 1,753 (37.0) | 0.03 | |
| Weight (kg), median (IQR) | 81.7 (69.0–96.8) | 81.0 (68.5–96.3) | 83.0 (70.0–97.8) | 0.050 | 69 (81.6–96.8) | 69.9 (83.0–97.3) | 0.006 | |
| Clinical data | ||||||||
| White blood cells (×109), median (IQR) | 12.1 (9.0–15.8) | 11.8 (8.8–15.5) | 12.7 (9.6–16.2) | 0.079 | 9.2 (12.3–16.0) | 9.1 (12.1–15.7) | 0.006 | |
| Platelets (×1012), median (IQR) | 177.7 (131.3–239.5) |
185.0 (136.3–246.0) |
162.8 (123.0–221.3) |
0.150 | 127.7 (171.5–232.5) |
126.5 (168.7–228.5) |
0.01 | |
| Creatinine (mg/dL), median (IQR) | 1.0 (0.8–1.4) | 1.0 (0.8–1.5) | 0.9 (0.7–1.3) | 0.069 | 0.7 (1.0–1.4) | 0.7 (1.0–1.4) | 0.016 | |
| Lactate (mmol/L), median (IQR) | 1.9 (1.4–2.7) | 1.9 (1.4–2.8) | 1.9 (1.5–2.6) | 0.107 | 1.4 (1.9–2.6) | 1.4 (1.9–2.5) | 0.028 | |
| SAPS II score, median (IQR) | 32.0 (24.0–41.0) | 32.0 (25.0–42.0) | 30.0 (23.0–38.0) | 0.207 | 24.0 (31.0–40.0) | 24.0 (31.0–39.0) | 0.042 | |
| SOFA score, median (IQR) | 5.0 (4.0–7.0) | 5.0 (3.0–7.0) | 5.0 (4.0–7.0) | 0.213 | 4.0 (5.0–7.0) | 4.0 (5.0–7.0) | 0.012 | |
| GCS score, median (IQR) | 14.0 (9.0–15.0) | 14.0 (8.0–15.0) | 14.0 (10.0–15.0) | 0.202 | 9.0 (14.0–15.0) | 10.0 (14.0–15.0) | 0.02 | |
| AKI, n (%) | 10,979 (59.1) | 7,430 (57.9) | 3549 (61.9) | 0.081 | 2,887 (60.9) | 2,869 (60.5) | 0.008 | |
| Medical history, n (%) | ||||||||
| Hypertension | 7,706 (41.5) | 5,160 (40.2) | 2,546 (44.4) | 0.085 | 2,014 (42.5) | 2,031 (42.9) | 0.007 | |
| Diabetes | 5,028 (27.1) | 3,334 (26.0) | 1,694 (29.5) | 0.079 | 1,330 (28.1) | 1,337 (28.2) | 0.003 | |
| Chronic heart failure | 4,610 (24.8) | 3,235 (25.2) | 1,375 (24.0) | 0.029 | 1,152 (24.3) | 1,137 (24.0) | 0.007 | |
| CKD | 3,059 (16.5) | 2,052 (16.0) | 1,007 (17.6) | 0.042 | 817 (17.2) | 816 (17.2) | 0.001 | |
| Chronic liver disease | 2,001 (10.8) | 1,390 (10.8) | 611 (10.7) | 0.006 | 330 (7.0) | 313 (6.6) | 0.014 | |
| COPD | 4,195 (22.6) | 3,026 (23.6) | 1,169 (20.4) | 0.077 | 1,001 (21.1) | 1,001 (21.1) | 0.001 | |
| Malignancy | 1,690 (9.1) | 995 (7.8) | 695 (12.1) | 0.146 | 571 (12.0) | 550 (11.6) | 0.014 | |
| Diagnosis, n (%) | ||||||||
| Surgery | 7,604 (41.1) | 4,238 (33.2) | 3,366 (59.0) | 0.537 | 2,466 (52.0) | 2,488 (52.5) | 0.009 | |
| Sepsis | 2,910 (15.7) | 2,198 (17.1) | 712 (12.4) | 0.133 | 685 (14.5) | 643 (13.6) | 0.026 | |
| ACS/AMI | 283 (1.5) | 231 (1.8) | 52 (0.9) | 0.077 | 51 (1.1) | 51 (1.1) | <0.001 | |
| Pneumonia | 3,729 (20.1) | 2,795 (21.8) | 934 (16.3) | 0.140 | 867 (18.3) | 854 (18.0) | 0.007 | |
| Treatments | ||||||||
| Fluid input on day 2 (L), median (IQR) | 6.9 (4.3–9.8) | 6.5 (3.8–9.8) | 7.5 (5.6–9.9) | 0.156 | 4.9 (7.3–10.2) | 4.9 (7.3–10.1) | 0.014 | |
| CRRT, n (%) | 930 (5.0) | 607 (4.7) | 323 (5.6) | 0.041 | 280 (5.9) | 275 (5.8) | 0.004 | |
| Glucocorticoids, n (%) | 3,277 (17.7) | 2,183 (17.0) | 1,094 (19.1) | 0.054 | 743 (15.7) | 965 (20.4) | 0.122 | |
| Vasopressor use, n (%) | 9,767 (52.6) | 6,524 (50.8) | 3,243 (56.5) | 0.115 | 2,586 (54.6) | 2,584 (54.5) | 0.001 | |
| Metoclopramide, n (%) | 4,920 (26.5) | 3,624 (28.2) | 1,296 (22.6) | 0.260 | 1,392 (29.4) | 1,249 (26.4) | 0.067 | |
| Antibiotics, n (%) | ||||||||
| Carbapenems | 1,416 (7.6) | 876 (6.8) | 540 (9.4) | 0.095 | 469 (9.9) | 457 (9.6) | 0.009 | |
| Glycopeptide | 11,489 (61.9) | 7,652 (59.6) | 3,837 (66.9) | 0.151 | 3,160 (66.7) | 3,117 (65.8) | 0.019 | |
| β-lactams | 5,647 (30.4) | 4,165 (32.5) | 1,482 (25.8) | 0.146 | 1,407 (29.7) | 1,367 (28.8) | 0.019 | |
| Aminoglycosides | 222 (1.2) | 126 (1.0) | 96 (1.7) | 0.060 | 68 (1.4) | 74 (1.6) | 0.01 | |
PSM, propensity score matching; SMD, standard mean difference; SD, standard deviation; IQR, interquartile range; SAPS II, simplified acute physiology score II; SOFA, sequential organ failure assessment; GCS, Glasgow coma scale; AKI, acute kidney disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ACS/AMI, acute coronary syndrome/acute myocardial infarction; CRRT, continuous renal replacement therapy.
Statistical analysis
Continuous variables are presented as the median and interquartile range (IQR), and categorical variables as numbers and percentages (%). Cox proportional hazard analysis was used to examine the association between in-hospital mortality, 60-day mortality and ondansetron use with hazard ratios (HRs) and their 95% confidence intervals (CIs). The confounders, including baseline clinical parameters and demographic characteristics in Table 1, were adjusted. To further consider the robustness of our results, 1:1 nearest propensity score matching (PSM) and an inverse probability of treatment weighting (IPTW) were performed with the same confounders adjusted in the multivariate logistic and Cox models. Using PSM (with a caliper of 0.2), individuals were matched, and the balance between matching groups was tested with the standard mean difference. Using the IPTW method, a pseudo-population was generated based on the propensity score and the HRs were calculated based on it. Kaplan-Meier (KM) curves with log-rank test were performed. Furthermore, subgroup analyses stratified by age, sex, surgery, acute kidney injury (AKI), sepsis, pneumonia, malignancy, and congestive heart failure (CHF) were performed to assess the robustness of the results. The percentage of missing value of all the participants is presented in Table S1. The Percentage of Missing Value of all the participants are less than 1%, except for lactic acid with 17.89% in non-ondansetron group and 15.01% in ondansetron group. Missing values were under the assumption of missing at random and imputed by multivariate imputation by chained equations. All analyses were performed using R (4.0.2) and Statistical Analysis System (9.4). A P value of <0.05 (two-sided) was considered statistically significant.
Results
Main result
In this study, 18,566 eligible patients on mechanical ventilation were identified, and of them 5,735 (30.89%) were administered ondansetron during their ICU stay. The flowchart of study patients is presented in Figure 1. All between-group comparisons used the non-ondansetron group as the reference unless otherwise indicated. The overall in-hospital mortality rate of patients on mechanical ventilation was 18.9% (3,512/18,566). Approximately 13.0% (746/5,735) and 21.6% (2,766/12,831) in-hospital mortality rates occurred in the ondansetron and non-ondansetron use groups, respectively (Table 2). HRs of the two groups in the multivariable Cox model before matching and after IPTW and PSM were 0.77 (95% CI: 0.70–0.85, P<0.001), 0.86 (95% CI: 0.78–0.95, P=0.002), and 0.62 (95% CI: 0.56–0.68, P<0.001), respectively, demonstrating a significant beneficial effect of ondansetron use on in-hospital death of patients on mechanical ventilation (Table 3).
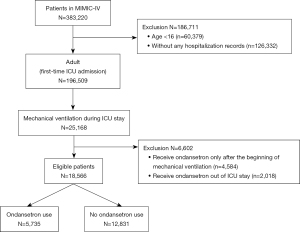
Table 2
| Outcome | Total | Non-ondansetron | Ondansetron | P value |
|---|---|---|---|---|
| Before PSM | ||||
| Patients, n | 18,566 | 12,831 | 5,735 | |
| In-hospital mortality, n (%) | 3,512 (18.9) | 2,766 (21.6) | 746 (13.0) | <0.001 |
| 60-day mortality, n (%) | 3,486 (18.8) | 2,752 (21.4) | 734 (12.8) | <0.001 |
| VAP, n (%) | 860 (4.6) | 647 (5.0) | 213 (3.7) | <0.001 |
| Duration of ventilator (h), median (IQR) | 18.0 (6.0–65.5) | 20.3 (7.0–73.9) | 12.7 (4.9–43.9) | <0.001 |
| Duration of vasopressor (h), median (IQR) | 24.2 (10.0–55.2) | 24.2 (9.9–53.8) | 24.6 (10.1–58.4) | 0.047 |
| After PSM | ||||
| Patients, n | 9,478 | 4,739 | 4,739 | |
| In-hospital mortality, n (%) | 1,657 (17.5) | 959 (20.2) | 698 (14.7) | <0.001 |
| 60-day mortality, n (%) | 1,643 (17.3) | 955 (20.2) | 688 (14.5) | <0.001 |
| VAP, n (%) | 412 (4.3) | 216 (4.6) | 196 (4.1) | 0.339 |
| Duration of ventilator (h), median (IQR) | 17.0 (5.78–64.0) | 17.2 (5.7–64.1) | 16.8 (5.9–64.0) | 0.007 |
| Duration of vasopressor (h), median (IQR) | 14.5 (6.1–31.6) | 14.8 (6.2–32.0) | 14.5 (6.1–31.2) | 0.170 |
PSM, propensity score matching; VAP, ventilator-associated pneumonia; IQR, interquartile range.
Table 3
| Model | HR | 95% CI | P value |
|---|---|---|---|
| In-hospital mortality | |||
| Unadjusted | 0.50 | 0.46–0.55 | <0.001 |
| Multivariable | 0.77 | 0.70–0.85 | <0.001 |
| IPTW | 0.86 | 0.78–0.95 | 0.002 |
| PSM | 0.62 | 0.56–0.68 | <0.001 |
| 60-day mortality | |||
| Unadjusted | 0.56 | 0.51–0.60 | <0.001 |
| Multivariable | 0.68 | 0.62–0.75 | <0.001 |
| IPTW | 0.76 | 0.68–0.83 | <0.001 |
| PSM | 0.69 | 0.62–0.77 | <0.001 |
Unadjusted: without adjustment; multivariable adjusted: adjusted for the baseline variables shown in Table 1. HR, hazard ratio; CI, confidence interval; IPTW, inverse probability treatment weighting; PSM, propensity score matching.
Secondary result
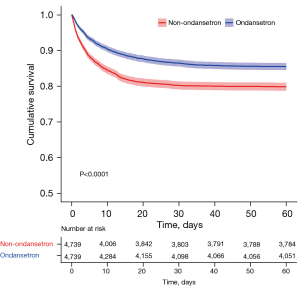
The 60-day hospital mortality rate was 18.8% (3,486/18,566), with 12.8% (734/5,735) and 21.4% (2,752/12,831) in the ondansetron and non-ondansetron use groups, respectively. HRs of the two groups in the multivariable Cox model before matching and after IPTW and PSM were 0.68 (95% CI: 0.62–0.75, P<0.001), 0.76 (95% CI: 0.68–0.83, P<0.001), and 0.69 (95% CI: 0.62–0.77, P<0.001), respectively, demonstrating a significant beneficial effect of the ondansetron use on the 60-day mortality rate for patients on mechanical ventilation (Table 3). Log-rank test for the KM curve of ondansetron use and the 60-day mortality rate before and after PSM was statistically significant (P<0.0001) (Figure 2).
The overall VAP incidence was 4.6% (860/18,566), with 3.7% (213/5,735) and 5.0% (647/12,831) in the ondansetron and non-ondansetron use groups, respectively (P<0.001) (Table 2). Moreover, the duration of ventilator use in the ondansetron group was statistically shorter than that in the non-ondansetron group [12.7 (4.9–43.9) vs. 20.3 (7.0–73.9), P<0.001] (Table 2). A statistical difference was also observed in the duration of ventilator use after PSM [16.8 (5.9–64.0) vs. 17.2 (5.7–64.1), P=0.007] in the two groups (Table 2). However, although VAP and vasopressor use duration decreased in the ondansetron group, there was no statistical difference of VAP (4.1% vs. 4.6%, P=0.339) and vasopressor use duration [14.5 (6.1–31.2) vs. 14.8 (6.2–32.0), P=0.170] in the two groups (Table 2).
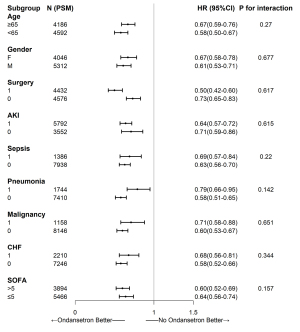
Subgroup and sensitivity analyses
Ondansetron has been associated with reduced mortality rates in critically ill patients using a ventilator, but it is not known whether this association exists in ventilated patients with different status. Therefore, we performed a subgroup analysis. As shown in Figure 3, among all the different subgroups, such as the subgroups of ventilated patients with and without sepsis, ondansetron use was associated with lower in-hospital mortality rates. For the association between ondansetron use and death, no interaction between stratification variables (age, sex, surgery, AKI, sepsis, pneumonia, malignancy, CHF, and SOFA) and ondansetron use was observed (Figure 3). Moreover, sensitivity analysis was performed to assess the association between ondansetron use within 48 h after ICU admission and outcomes, with the results showing similar conclusions (Table S2).
Discussion
This retrospective cohort study found that ondansetron use was associated with reduced mortality rates for patients on mechanical ventilation. This association was robust because of the additional models for controlling the indicated bias and subgroups stratified by age, sex, surgery, AKI, sepsis, pneumonia, malignancy, CHF, and SOFA. We believe this finding can be extended to the larger population of ICU ventilated patients.
The worldwide VAP incidence is 15.6% (28), but in our study of 18,566 patients on mechanical ventilation in the MIMIC-IV database during 2008 and 2019, VAP occurred in 4.63% (860/18,566) of patients. Consistent with our finding, Su et al. (29) analyzed 9,457 patients on mechanical ventilation during 2001 and 2012 in the MIMIC-III database and found that the VAP incidence was 4.42% (418/9,457). VAP incidence varies in critical patients with different diseases (30,31), and there exists certain diagnostic difficulties. Maybe because of the above reasons, the record of VAP in the “Diagnosis” section in the MIMIC-IV database is more likely unrecorded, and therefore, the VAP incidence was lower in our study than that in other previous studies. Furthermore, although no statistical difference was observed in the two groups after PSM in this study, the VAP incidence tended to decrease in the ondansetron group before and after PSM. Madineh et al.’s (32) study also showed that VAP in the ondansetron group was less prevalent than in the non-ondansetron group (12.5% vs. 15%). However, the sample size of 40 participants in their study was relatively small.
In obstetric-related studies, 4 mg of intravenous ondansetron reduced the median effective dose (ED50) of a prophylactic phenylephrine infusion by approximately 26% in patients undergoing cesarean delivery under combined spinal-epidural anesthesia (33), and prophylactic ondansetron of 8 mg reduced the number of hypotensive events per patient by 50% during elective cesarean delivery under spinal anesthesia in a double-blinded, randomized, placebo-controlled trial (34). Ondansetron affected the plasma vasopressin level in a study of patients with cancer, indicating that serotoninergic mechanisms, which regulate vasopressin secretion in humans, may activate serotonin receptors that ondansetron recognizes (35). In our study, ondansetron use was also associated with shorter vasopressor duration before PSM, although this result was not statistically significant after PSM.
The mortality rate of patients on mechanical ventilation is high. It was 26.0% in a study of a machine learning model for predicting in-hospital death of patients on mechanical ventilation following a moderate to severe traumatic brain injury (1). In critically ill children on mechanical ventilation, the in-hospital mortality rate varies from 7% to 33.7% (36). In our study, the in-hospital mortality rate of patients on mechanical ventilation was 21.6%, which was statistically decreased to 13.0% in the ondansetron group.
There are several possible reasons for patients on mechanical ventilation benefiting from ondansetron use. First, ondansetron may reduce excessive inflammatory reactions in the lungs. Inflammation plays a critical role in the need for invasive mechanical ventilation (37), ventilator-induced lung injury (38-40), prolonged mechanical ventilation (41,42), and death risk of patients on mechanical ventilation (37,43). Recently, 5-HT3 receptor antagonists were suggested as important potential agents for regulating inflammation and immune disorders (7). Administration of granisetron to septic mice was shown to significantly decrease lung damage by suppressing macrophage C-X-C motif chemokine ligand (CXCL)1/CXCL2 expression and neutrophil recruitment (18). Second, different from patients who undergo elective surgery with an empty stomach and under general anesthesia, critically ill patients who need emergency intubation and ventilation have a high risk of reflux aspiration. Previous studies found that the 5-HT3 receptors may mediate relaxation of rat esophageal smooth muscle (44) and esophageal motility (45), thus 5-HT3 receptor antagonists might reduce the relaxation effect (44,46). Ondansetron use before ventilation may prevent reflux aspiration during the intubation process. Our study also showed that ondansetron use was associated with shorter ventilator duration. Third, 5-HT3 receptor antagonists have many other effects, such as antiplatelet aggregation effect (47), reduction of cell apoptosis and oxidative stress (48) and kidney protection (49). They also affect plasma vasopressin secretion (35), have concomitant effects on autonomic nervous function (50) and inhibit sepsis by regulating the cardiac action potential (51). These mechanisms may be partly involved in reducing the mortality rate of patients on mechanical ventilation. However, the actual mechanism of the benefit for ventilated patients from ondansetron administration needs further investigation.
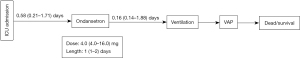
Several limitations should be considered. First, potential and unknown confounders may exist, as expected in all retrospective studies. However, we adjusted as many possible confounders and achieved a good balance in the PSM and IPTW cohorts in this study. Second, ondansetron is not a routine medication before mechanical ventilation, and specific indications for ondansetron administration are still unclear. However, clinically common diseases, such as malignant tumors and operation, with possible ondansetron use to prevent vomiting were analyzed in the subgroup analysis. Moreover, our results indicated that ondansetron could reduce the mortality rate of patients on mechanical ventilation in these two subgroups and other subgroups. Third, this study did not specify the amount and time of ondansetron administration, although we provided a range in the descriptive statistics (Figure 4). Fourth, the results reported in this study need to be further verified by further randomized trials because we obtained our data from an observational database.
Conclusions
This cohort study suggested that ondansetron use was associated with lower risk-adjusted mortality rates of patients on mechanical ventilation. 5-HT3 receptor antagonist use is may be new potential adjunctive therapeutic strategy for patients on mechanical ventilation in the ICU.
Acknowledgments
We thank Mr. H. Wu (Southern Medical University, China) for his guidance on the MIMIC-IV data extraction. We also thank Mr. J. Liu (PLA General Hospital 301, China) and Qilin Yang (The Second Affiliated Hospital of Guangzhou Medical University, China) for their guidance on the design of the study.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81971883).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6256/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6256/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abujaber A, Fadlalla A, Gammoh D, et al. Prediction of in-hospital mortality in patients on mechanical ventilation post traumatic brain injury: machine learning approach. BMC Med Inform Decis Mak 2020;20:336. [Crossref] [PubMed]
- Khalid I, Doshi P, DiGiovine B. Early enteral nutrition and outcomes of critically ill patients treated with vasopressors and mechanical ventilation. Am J Crit Care 2010;19:261-8. [Crossref] [PubMed]
- Grasselli G, Greco M, Zanella A, et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med 2020;180:1345-55. [Crossref] [PubMed]
- Estenssoro E, Loudet CI, Ríos FG, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med 2021;9:989-98. [Crossref] [PubMed]
- Löfdahl A, Wenglén C, Rydell-Törmänen K, et al. Effects of 5-Hydroxytryptamine Class 2 Receptor Antagonists on Bronchoconstriction and Pulmonary Remodeling Processes. Am J Pathol 2018;188:1113-9. [Crossref] [PubMed]
- Yang T, Wang H, Li Y, et al. Serotonin receptors 5-HTR2A and 5-HTR2B are involved in cigarette smoke-induced airway inflammation, mucus hypersecretion and airway remodeling in mice. Int Immunopharmacol 2020;81:106036. [Crossref] [PubMed]
- Gong S, Yan Z, Liu Z, et al. Intestinal Microbiota Mediates the Susceptibility to Polymicrobial Sepsis-Induced Liver Injury by Granisetron Generation in Mice. Hepatology 2019;69:1751-67. [Crossref] [PubMed]
- Gillis CN, Pitt BR, Wiedemann HP, et al. Depressed prostaglandin E1 and 5-hydroxytryptamine removal in patients with adult respiratory distress syndrome. Am Rev Respir Dis 1986;134:739-44. [PubMed]
- Abid S, Houssaini A, Chevarin C, et al. Inhibition of gut- and lung-derived serotonin attenuates pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 2012;303:L500-8. [Crossref] [PubMed]
- Arreola-Ramírez JL, Alquicira-Mireles J, Morales-Hernández PE, et al. 5-HT Receptor Antagonism Attenuates the Ischemia-Reperfusion Injury After Rabbit Lung Preservation. Transplant Proc 2015;47:1653-6. [Crossref] [PubMed]
- Löfdahl A, Tornling G, Wigén J, et al. Pathological Insight into 5-HT(2B) Receptor Activation in Fibrosing Interstitial Lung Diseases. Int J Mol Sci 2020;22:225. [Crossref] [PubMed]
- McParlin C, O'Donnell A, Robson SC, et al. Treatments for Hyperemesis Gravidarum and Nausea and Vomiting in Pregnancy: A Systematic Review. JAMA 2016;316:1392-401. [Crossref] [PubMed]
- Sakran R, Shechtman S, Arnon J, et al. Pregnancy outcome following in-utero exposure to ondansetron: A prospective comparative observational study. Reprod Toxicol 2021;99:9-14. [Crossref] [PubMed]
- Suarez EA, Boggess K, Engel SM, et al. Ondansetron use in early pregnancy and the risk of miscarriage. Pharmacoepidemiol Drug Saf 2021;30:103-13. [Crossref] [PubMed]
- van Gelder MMHJ, Nordeng H. Antiemetic Prescription Fills in Pregnancy: A Drug Utilization Study Among 762,437 Pregnancies in Norway. Clin Epidemiol 2021;13:161-74. [Crossref] [PubMed]
- Fu XW, Wang D, Pan J, et al. Neuroepithelial bodies in mammalian lung express functional serotonin type 3 receptor. Am J Physiol Lung Cell Mol Physiol 2001;281:L931-40. [Crossref] [PubMed]
- Wu H, Denna TH, Storkersen JN, et al. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol Res 2019;140:100-14. [Crossref] [PubMed]
- Wang J, Gong S, Wang F, et al. Granisetron protects polymicrobial sepsis-induced acute lung injury in mice. Biochem Biophys Res Commun 2019;508:1004-10. [Crossref] [PubMed]
- Liu Y, Zhang H, Wang Z, et al. 5-Hydroxytryptamine1a receptors on tumour cells induce immune evasion in lung adenocarcinoma patients with depression via autophagy/pSTAT3. Eur J Cancer 2019;114:8-24. [Crossref] [PubMed]
- Du X, Wang T, Wang Z, et al. 5-HT(7) Receptor Contributes to Proliferation, Migration and Invasion in NSCLC Cells. Onco Targets Ther 2020;13:2139-51. [Crossref] [PubMed]
- Tone M, Tahara S, Nojima S, et al. HTR3A is correlated with unfavorable histology and promotes proliferation through ERK phosphorylation in lung adenocarcinoma. Cancer Sci 2020;111:3953-61. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Johnson A, Bulgarelli L, Pollard T, et al. MIMIC-IV (version 0.4). 2020. Available online: https://physionet.org/content/mimiciv/0.4/
- Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2000;101:E215-20. [Crossref] [PubMed]
- Aegerter P, Boumendil A, Retbi A, et al. SAPS II revisited. Intensive Care Med 2005;31:416-23. [Crossref] [PubMed]
- Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 2008;12:R161. [Crossref] [PubMed]
- Reith FC, Van den Brande R, Synnot A, et al. The reliability of the Glasgow Coma Scale: a systematic review. Intensive Care Med 2016;42:3-15. [Crossref] [PubMed]
- Kollef MH, Chastre J, Fagon JY, et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med 2014;42:2178-87. [Crossref] [PubMed]
- Su L, Zhang Z, Zheng F, et al. Five novel clinical phenotypes for critically ill patients with mechanical ventilation in intensive care units: a retrospective and multi database study. Respir Res 2020;21:325. [Crossref] [PubMed]
- Forel JM, Voillet F, Pulina D, et al. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care 2012;16:R65. [Crossref] [PubMed]
- Asehnoune K, Seguin P, Allary J, et al. Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomised placebo-controlled trial. Lancet Respir Med 2014;2:706-16. [Crossref] [PubMed]
- Madineh H, Rahimi O, Zadeh MA, et al. Effect of Ondansetron on Prevention of Ventilator Associated Pneumonia in Intensive Care Unit Patients in Kashani Hospital in 2013. J Clin Diagn Res 2017;11:UC05-8. [Crossref] [PubMed]
- Xiao F, Wei C, Chang X, et al. A Prospective, Randomized, Double-Blinded Study of the Effect of Intravenous Ondansetron on the Effective Dose in 50% of Subjects of Prophylactic Phenylephrine Infusions for Preventing Spinal Anesthesia-Induced Hypotension During Cesarean Delivery. Anesth Analg 2020;131:564-9. [Crossref] [PubMed]
- Ortiz-Gómez JR, Palacio-Abizanda FJ, Morillas-Ramirez F, et al. Reducing by 50% the incidence of maternal hypotension during elective caesarean delivery under spinal anesthesia: Effect of prophylactic ondansetron and/or continuous infusion of phenylephrine - a double-blind, randomized, placebo controlled trial. Saudi J Anaesth 2017;11:408-14. [Crossref] [PubMed]
- Barreca T, Corsini G, Cataldi A, et al. Effect of the 5-HT3 receptor antagonist ondansetron on plasma AVP secretion: a study in cancer patients. Biomed Pharmacother 1996;50:512-4. [Crossref] [PubMed]
- Jung M, Park H, Kang D, et al. The effect of bed-to-nurse ratio on hospital mortality of critically ill children on mechanical ventilation: a nationwide population-based study. Ann Intensive Care 2020;10:159. [Crossref] [PubMed]
- Scully EP, Gupta A, Klein SL. Sex-biased clinical presentation and outcomes from COVID-19. Clin Microbiol Infect 2021;27:1072-3. [Crossref] [PubMed]
- Liu H, Gu C, Liu M, et al. Ventilator-induced lung injury is alleviated by inhibiting NLRP3 inflammasome activation. Mol Immunol 2019;111:1-10. [Crossref] [PubMed]
- Koh MW, Baldi RF, Soni S, et al. Secreted Extracellular Cyclophilin A Is a Novel Mediator of Ventilator-induced Lung Injury. Am J Respir Crit Care Med 2021;204:421-30. [Crossref] [PubMed]
- Su K, Wang J, Lv Y, et al. YAP expression in endothelial cells prevents ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2021;320:L568-82. [Crossref] [PubMed]
- Hagman C, Björklund LJ, Bjermer L, et al. Perinatal inflammation relates to early respiratory morbidity and lung function at 12 years of age in children born very preterm. Acta Paediatr 2021;110:2084-92. [Crossref] [PubMed]
- Rognes IN, Pischke SE, Ottestad W, et al. Increased complement activation 3 to 6 h after trauma is a predictor of prolonged mechanical ventilation and multiple organ dysfunction syndrome: a prospective observational study. Mol Med 2021;27:35. [Crossref] [PubMed]
- Dennison D, Al Khabori M, Al Mamari S, et al. Circulating activated neutrophils in COVID-19: An independent predictor for mechanical ventilation and death. Int J Infect Dis 2021;106:155-9. [Crossref] [PubMed]
- Ohia SE, Cheung YD, Bieger D, et al. Pharmacological profile of the 5-hydroxytryptamine receptor that mediates relaxation of rat oesophageal smooth muscle. Gen Pharmacol 1992;23:649-58. [Crossref] [PubMed]
- Koutsoumbi P, Epanomeritakis E, Tsiaoussis J, et al. The effect of erythromycin on human esophageal motility is mediated by serotonin receptors. Am J Gastroenterol 2000;95:3388-92. [Crossref] [PubMed]
- Blackshaw LA, Nisyrios V, Dent J. Responses of ferret lower esophageal sphincter to 5-hydroxytryptamine: pathways and receptor subtypes. Am J Physiol 1995;268:G1004-11. [PubMed]
- Liu FC, Liou JT, Liao HR, et al. The anti-aggregation effects of ondansetron on platelets involve IP3 signaling and MAP kinase pathway, but not 5-HT3-dependent pathway. Thromb Res 2012;130:e84-94. [Crossref] [PubMed]
- Aminzadeh A. Protective effect of tropisetron on high glucose induced apoptosis and oxidative stress in PC12 cells: roles of JNK, P38 MAPKs, and mitochondria pathway. Metab Brain Dis 2017;32:819-26. [Crossref] [PubMed]
- Tao L, Zhou S, Chang P, et al. Effects of ondansetron use on outcomes of acute kidney injury in critically ill patients: An analysis based on the MIMIC-IV database. J Crit Care 2021;66:117-22. [Crossref] [PubMed]
- Setoguchi D, Nakamura M, Yatsuki H, et al. Experimental examination of anti-inflammatory effects of a 5-HT3 receptor antagonist, tropisetron, and concomitant effects on autonomic nervous function in a rat sepsis model. Int Immunopharmacol 2011;11:2073-8. [Crossref] [PubMed]
- Liu Z, Zeng Z, Wu C, et al. Tropisetron inhibits sepsis by repressing hyper-inflammation and regulating the cardiac action potential in rat models. Biomed Pharmacother 2019;110:380-8. [Crossref] [PubMed]
(English Language Editor: K. Brown)


