
Thyroid stimulating hormone correlates with triglyceride levels but is not associated with the severity of acute ischemic stroke in patients with euthyroidism: a cross-sectional study
Highlight box
Key findings
• Thyroid stimulating hormone (TSH) levels are associated with the severity of acute ischemic stroke (AIS) patients, but not in patients with euthyroidism. TSH levels are correlated with triglycerides (TG) levels in AIS patients.
What is known and what is new?
• Growing evidence suggests an association between thyroid stimulating hormone (TSH) and severity of AIS.
• We found that TSH levels are associated with the severity of AIS patients, but not in patients with euthyroidism. Also, we found TSH levels are correlated with TG levels in patients with AIS, which has not been previously studied.
What is the implication, and what should change now?
• Stratified management of TSH may be beneficial in patients with AIS. TSH may affect AIS by regulating the lipid profiles.
Introduction
Stroke has become one of the most important causes of morbidity and mortality worldwide. The incidence of stroke has shown a gradual increase over the past years, causing a remarkable economic and social burden (1,2). Several factors, including traditional and non-traditional vascular risk factors, such as age, cigarette smoking, homocysteine, inflammation, diabetes mellitus, hyperlipidemia, obesity, ischemic heart disease, and hypertension, have been found to be associated with the severity and outcome of ischemic stroke (3-8). Researching the risk factors of ischemic stroke is of great significance to guide medical and rehabilitation treatment.
Several studies have discovered an association between thyroid dysfunction and prognosis of acute ischemic stroke (AIS) patients in recent years (9-19). The association between lower serum triiodothyronine (T3) and poor functional outcome in stroke patients has been revealed in several studies (10-13). At present, the results of studies on the relationship between thyroid stimulating hormone (TSH) and AIS patients are controversial. Some studies have reported the protective effects of elevated TSH on the severity and prognosis in AIS patients (14-17). Subclinical hyperthyroidism is associated with poor prognosis in AIS patients (18). Patients with subclinical hypothyroidism tend to have a milder stroke and a favorable functional outcome (19). Contrarily, a study on patients with acute cerebrovascular disease reported that TSH levels in severe patients were higher than those in mild severity patients, and that the TSH levels were higher in the poor outcomes group (20). Further studies are needed to better understand the association between TSH and AIS. As the most sensitive index for assessing the thyroid function, TSH also interacts with thyroid hormones. Regrettably, few studies have ruled out the potential influences of abnormal thyroid hormones when exploring the association between TSH and stroke. Moreover, the mechanism of TSH affecting stroke is still unclear. Many studies have found that TSH levels are associated with serum lipid profiles in healthy individuals (21,22), and the association still exists in patients with coronary heart disease and type 2 diabetes mellitus (23,24). In the acute phase of COVID-19 TSH levels are also associated with total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C) reduction (25). However, the association between TSH and lipid profiles has not been assessed in patients with AIS.
In this study, we created a stricter criterion for inclusion and focused on the relationship between TSH and severity in patients with AIS, especially in those with euthyroidism, to further detailed study the association of TSH and the severity of AIS in order to stratified management of TSH in patients with AIS. We further analyzed the correlations of TSH levels and lipid profiles to preliminarily explore the mechanism of TSH on AIS. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6374/rc).
Methods
Ethical approval
This retrospective cross-sectional study was approved by the Ethics Committee of the Shandong Second Provincial General Hospital (No. 20220402) and was performed in accordance with the Declaration of Helsinki (as revised in 2013). Patient identity remained anonymous, and the requirement for informed consent was waived due to the retrospective nature of the study.
Patients
This retrospective study enrolled patients who had experienced their first AIS and were admitted to the Neurology Unit of Shandong Second Provincial General Hospital from March 2020 to March 2021. The inclusion criteria included patients older than 18 years old, and AIS diagnosed by a new focal neurological symptom and a corresponding lesion on magnetic resonance imaging (MRI) assessment. The exclusion criteria were as follows: (I) evidence of preexisting thyroid disease except for benign nodules; (II) patients with abnormal FT3 and FT4 levels; (III) use of thyroxine therapy or thyroid-affecting medication that could influence thyroid functions within the past 3 months (including antithyroid medication, antidepressants, estrogen, androgen, and amiodarone); (IV) use of corticosteroids or lipid-lowering drugs before the study; (V) acute myocardial infarction at the moment of hospitalization; (VI) with a malignant tumor; (VII) with hereditary hyperlipidemia; (VIII) with serious liver and renal dysfunctions; (IX) pregnancy; (X) with other serious conditions or staying in the intensive care unit. In addition, patients with incomplete clinical data were also excluded. The number of cases, according to inclusion and exclusion criteria, admitted to our Neurology Unit during the study period determined the sample size.
Clinical and laboratory data collection
Clinical data were obtained from patients’ original medical records by 2 investigators. All clinical data and neurologic function scale scores were collected within 24 h of admission. Baseline clinical characteristics included demographic information (age and sex), vascular risk factors (regular cigarette smoking, previous history of hypertension, diabetes mellitus, ischemic heart disease, and transient ischemic attack or stroke). Stroke severity was determined using the National Institutes of Health Stroke Scale (NIHSS) by a neurologist at presentation. An NIHSS score <5 was considered as mild severity, and a score ≥5 was considered as a severe stroke.
Blood samples were collected after an overnight fast at least 8 h after admission. All the measurements were performed in our hospital’s clinical laboratory by the laboratory staff, who were unaware of the patients’ clinical symptoms. In addition to general blood tests, serum levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSH were also determined for each patient. The reference intervals of thyroid indicators in our laboratory are as follows: FT3 1.8–4.2 pg/mL, FT4 0.5–1.4 ng/dL, and TSH 0.35–5.6 µIU/mL. Patients were categorized according to thyroid function: (I) subclinical hypothyroidism: TSH >5.6 µIU/mL and/or FT3 and FT4 levels less than the reference ranges; (II) euthyroidism: FT3, FT4, and TSH within the reference ranges; (III) subclinical hyperthyroidism: TSH <0.35 µIU/mL and/or FT3 and FT4 levels above the reference ranges. The levels of TC, triglycerides (TG), high density lipoprotein cholesterol (HDL-C), and LDL-C were also determined using an Auto Biochemical Analyzer (HITACHI LABOSPECT 008 AS, Japan). Hypertriglyceridemia was defined as a TG value over 1.88 mmol/L.
All patients underwent brain MRI. Sequences included T1-weighted, T2-weighted, diffusion-weighted imaging (DWI), and fluid-attenuated inversion recovery (FLAIR). The AIS was confirmed with high signal on DWI. Transcranial Doppler ultrasonography, computed tomography (CT) angiography, and magnetic resonance angiography (MRA) were also performed for selected patients.
Statistical analysis
Statistical analyses in our study were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
Continuous variables were expressed as the mean ± standard deviation (SD) if the data were normally distributed, or as medians [interquartile range (IQR)] for the skewed data. Categorical variables were expressed as counts (%). The differences between groups at baseline was compared using the one-way analysis of variance (ANOVA) for normally distributed continuous variables, the Kruskal-Wallis test for skewed data, and the chi-square test for categorical variables. Univariate logistic regression analysis was used to derive potential factors, especially TSH levels, associated with the severity of AIS patients. And the candidate variables with a univariate relationship (P<0.05) were included in a multivariate logistic regression to deal with confounding factors and determine the independent predictors of the severity of AIS patients. The correlations between thyroid function and lipid profiles were evaluated with Spearman’s rank correlation analysis. All these analyses were performed in total patients and in patients with euthyroidism, respectively.
Results
Characteristics of patients with AIS according to serum TSH concentrations
A total of 438 AIS patients in our hospital from March 2020 to March 2021 were included in the study. After application of the aforementioned eligibility criteria, 345 patients met the inclusion criteria and were finally enrolled in the analysis. For these patients with normal T3 and T4 levels, the median age at study entry was 63 years (63±12 years), and 106 patients (30.7%) were female. The prevalence of thyroid dysfunction was 7.8%, including subclinical hypothyroidism (1.4%) and subclinical hyperthyroidism (6.4%). The prevalence of euthyroidism was 93.6%. Patients were stratified into 5 groups according to the serum TSH concentration, as shown in Table 1. The proportions of the 5 groups of patients were 1.4%, 53.0%, 33.6%, 5.5%, and 6.4%, respectively.
Table 1
| Variables | Serum TSH concentration (μIU/mL) | P1 value | P2 value | ||||
|---|---|---|---|---|---|---|---|
| Subclinical hyperthyroidism <0.35 | Euthyroidism | Subclinical hypothyroidism >5.6 | |||||
| 0.35–2.10 | 2.11–3.85 | 3.86–5.60 | |||||
| Total | 5 (1.4) | 183 (53.0) | 116 (33.6) | 19 (5.5) | 22 (6.4) | ||
| Age (years) | 60±3 | 63±12 | 63±11 | 66±11 | 60±13 | 0.392 | 0.597 |
| Sex, female | 0 (0.0) | 48 (26.2) | 36 (31.0) | 7 (36.8) | 15 (68.2) | 0.001 | 0.479 |
| Hypertension | 0 (0.0) | 121 (66.1) | 68 (58.6) | 10 (52.6) | 13 (59.1) | 0.030 | 0.278 |
| Diabetes | 2 (40.0) | 62 (33.9) | 30 (25.9) | 6 (31.6) | 5 (22.7) | 0.555 | 0.342 |
| Smoking | 4 (80.0) | 83 (45.4) | 50 (43.1) | 7 (36.8) | 8 (36.4) | 0.445 | 0.752 |
| NIHSS score at admission | 4 [2–11.5] | 4 [2–5] | 3 [1–5] | 2 [0–4] | 2 [1–3] | 0.042 | 0.078 |
| FT3 (pg/mL) | 2.41 [2.28–2.65] | 2.43 [2.24–2.73] | 2.57 [2.31–2.78] | 2.71 [2.38–2.82] | 2.50 [2.25–2.78] | 0.153 | 0.039 |
| FT4 (ng/dL) | 0.92 [0.73–1.14] | 0.98 [0.87–1.11] | 0.94 [0.84–1.05] | 0.94 [0.85–1.09] | 0.84 [0.74–1.00] | 0.008 | 0.156 |
| TSH (μIU/mL) | 0.31 [0.19–0.34] | 1.37 [0.94–1.73] | 2.69 [2.43–3.08] | 4.68 [4.43–5.11] | 6.63 [5.98–8.72] | 0.000 | 0.000 |
| TG (mmol/L) | 1.16 [0.82–1.80] | 1.31 [0.93–1.79] | 1.32 [1.02–1.95] | 1.53 [0.98–2.52] | 1.49 [1.03–2.12] | 0.466 | 0.249 |
| TC (mmol/L) | 4.46 [3.79–5.12] | 4.47 [3.70–5.12] | 4.44 [3.77–5.05] | 4.71 [4.10–5.31] | 4.70 [3.74–5.56] | 0.659 | 0.445 |
| HDL-C (mmol/L) | 1.22 [1.03–1.47] | 1.15 [1.00–1.30] | 1.14 [1.00–1.31] | 1.16 [1.02–1.28] | 1.20 [0.99–1.34] | 0.968 | 0.892 |
| LDL-C (mmol/L) | 2.72 [2.27–3.45] | 2.79 [2.19–3.31] | 2.78 [2.25–3.33] | 2.80 [2.46–3.60] | 2.84 [2.35–3.51] | 0.729 | 0.506 |
Values are presented as numbers (%), median [interquartile range], or mean ± standard deviation. The P1 value for the differences among the 5 groups of total patients. The P2 value for the difference between 3 groups of patients with euthyroidism. AIS, acute ischemic stroke; NIHSS, National Institutes of Health Stroke Scale; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TG, triglycerides; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
As shown in Table 1, significant differences were detected among the 5 groups with respect to sex (P=0.001), hypertension (P=0.030), NIHSS scores (P=0.042), and FT4 levels (P=0.008) in total AIS patients. No significant differences were found regarding age, diabetes, smoking, FT3 levels, TC, TG, HDL-C, and LDL-C levels. Patients with elevated serum TSH levels tended to have a milder stroke on admission in total patients, as well as in patients with euthyroidism. However, the difference was not significant (P=0.078) in patients with euthyroidism.
Factors associated with the severity of AIS patients
Among the 345 patients with AIS, 237 (68.7%) patients were mild-severity and 108 (31.3%) patients were severity. To explore the factors that may affect the severity of AIS patients at study entry, univariate logistic regression analysis was performed. As shown in Table 2, lower levels of age [odds ratio (OR) =1.03, 95% confidence interval (CI): 1.01–1.05, P=0.012], higher levels of TSH (OR =0.84, 95% CI: 0.72–0.98, P=0.025), higher levels of TC (OR =0.75, 95% CI: 0.59–0.95, P=0.016), and higher levels of HDL-C (OR =0.17, 95% CI: 0.06–0.52, P=0.002) were associated with a mild severity of AIS in total patients. In contrast, sex, hypertension, diabetes, smoking, FT3 levels, FT4 levels, TG levels, and LDL-C levels were not associated with the severity of total AIS patients. Among the patients with euthyroidism, age (OR =1.03, 95% CI: 1.01–1.05, P=0.017), FT3 levels (OR =0.49, 95% CI: 0.24–1.00, P=0.049), TC (OR =0.77, 95% CI: 0.61–0.98, P=0.032), and HDL-C (OR =0.18, 95% CI: 0.06–0.55, P=0.003) were associated with the severity of AIS. Sex, hypertension, diabetes, smoking, FT4 levels, TSH levels, TG levels, and LDL-C levels were not associated with the severity of AIS.
Table 2
| Variables | Total patients | Patients with euthyroidism | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (years) | 1.03 (1.01–1.05) | 0.012 | 1.03 (1.01–1.05) | 0.017 | |
| Sex, female | 0.60 (0.35–1.00) | 0.051 | 1.57 (0.91–2.71) | 0.108 | |
| Hypertension | 1.09 (0.68–1.74) | 0.722 | 0.92 (0.56–1.49) | 0.726 | |
| Diabetes | 1.42 (0.87–2.31) | 0.160 | 0.78 (0.47–1.29) | 0.336 | |
| Smoking | 1.44 (0.91–2.27) | 0.123 | 0.71 (0.44–1.14) | 0.160 | |
| FT3 (pg/mL) | 0.51 (0.25–1.02) | 0.056 | 0.49 (0.24–1.00) | 0.049 | |
| FT4 (ng/dL) | 3.99 (0.97–16.46) | 0.056 | 2.66 (0.61–11.62) | 0.193 | |
| TSH (μIU/mL) | 0.84 (0.72–0.98) | 0.025 | 0.90 (0.72–1.12) | 0.337 | |
| TG (mmol/L) | 1.01 (0.79–1.29) | 0.94 | 1.04 (0.81–1.34) | 0.745 | |
| TC (mmol/L) | 0.75 (0.59–0.95) | 0.016 | 0.77 (0.61–0.98) | 0.032 | |
| HDL-C (mmol/L) | 0.17 (0.06–0.52) | 0.002 | 0.18 (0.06–0.55) | 0.003 | |
| LDL-C (mmol/L) | 0.80 (0.60–1.06) | 0.119 | 0.83 (0.62–1.11) | 0.202 | |
AIS, acute ischemic stroke; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TG, triglycerides; TC, total cholesterol; HDL-C high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; CI, confidence interval.
A multiple logistic regression model was used to analyze the factors associated with severity of total AIS patients (Table 3). Factors with a P<0.05 in Table 2 were included in the final model. Multivariate logistic regression analysis showed that age (OR =1.03, 95% CI: 1.01–1.05, P=0.005), TSH levels (OR =0.85, 95% CI: 0.72–1.00, P=0.044), and HDL-C (OR =0.16, 95% CI: 0.05–0.58, P=0.005) were significantly associated with the severity of AIS. The TC levels did not retain significance in the final model.
Table 3
| Variables | OR (95% CI) | P value |
|---|---|---|
| Age (years) | 1.03 (1.01–1.05) | 0.005 |
| TSH (μIU/mL) | 0.85 (0.72–1.00) | 0.044 |
| TC (mmol/L) | 0.95 (0.72–1.25) | 0.710 |
| HDL-C (mmol/L) | 0.16 (0.05–0.58) | 0.005 |
AIS, acute ischemic stroke; TSH, thyroid stimulating hormone; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; OR, odds ratio; CI, confidence interval.
The correlations between thyroid function and the serum lipid profiles
As shown in Table 4, in total AIS patients, Spearman’s rank correlation analysis showed that TSH levels were positively correlated with TG levels (r=0.135, P=0.012). The correlations between TSH and TC, HDL-C, and LDL-C levels were not significant. Interestingly, the correlations between TSH levels and TG levels remained significant in patients with euthyroidism (r=0.133, P=0.018). There was no significant correlation between FT3 and FT4 levels with the serum lipid profiles.
Table 4
| Thyroid function | TG | TC | HDL-C | LDL-C |
|---|---|---|---|---|
| Total patients | ||||
| FT3 | ||||
| r | −0.017 | −0.005 | 0.015 | 0.013 |
| P | 0.747 | 0.924 | 0.775 | 0.809 |
| FT4 | ||||
| r | −0.034 | −0.024 | 0.030 | −0.016 |
| P | 0.525 | 0.662 | 0.574 | 0.767 |
| TSH | ||||
| r | 0.135 | 0.090 | 0.037 | 0.075 |
| P | 0.012 | 0.096 | 0.490 | 0.164 |
| Patients with euthyroidism | ||||
| FT3 | ||||
| r | 0.011 | 0.019 | 0.028 | 0.041 |
| P | 0.842 | 0.732 | 0.625 | 0.464 |
| FT4 | ||||
| r | −0.018 | −0.042 | 0.007 | −0.029 |
| P | 0.753 | 0.454 | 0.903 | 0.609 |
| TSH | ||||
| r | 0.133 | 0.081 | 0.042 | 0.069 |
| P | 0.018 | 0.151 | 0.452 | 0.222 |
AIS, acute ischemic stroke; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
The prevalence of hypertriglyceridemia in AIS patients with euthyroidism stratified according to the serum TSH levels
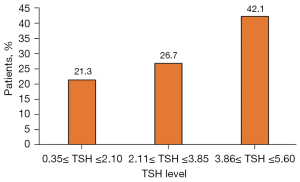
Among the 318 AIS patients with euthyroidism, there were 78 (24.5%) with hypertriglyceridemia. As shown in Figure 1, the prevalence of hypercholesterolemia was 21.3%, 26.7%, and 42.1%, respectively, in patients with the serum TSH levels between 0.35 and 2.10 µIU/mL, between 2.11 and 3.85 µIU/mL, and between 3.86 and 5.60 µIU/mL. The prevalence of hypertriglyceridemia showed an increase with elevations of the serum TSH levels even though the difference was not significant (P=0.106).
Discussion
The results obtained in this study demonstrate that the higher serum TSH levels were associated with the mild severity of patients with AIS, but not in patients with euthyroidism. We also observed significant correlations between TSH levels and TG levels in total patients with AIS, as well as in the patients with euthyroidism.
The purposes of this study included determining whether TSH can predict severity of patients with AIS, especially in patients with euthyroidism, and analyzing the correlations between TSH levels and lipid profiles to preliminarily explore the mechanism of TSH on this disease. Our study suggests that TSH may be used as an indicator of disease severity of AIS, which is in agreement with the study by Delpont et al. (15), wherein higher TSH levels were independently associated with a decreased risk of severity in patients with ischemic stroke. However, they did not exclude the potential influences of abnormal thyroid hormones when assessing the association between TSH and disease severity, nor did they demonstrate the association in patients with euthyroidism. Akhoundi et al. (19) also found that patients with higher levels of serum TSH tended to have a milder stroke on admission and a better outcome than patients with normal serum TSH levels. However, their study was conducted on patients with subclinical hypothyroidism, not on patients with euthyroidism. In our study, we found that higher TSH was associated with the mild severity of AIS, but we did not find this association in patients with euthyroidism, which has not been assessed in previous studies. These studies predict that stratified management of TSH may be beneficial for patients with AIS, especially for those with low levels of TSH.
The potential mechanism by which TSH is associated with AIS is not well understood. Several hypotheses have been put forward to explain the favorable effect of elevated TSH on stroke severity. One possible mechanism is hypometabolism. It is well known that higher TSH levels lead to a decreased basal metabolic rate (26). Many studies have found that hypometabolism, induced by hypothermia, has a protective effect on brain tissues at risk after an ischemic event (27,28). Reduced response to physical stress may also account for the decreased severity in patients with higher TSH levels. A decreased sensitivity to adrenergic stimulation has been observed in hypothyroid patients (29,30). Another possible explanation is that higher TSH levels can increase ischemic tolerance, which relies on a theory that sublethal ischemia renders the affected tissues resistant to subsequent, more severe, ischemic attacks. Several studies have provided evidence that subclinical hypothyroidism could increase the risk of atherosclerosis by increasing systemic vascular resistance, arterial stiffness, endothelial function, lipid abnormalities, and insulin resistance (30,31). Therefore, a higher level of TSH induces atherosclerosis and a neuroprotective mechanism of ischemic preconditioning is initiated. In our study, a correlation between TSH and TG levels was identified, and this correlation persisted in patients with euthyroidism, which further confirms the above theory. However, there were no significant associations detected between TSH and disease severity in patients with euthyroidism. We speculate that the fluctuation of TSH levels within the normal range is not enough to cause a difference in clinical outcome.
A series of studies have reported that a high level of TSH is associated with lipid metabolism and cardiovascular risk (21,22,32-36). The association between TSH and lipid profiles has also been confirmed in coronary heart disease and type 2 diabetes mellitus (23,24), but not has been studied in AIS. In our study, we found an association between TSH and TG levels, which is in agreement with the study by Xu et al. (23) in coronary heart disease. The possible explanations for the effects of TSH on lipid profiles have not been fully elucidated. Studies have demonstrated that liver cells express TSH receptor (37,38). A study found that TSH can act on the TSH receptor in hepatocyte membranes and promote the expression of hepatic 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, a rate-limiting enzyme in cholesterol synthesis, thereby increasing cholesterol synthesis and elevating cholesterol levels (39). TSH can also act on TSH receptors expressed on adipocytes and greatly increase adipogenesis and lipolysis, which can also elevate serum free fatty acid levels (40,41). Insulin resistance may also be the mechanism by which TSH affects lipid profiles (31,42). The direct effects of TSH on lipids require further investigation.
Our study has several strengths. Firstly, to the best of our knowledge, this is the first study to concentrate on the correlations between TSH and lipid profiles in patients with AIS, which provides a basis for further study on the mechanism of TSH on AIS. Secondly, we created a stricter criterion for inclusion and adjusted for additional potential confounders that influence lipid profiles and TSH levels, making the results more accurate. In this study, we focused on AIS patients with euthyroidism, which can exclude the potential influences of the abnormal thyroid hormones when studying the association between TSH and AIS. Thirdly, the range of patients in our study was broad, included men and women, from teenagers to elderly people, which makes the results well represented. Our study would be further strengthened if we assessed the effects of TSH levels on prognosis of AIS patients. In future study, we will focus on this aspect.
There are several limitations that should be considered in our study. Firstly, from this retrospective study, we could not draw any causal relationship between TSH and severity of AIS. Furthermore, we did not take all the possible confounders into consideration, such as sleep disorder, medications, diet, and physical activity, because some data could not be obtained in the retrospective analysis, and these should be considered in further study. In addition, individuals that received lipid-lowering drugs and with abnormal FT3 and FT4 levels were excluded from our study, thus the sample does not represent all patients with AIS. Prospective studies with larger sample size are needed.
Conclusions
In conclusion, our findings demonstrated that TSH levels are associated with the severity of patients with AIS, but not in patients with euthyroidism, predicting that stratified management of TSH may be beneficial in patients with AIS. Moreover, the correlations between TSH and TG levels were found in patients with AIS, and also in patients with euthyroidism, suggesting that TSH maybe affect the disease by regulating the lipid profiles. Further studies, especially prospective studies with a larger study sample and a longer follow-up, are needed to better understand the association between TSH and AIS.
Acknowledgments
The authors thank the participating patients, their families, and staff at the study site.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6374/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6374/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6374/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Ethics Committee of the Shandong Second Provincial General Hospital (ethical approval No. 20220402) and was performed in accordance with the Declaration of Helsinki (as revised in 2013). Patient identity remained anonymous, and the requirement for informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kalkonde YV, Deshmukh MD, Sahane V, et al. Stroke Is the Leading Cause of Death in Rural Gadchiroli, India: A Prospective Community-Based Study. Stroke 2015;46:1764-8. [Crossref] [PubMed]
- Wang W, Jiang B, Sun H, et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017;135:759-71. [Crossref] [PubMed]
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160-236. [Crossref] [PubMed]
- Guzik A, Bushnell C. Stroke Epidemiology and Risk Factor Management. Continuum (Minneap Minn) 2017;23:15-39. [Crossref] [PubMed]
- Zhou P, Liu J, Wang L, et al. Association of Small Dense Low-Density Lipoprotein Cholesterol with Stroke Risk, Severity and Prognosis. J Atheroscler Thromb 2020;27:1310-24. [Crossref] [PubMed]
- Tan YF, Zhan LX, Chen XH, et al. Risk Factors, Clinical Features and Prognosis for Subtypes of Ischemic Stroke in a Chinese Population. Curr Med Sci 2018;38:296-303. [Crossref] [PubMed]
- Ma LZ, Sun FR, Wang ZT, et al. Metabolically healthy obesity and risk of stroke: a meta-analysis of prospective cohort studies. Ann Transl Med 2021;9:197. [Crossref] [PubMed]
- Liu L, Wang W. Risk factors for acute ischemic stroke following intravenous thrombolysis: a 2-center retrospective cohort study. Ann Palliat Med. 2022;11:185-200. [Crossref] [PubMed]
- Jiang X, Xing H, Wu J, et al. Prognostic value of thyroid hormones in acute ischemic stroke - a meta analysis. Sci Rep 2017;7:16256. [Crossref] [PubMed]
- Suda S, Muraga K, Kanamaru T, et al. Low free triiodothyronine predicts poor functional outcome after acute ischemic stroke. J Neurol Sci 2016;368:89-93. [Crossref] [PubMed]
- Huang GQ, Zeng YY, Cheng QQ, et al. Low triiodothyronine syndrome is associated with hemorrhagic transformation in patients with acute ischaemic stroke. Aging (Albany NY) 2019;11:6385-97. [Crossref] [PubMed]
- Chen H, Wu Y, Huang G, et al. Low Tri-iodothyronine Syndrome Is Associated With Cognitive Impairment in Patients With Acute Ischemic Stroke: A Prospective Cohort Study. Am J Geriatr Psychiatry 2018;26:1222-30. [Crossref] [PubMed]
- Song Y, Yang C, Wang H. Free Triiodothyronine Is Associated with Poor Outcomes after Acute Ischemic Stroke. Int J Clin Pract 2022;2022:1982193. [Crossref] [PubMed]
- Wang J, Li F, Xiao L, et al. Depressed TSH level as a predictor of poststroke fatigue in patients with acute ischemic stroke. Neurology 2018;91:e1971-8. [Crossref] [PubMed]
- Delpont B, Aboa-Eboulé C, Durier J, et al. Associations between Thyroid Stimulating Hormone Levels and Both Severity and Early Outcome of Patients with Ischemic Stroke. Eur Neurol 2016;76:125-31. [Crossref] [PubMed]
- Chen Z, Sun Y, Zhang Y, et al. Low TSH level predicts a poor clinical outcome in patients with anterior circulation ischemic stroke after endovascular thrombectomy. Neurol Sci 2020;41:1821-8. [Crossref] [PubMed]
- Møllehave LT, Skaaby T, Linneberg A, et al. The association of thyroid stimulation hormone levels with incident ischemic heart disease, incident stroke, and all-cause mortality. Endocrine 2020;68:358-67. [Crossref] [PubMed]
- Lee SH, Jang MU, Kim Y, et al. Subclinical Hyperthyroidism Could Predict Poor Outcomes in Patients With Acute Ischemic Stroke Treated With Reperfusion Therapy. Front Neurol 2019;10:782. [Crossref] [PubMed]
- Akhoundi FH, Ghorbani A, Soltani A, et al. Favorable functional outcomes in acute ischemic stroke patients with subclinical hypothyroidism. Neurology 2011;77:349-54. [Crossref] [PubMed]
- Zhu J, Chen M, Li N, et al. Correlation analysis of serum thyroid stimulating hormone with acute cerebrovascular disease. Eur J Med Res 2019;24:35. [Crossref] [PubMed]
- Ahi S, Amouzegar A, Gharibzadeh S, et al. The Association Between Normal Range TSH and Lipid Profile. Horm Metab Res 2017;49:424-9. [Crossref] [PubMed]
- Wang F, Tan Y, Wang C, et al. Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. J Clin Endocrinol Metab 2012;97:2724-31. [Crossref] [PubMed]
- Xu C, Yang X, Liu W, et al. Thyroid stimulating hormone, independent of thyroid hormone, can elevate the serum total cholesterol level in patients with coronary heart disease: a cross-sectional design. Nutr Metab (Lond) 2012;9:44. [Crossref] [PubMed]
- Sun X, Chen L, Wu R, et al. Association of thyroid hormone with body fat content and lipid metabolism in euthyroid male patients with type 2 diabetes mellitus: a cross-sectional study. BMC Endocr Disord 2021;21:241. [Crossref] [PubMed]
- Mannarino MR, Bianconi V, Cosentini E, et al. Thyroid-Stimulating Hormone Predicts Total Cholesterol and Low-Density Lipoprotein Cholesterol Reduction during the Acute Phase of COVID-19. J Clin Med 2022;11:3347. [Crossref] [PubMed]
- Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 2008;18:141-4. [Crossref] [PubMed]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 2008;371:1955-69. [Crossref] [PubMed]
- Hoesch RE, Geocadin RG. Therapeutic hypothermia for global and focal ischemic brain injury--a cool way to improve neurologic outcomes. Neurologist 2007;13:331-42. [Crossref] [PubMed]
- Pantos C, Mourouzis C, Katramadou M, et al. Decreased vascular reactivity to alpha1 adrenergic stimulation in the presence of hypothyroid state: a part of an adaptive response? Int Angiol 2006;25:216-20. [PubMed]
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008;29:76-131. [Crossref] [PubMed]
- Sun X, Sun Y, Li WC, et al. Association of thyroid-stimulating hormone and cardiovascular risk factors. Intern Med 2015;54:2537-44. [Crossref] [PubMed]
- Lee YK, Kim JE, Oh HJ, et al. Serum TSH level in healthy Koreans and the association of TSH with serum lipid concentration and metabolic syndrome. Korean J Intern Med 2011;26:432-9. [Crossref] [PubMed]
- Karbownik-Lewińska M, Stępniak J, Żurawska A, et al. Less Favorable Lipid Profile and Higher Prevalence of Thyroid Antibodies in Women of Reproductive Age with High-Normal TSH-Retrospective Study. Int J Environ Res Public Health 2020;17:2122. [Crossref] [PubMed]
- Inoue K, Ritz B, Brent GA, et al. Association of Subclinical Hypothyroidism and Cardiovascular Disease With Mortality. JAMA Netw Open 2020;3:e1920745. [Crossref] [PubMed]
- Du FM, Kuang HY, Duan BH, et al. Associations Between Thyroid Hormones Within the Euthyroid Range and Indices of Obesity in Obese Chinese Women of Reproductive Age. Metab Syndr Relat Disord 2019;17:416-22. [Crossref] [PubMed]
- Jain RB. Associations between the levels of thyroid hormones and lipid/lipoprotein levels: Data from National Health and Nutrition Examination Survey 2007-2012. Environ Toxicol Pharmacol 2017;53:133-44. [Crossref] [PubMed]
- Zhang W, Tian LM, Han Y, et al. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med 2009;13:4636-42. [Crossref] [PubMed]
- Huang B, Wen W, Ye S. TSH-SPP1/TRβ-TSH positive feedback loop mediates fat deposition of hepatocyte: Crosstalk between thyroid and liver. Front Immunol 2022;13:1009912. [Crossref] [PubMed]
- Tian L, Song Y, Xing M, et al. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology 2010;52:1401-9. [Crossref] [PubMed]
- Lu M, Lin RY. TSH stimulates adipogenesis in mouse embryonic stem cells. J Endocrinol 2008;196:159-69. [Crossref] [PubMed]
- Gagnon A, Antunes TT, Ly T, et al. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism 2010;59:547-53. [Crossref] [PubMed]
- Bulum T, Kolarić B, Duvnjak L. Insulin sensitivity modifies the relationship between thyroid function and lipid profile in euthyroid type 1 diabetic patients. Endocrine 2012;42:139-45. [Crossref] [PubMed]
(English Language Editor: J. Jones)


