
A novel nomogram to predict survival of patients with hepatocellular carcinoma after transarterial chemoembolization: a tool for retreatment decision making
Highlight box
Key findings
• This study developed and validated a prognostic nomogram model for HCC patients undergoing TACE.
What is known and what is new?
• There is still no standardized policy regarding whether patients treated after TACE should be retreated or switch to systemic therapies.
• This study generated and validated a nomogram model using independent risk factors before or after TACE to help guide further treatment strategies in HCC patients undergoing TACE.
What is the implication, and what should change now?
• According to the death risk predicted by the nomogram, we can cautiously speculate that HCC patients who are predicted to have poor survival outcomes may not profit from further TACE treatments and should be recommended to switch to another treatment schedule in a timely manner.
Introduction
Primary hepatocellular carcinoma (HCC) is the sixth most common type of cancer worldwide and one of the leading causes of cancer-related death (1). As the most widely used treatment for HCC, transarterial chemoembolization (TACE) is an interventional procedure for injecting chemotherapeutic drugs at the HCC site while impeding blood supply to induce the ischemic tumor necrosis. TACE is the first-line treatment for HCC patients with unresectable large or multinodular tumors without vascular infiltration or extrahepatic spread, which is classified as the Barcelona Clinic Liver Cancer intermediate stage (BCLC-B) (2). However, emerging evidence has supported that HCC patients benefit from TACE treatment even if they are in the early or advanced stage (3,4). In patients with segmental vascular invasion, TACE treatment has a higher survival rate than conservative treatment (5).
Although TACE is considered a relatively safe and effective procedure, repeated treatments have been reported to be associated with increased adverse events (e.g., liver dysfunction) and reduced efficacy (6,7). As novel molecular targeted agents have shown clinical benefits in phase 3 trials, systemic therapies have significantly prolonged the survival of HCC patients compared with the era of sorafenib (8-10). However, there is still no standardized policy regarding whether patients treated after TACE should be retreated or switched to systemic therapies. If TACE is continued without proper assessments, patients may lose the opportunity for switching to systemic therapies due to deterioration of liver function (11).
To identify patients who are not benefiting from TACE, researchers have investigated factors that impact overall survival (OS) in TACE-treated HCC patients, such as tumor burden (tumor number, size, portal vein invasion), liver function, alpha-fetoprotein (AFP) level, and performance status (12). Particularly, the tumor response after TACE has been recognized as an indispensable factor to provide further guidance, which is widely recommended using the modified Response Evaluation Criteria In Solid Tumors (mRECIST) or the European Association for the Study of the Liver (EASL) response evaluation criteria for assessments (13,14). Based on these predictors, several prognostic models such as the Assessment for Retreatment with TACE (ART) score, AFP level BCLC stage, Child-Pugh class, Response after TACE (ABCR) score, Tumor Size and Number, baseline AFP, Child-Pugh and Objective radiological Response (SNACOR) score and the post-TACE-Predict Model (15-18), have been established to help clinicians decide whether to continue TACE or transition to systemic treatment. However, the predictive power and applicability of these models are limited in practice (19).
It has been shown that nomograms are more practical and accurate than traditional tumor staging systems (20). Prognostic nomogram has been implemented to predict treatment outcome in HCC patients after TACE (21,22), but these studies ignored the negative impact of liver function deterioration after TACE on the patient prognosis, which has been confirmed as an independent prognostic factor. Therefore, in this study, we generated and validated a nomogram model using independent risk factors before or after TACE to guide further treatment strategies in HCC patients undergoing TACE. We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6513/rc).
Methods
Study design and population
A total of 1,738 HCC patients who received TACE treatment in the First Affiliated Hospital of Wenzhou Medical University from 12 June 2015 to 30 May 2019 were included. Patients who met the following criteria were included: age 18–75 years; the diagnosis of HCC fulfilled the clinical standards of the American Association for the Study of Liver Diseases (AASLD); and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1. The exclusion criteria were as follows: patients underwent other therapy before or during the first TACE therapy; concurrent malignancies; extrahepatic metastasis; tumor rupture; and missing essential follow-up data. As selective portal vein invasion is no longer contraindicated for TACE, we included patients with segmental vein thrombosis in the analysis. Finally, 578 patients were randomly allocated to 1 of 2 cohorts: a training cohort for establishing the nomogram and a validation cohort for confirming the new model's performance, in a 7:3 ratio (Figure S1). Written informed consent was provided by each patient included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (approval number: KY2022-R108).
Data collection and definition
The following clinical data were recorded: gender, age, etiology of liver disease [hepatitis B virus (HBV) infection or others], tumor characteristics (tumor number, size, portal vein invasion), levels of AFP, hemoglobin (Hb), white blood cell (WBC), platelet count (PLT), albumin (ALB), alanine aminotransferase (ALT), aspartate transaminase (AST), total bilirubin (TB), international normalized ratio (INR), and fibrinogen (Fbg). Diagnosis of cirrhosis was based on clinical, radiological [computed tomography (CT) or magnetic resonance imaging (MRI)], and histological criteria. The period from the time of TACE to death by any cause or last follow-up was defined as OS.
The ALBI was calculated according to the following formula: ALBI = [log10 bilirubin (µmol/L) × 0.66] + [albumin (g/L) × −0.085]. ALBI was divided into 3 grades: grade I (<−2.60), grade II (−2.60≤ ALBI ≤−1.39), and grade III (>−1.39). The ART score, ABCR score, SNACOR score, and the post-TACE-Predict Model were calculated according to each criterion.
Treatment procedure and follow-up
All conventional TACE (cTACE) operations were conducted by experienced interventional radiologists. All patients were treated with a modified Seldinger puncture of the right femoral artery and a microcatheter was super-selected to the tumor-supplying artery. After the catheter was properly positioned, a mixture of doxorubicin (10–50 mg) and lipiodol (2–20 mL) was injected, adjusted by the number, size, and embolization degree of the tumor, followed by embolization with gelatin sponge particles until the tumor had no contrast staining. Sequential TACE was performed ‘‘on demand’’ according to each patient's tumor burden, previous treatment response, and liver function at 6- to 8-week intervals. Within 1 week before each TACE, serum AFP, liver function tests, and CT or MRI evaluation were conducted. The tumor radiologic response was assessed by mRECIST with 4 response categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Statistical analysis
The Student’s t-test or Mann-Whitney U-test was used to compare continuous variables between different sets, and the chi-square test or Fisher’s exact test was used to compare binary categorical variables. The independent risk factors were identified by univariate and multiple Cox proportional hazard regression analyses. Statistically significant variables (P<0.01) obtained from univariate analysis were incorporated into a stepwise backward multivariate regression analysis (entry criteria for selection into the final risk prediction model was P<0.05). Based on the independent risk factors, we used the “rms” package of R software (R Foundation for Statistical Computing, Vienna, Austria) to draw a nomogram. Calibration curves were depicted to assess the calibration ability of the nomogram, and the predictive performance in continuous time was presented with the area under time-dependent receiver operating characteristic (ROC) curve (AUC) by the “timeROC” package. To assess the discrimination ability and clinical value of the nomogram and other models, the concordance index (C-index) was carried out by the survival R package and the decision curve analysis (DCA) was performed by source file “stdca.R”. Survival curves were plotted by the Kaplan-Meier method and compared using the log-rank test. All analyses were performed using R version 3.5.1.
Results
Baseline characteristics
The baseline characteristics of the training cohort and validation cohort in this study are shown in Table 1. The median OS for all patients was 26 months [95% confidence interval (CI): 21.296, 30.704]; it was 25 months (95% CI: 20.071, 29.929) in the training cohort and 30 months (95% CI: 19.016, 40.984) in the internal validation cohort. The patients were predominantly male (83.0%), with an average age of 62.5 (50.5–74.5) years. According to the cause of disease, the majority (72.1%) of the participants were HBV infected, and 68.1% had cirrhosis. In terms of tumor characteristics, about half of the patients had multiple tumors (47.1%) and had a tumor larger than 50 mm in diameter (55.7%). Many patients experienced chronic deterioration in liver function after TACE, presented as a 19.3% increase in ALBI grade 2/3. Besides, about half of the patients (47.9%) showed PR to the first TACE treatment. These clinical characteristics did not differ significantly (P>0.05) between the training and validation cohorts.
Table 1
| Characteristics | Total (n=578) | Training set (n=405) | Validation set (n=173) | P value |
|---|---|---|---|---|
| Age (years) | 62.5±12.0 | 63.1±11.8 | 61.2±12.3 | 0.074 |
| Hb (g/L) | 127.6±21.4 | 127.6±21.7 | 127.6±20.9 | 0.990 |
| WBC (109/L) | 5.8±4.4 | 5.9±4.9 | 5.5±2.7 | 0.367 |
| PLT (109/L) | 165.3±96.1 | 168.1±96.6 | 158.7±95.0 | 0.279 |
| Pre-ALT (U/L) | 43.5±43.1 | 41.4±39.2 | 48.4±50.9 | 0.073 |
| Post-ALT (U/L) | 106.6±140.5 | 109.3±149.0 | 1,100.2±118.3 | 0.473 |
| Pre-AST (U/L) | 62.1±58.0 | 62.0±61.6 | 62.5±48.5 | 0.922 |
| Post-AST (U/L) | 150.0±220.5 | 156.1±241.0 | 136.0±162.6 | 0.317 |
| Pre-AFP (ng/mL) | 4,514.4±1,2777.6 | 4,560.3±12,879.5 | 4,408.0±12,574.7 | 0.896 |
| Post-AFP (ng/mL) | 4,190.0±1,2551.9 | 4,460.1±13,133.9 | 3,584.2±11,160.5 | 0.506 |
| INR | 1.2±0.2 | 1.2±0.2 | 1.2±0.2 | 0.754 |
| Fbg (g/L) | 3.6±1.5 | 3.7±1.5 | 3.4±1.4 | 0.074 |
| Gender | 0.686 | |||
| Male | 480 (83.0) | 338 (83.5) | 142 (82.1) | |
| Female | 98 (17.0) | 67 (16.5) | 31 (17.9) | |
| Etiology | 0.518 | |||
| HBV | 417 (72.1) | 289 (71.4) | 128 (74.0) | |
| Others | 161 (27.9) | 116 (28.6) | 45 (26.0) | |
| Tumor diameter (mm) | 0.664 | |||
| ≤50 | 256 (44.3) | 177 (43.7) | 79 (45.7) | |
| >50 | 322 (55.7) | 228 (56.3) | 94 (54.3) | |
| Number of tumors | 0.940 | |||
| ≤3 | 306 (52.9) | 214 (52.8) | 92 (53.2) | |
| >3 | 272 (47.1) | 191 (47.2) | 81 (46.8) | |
| Portal vein invasion | 0.711 | |||
| Yes | 105 (18.2) | 72 (17.8) | 33 (19.1) | |
| No | 473 (81.8) | 333 (82.2) | 140 (80.9) | |
| Liver cirrhosis | 0.959 | |||
| Yes | 392 (68.1) | 274 (70.0) | 118 (68.2) | |
| No | 184 (31.9) | 129 (32.0) | 55 (31.8) | |
| Tumor response | 0.603 | |||
| CR | 127 (22.0) | 95 (23.5) | 32 (18.5) | |
| PR | 277 (47.9) | 192 (47.4) | 85 (49.1) | |
| SD | 127 (22.0) | 86 (21.2) | 41 (23.7) | |
| PD | 47 (8.1) | 32 (7.9) | 15 (8.7) | |
| Pre-ALBI grade | 0.830 | |||
| I | 191 (33.3) | 136 (33.7) | 55 (32.2) | |
| II | 360 (62.7) | 252 (62.5) | 108 (63.2) | |
| III | 23 (4.0) | 15 (3.7) | 8 (4.7) | |
| Post-ALBI grade | 0.465 | |||
| I | 79 (13.7) | 60 (14.8) | 19 (11.0) | |
| II | 439 (76.0) | 303 (74.8) | 136 (78.6) | |
| III | 60 (10.4) | 42 (10.4) | 18 (10.4) |
Variables are expressed as mean ± standard deviation or n (%). Hb, hemoglobin; WBC, white blood cell; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate transaminase; AFP, alpha-fetoprotein; INR, international normalized ratio; Fbg, fibrinogen; HBV, hepatitis B virus; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ALBI, albumin-bilirubin.
Development of the prognostic nomogram
As shown in Table 2, the univariate analyses in the training cohort revealed that PLT, pre-ALT, pre-AST, post-AST, pre-AFP, post-AFP, INR, Fbg, pre-ALBI grade, post-ALBI grade, tumor diameter, number of tumors, portal vein invasion, and tumor response were related to OS (P<0.05). The further multivariate analyses identified that post-ALBI grade, tumor diameter, number of tumors, portal vein invasion, and tumor response were the final independent risk factors of HCC patients undergoing TACE.
Table 2
| Characteristics | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Gender (male/female) | 1.208 | 0.846, 1.726 | 0.298 | ||||
| Age (years) | 1.005 | 0.993, 1.017 | 0.382 | ||||
| Etiology (HBV/others) | 0.941 | 0.698, 1.269 | 0.692 | ||||
| Hb (g/L) | 0.995 | 0.989, 1.002 | 0.152 | ||||
| PLT (109/L) | 1.001 | 1.000, 1.003 | 0.038 | ||||
| WBC (109/L) | 1.010 | 0.990, 1.031 | 0.345 | ||||
| Pre-ALT (U/L) | 1.004 | 1.002, 1.007 | <0.001 | ||||
| Post-ALT (U/L) | 1.000 | 0.999, 1.001 | 0.485 | ||||
| Pre-AST (U/L) | 1.004 | 1.003, 1.005 | <0.001 | ||||
| Post-AST (U/L) | 1.001 | 1.000, 1.001 | 0.020 | ||||
| Pre-AFP (ng/mL) | 1.000 | 1.000, 1.000 | <0.001 | ||||
| Post-AFP (ng/mL) | 1.000 | 1.000, 1.000 | <0.001 | ||||
| INR | 1.685 | 0.815, 3.484 | 0.159 | ||||
| Fbg (g/L) | 1.155 | 1.065, 1.252 | <0.001 | ||||
| Liver cirrhosis (yes/no) | 0.971 | 0.720, 1.308 | 0.844 | ||||
| Pre-ALBI grade | |||||||
| I | 1.0 | ||||||
| II | 2.155 | 1.560, 2.976 | <0.001 | ||||
| III | 1.476 | 0.669, 3.256 | 0.335 | ||||
| Post-ALBI grade | |||||||
| I | 1.0 | ||||||
| II | 2.475 | 1.529, 4.008 | <0.001 | 1.794 | 1.100, 2.926 | 0.019 | |
| III | 3.424 | 1.907, 6.149 | <0.001 | 2.647 | 1.464, 4.787 | 0.001 | |
| Tumor diameter (>50/≤50, mm) | 2.575 | 1.909, 3.473 | <0.001 | 1.462 | 1.054, 2.029 | 0.023 | |
| Number of tumors (>3/≤3) | 2.805 | 2.111, 3.728 | <0.001 | 2.054 | 1.522, 2.772 | <0.001 | |
| Portal vein invasion (yes/no) | 2.528 | 1.831, 3.491 | <0.001 | 1.588 | 1.137, 2.216 | 0.007 | |
| Tumor response | |||||||
| CR | 1.0 | 1.0 | |||||
| PR | 2.903 | 1.850, 4.554 | <0.001 | 2.117 | 1.313, 3.414 | 0.002 | |
| SD | 4.917 | 3.048, 7.933 | <0.001 | 2.939 | 1.773, 4.873 | <0.001 | |
| PD | 9.744 | 5.516, 17.214 | <0.001 | 5.014 | 2.722, 9.238 | <0.001 | |
HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; Hb, hemoglobin; PLT, platelet count; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate transaminase; AFP, alpha-fetoprotein; INR, international normalized ratio; Fbg, fibrinogen; ALBI, albumin-bilirubin; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
The 5 identified risk factors were used to construct a nomogram to predict the 1-, 3-, and 5-year OS (Figure 1). According to the results of the nomogram, tumor response had the greatest impact on the prognosis of HCC patients, followed by postoperative ALBI grade, number of tumors, portal vein invasion, and tumor diameter. The subtypes of each risk factor were given various scores, which were then summed together to yield a total score. Finally, the specific 1-, 3-, and 5-year survival probability of each patient could be calculated by vertical lines of the total score.

Validation of the prognostic nomogram
The calibration plots for the 1-, 3-, and 5-year OS showed optimal agreements between the nomogram predictions and actual observations in the training cohort and the validation cohort (Figure 2). The discrimination ability of the nomogram was measured by the time-dependent ROC curves and C-index values. In the training cohort, the time-dependent AUCs of the nomogram for OS at 1-, 3-, and 5-year were 0.803 (95% CI: 0.756, 0.851), 0.840 (95% CI: 0.779, 0.901), and 0.872 (95% CI: 0.791, 0.952), respectively (Figure 3A). The 1-, 3-, and 5-year AUCs of the nomogram for OS in the validation cohort were 0.833 (95% CI: 0.767, 0.899), 0.825 (95% CI: 0.748, 0.901), and 0.878 (95% CI: 0.812, 0.945), respectively (Figure 3B). The C-indexes for OS prediction in the training and validation cohorts were 0.753 (95% CI: 0.722, 0.784) and 0.770 (95% CI: 0.717, 0.823), respectively (Table 3).

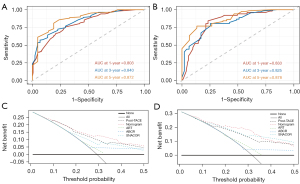
Table 3
| Models | Training cohort | Validation cohort | |||
|---|---|---|---|---|---|
| C-index | 95% CI | C-index | 95% CI | ||
| Nomogram | 0.753 | 0.722, 0.784 | 0.770 | 0.717, 0.823 | |
| Post-TACE model | 0.711 | 0.678, 0.744 | 0.709 | 0.654, 0.764 | |
| ART | 0.557 | 0.524, 0.590 | 0.569 | 0.516, 0.622 | |
| ABCR | 0.56 | 0.535, 0.585 | 0.562 | 0.525, 0.599 | |
| SNACOR | 0.662 | 0.625, 0.699 | 0.703 | 0.652, 0.754 | |
C-index, concordance index; CI, confidence interval; TACE, transarterial chemoembolization; ART, Assessment for Retreatment with TACE; ABCR, AFP level BCLC stage, Child-Pugh class, Response after TACE; SNACOR, Tumor Size and Number, baseline AFP, Child-Pugh and Objective radiological Response.
The performance of the nomogram compared to other prognostic models
The C‐indexes of the prognostic models including the post-TACE model, ART score, ABCR score, and SNACOR score in both cohorts were also calculated, among which only the post-TACE model showed a C‐index higher than 0.7 (Table 3). The C-index of the nomogram was superior to that of other models in the validation cohort (P<0.001 for all), indicating the predictive power of the new model for HCC patients.
DCA can clearly show the clinical utility of models for patients. The DCAs for the nomogram and other prognostic models are plotted in Figure 3. The results presented that if the threshold probability of a patient range was 0.1 to 0.5, the nomogram, the post-TACE model, and the SNACOR score in predicting OS were more beneficial than an all patients dead strategy or no patients dead strategy in both the training and validation cohorts. Furthermore, the nomogram exhibited higher net benefits than other models, implying that it had superior clinical value.
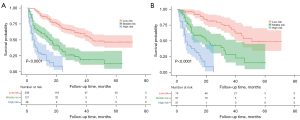
Based on the OS rate predicted by the nomogram, Kaplan-Meier curves were drawn to stratify the patients into 3 risk subgroups in both cohorts (Figure 4). For the low-, medium-, and high-risk group in the training cohort, the OS were (45.752±2.097), (22.003±2.296), and (7.493±1.059) months, respectively, and in the validation cohort were (50.161±3.393), (25.656±3.153), and (9.732±1.301) months, respectively (both P<0.001). Besides, we also drew Kaplan-Meier curves of the other 4 models to compare the discrimination performance (Figure S2).
Discussion
Timely identification of HCC patients who will not benefit from continuing TACE or who have poor prognosis is important as these patients can still benefit from systemic therapies as long as the tumor burden and liver function allow for it (23). As there are no guidelines concerning standardized re-treatment policy, we established and validated a reliable, easy-to-use, and accurate risk prediction model to help guide the decision process for the retreatment with TACE.
The final nomogram model that we established integrated 5 independent risk factors: post-ALBI grade, tumor diameter, number of tumors, portal vein invasion, and tumor response, showing outstanding predictive performances for OS, which were verified by its discrimination and calibration in both training and validation cohorts. Compared with the training cohort, the validation cohort showed better discrimination ability (C-index: 0.770 vs. 0.753), suggesting that the nomogram might have adequate external utility. According to the death risk predicted by the nomogram, HCC patients could be divided into 3 distinct risk groups, and the OS in both cohorts was considerably reduced as the death risk increased. As systemic therapies have become the main treatment strategy for unresectable HCC, the postoperative nomogram can provide guidance for identifying patients who are not benefiting from TACE and switching to systemic therapies or other evidence-based treatments timely if the patient is estimated to have poor survival outcomes.
Different from other solid tumors, not only tumor characteristics, but liver function reserve is crucial to the prognosis and the treatment options for HCC patients. Guidelines have underlined the necessity of liver function reserve during TACE treatments for underlying conversion to systemic therapies (23). Acute liver function deterioration after TACE treatment generally resolves within 1 month, otherwise it may become chronic, potentially compromising the survival benefits of TACE (6). Although the Child-Pugh score (CPS) has been widely used in liver function assessment of HCC patients, there is no clear standard for subjective factors such as ascites and hepatic encephalopathy, and variables of ALB and ascites have a mutual influence. Including only 2 laboratory parameters, the ALBI grade has been considered a simple and objective indicator of liver function, and its predictive value in HCC patients is comparable to that of the CPS (24). The postoperative ALBI grade was strongly linked with OS in univariate and multivariate analyses, indicating that postoperative chronic liver function impairment was a prognostic factor for TACE-treated HCC patients.
Instead of the World Health Organization (WHO) criteria or RECIST, the mRECIST or EASL criteria have been already recommended by guidelines as the standard tool for measuring radiological endpoints of HCC (25-27). The degree of tumor response reflected by mRECIST has been shown to be a strong predictor of median OS in HCC patients, which was also validated in this study (28). There is no standard for the number of TACE sessions and the interval between 2 TACEs required to achieve an individual’s best response. The over-pursuit of the best response through repeated TACE courses may miss the opportunity for more prompt optimization of treatment strategies. Thus, we included the tumor response after the first TACE rather than the best response as a prognostic variable in our nomogram model. However, the traditional monitoring indicator AFP, which also remains a controversial biomarker for HCC, did not have a significant prognostic value in this study (29).
To date, a series of prognostic models or scores have been developed for HCC patients undergoing TACE. The ART score, first proposed by Sieghart et al. in 2013, includes tumor response (EASL criteria) and liver function (CPS, AST) (15). The ABCR score [AFP, BCLC, CPS, and tumor radiological response (EASL criteria)] was introduced by Adhoute et al. in 2015 (16). Similar to the ABCR score, the SNACOR score was developed by Kim et al. in 2016 including liver function (CPS), tumor characteristics (size, number), baseline AFP and tumor response (mRECIST criteria) (17). Recently, Han et al. proposed the Post-TACE-Prediction model, based on the baseline AFP, bilirubin, tumor characteristics (size, number), and tumor response (mRECIST criteria), which were summarized in a complex formula (18). Compared to these systems, our nomogram has the following merits: First, simple, objective, and readily available clinical indicators are included, and it can be applied in patients with different stages in adaptation to the clinical practice that TACE has been widely used beyond the guideline criteria (30). Second, the importance of chronic rather than acute liver function deterioration after TACE was pointed out. Third, compared with simple grades or complex online calculators, the nature of nomograms could provide an easy-to-access and accurate probability to predict OS. Above all, our nomogram showed a more powerful and accurate predictive ability for OS, which was indicated by the C-index comparison and DCAs.
This study had some limitations. First, although the nomogram performed better in the validation cohort, prospective and multicenter external validation is necessary to validate the external utility. Second, similar to other prognostic tools, the variables included in the nomogram were traditional indicators. With the rapid development of big data technology, high sensitivity and specificity tumor markers of HCC have been found in genomics and radiomics analysis, which may improve the predictive ability if included in the model, but these indicators still need to be further screened and verified (31-34). Third, only patients undergoing initial conventional (c)TACE were enrolled, though until today no significant difference in tumor response or survival has been observed between cTACE and drug-eluting bead TACE (DEB-TACE) (35,36). However, further validation studies might be necessary for HCC patients treated with DEB-TACE and patients underwent other therapy before or during the TACE to generalize our results.
Conclusions
In conclusion, this study generated and validated a reliable, easy-to-use, and accurate nomogram model to predict prognosis of HCC patients undergoing TACE. According to the death risk predicted by the nomogram, we can cautiously speculate that HCC patients who are estimated to have poor survival outcomes may not profit from further TACE treatments and should be promptly recommended switch to another treatment schedule.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81972233) and Major Scientific and Technological Innovation Project of Wenzhou Science and Technology Bureau (No. ZY2021009).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6513/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6513/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6513/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (approval number: KY2022-R108) and written informed consent was provided by each patient included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Han K, Kim JJWjog. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. 2015;21:10327-35.
- Tay BWR, Huang DQ, Mark M, et al. Comparable Outcomes in Early Hepatocellular Carcinomas Treated with Trans-Arterial Chemoembolization and Radiofrequency Ablation. Biomedicines 2022;10:2361. [Crossref] [PubMed]
- Yang XG, Sun YY, Wang HQ, et al. Efficacy and safety of transarterial chemoembolization combining sorafenib with or without immune checkpoint inhibitors in previously treated patients with advanced hepatocellular carcinoma: A propensity score matching analysis. Front Oncol 2022;12:914385. [Crossref] [PubMed]
- Zane KE, Makary MS. Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers (Basel) 2021;13:5430. [Crossref] [PubMed]
- Miksad RA, Ogasawara S, Xia F, et al. Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: the LiverT study. BMC Cancer 2019;19:795. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Kudo M, et al. Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig Dis 2017;35:602-10. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Piscaglia F, Ogasawara S. Patient Selection for Transarterial Chemoembolization in Hepatocellular Carcinoma: Importance of Benefit/Risk Assessment. Liver Cancer 2018;7:104-19. [Crossref] [PubMed]
- Müller L, Stoehr F, Mähringer-Kunz A, et al. Current Strategies to Identify Patients That Will Benefit from TACE Treatment and Future Directions a Practical Step-by-Step Guide. J Hepatocell Carcinoma 2021;8:403-19. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2013;57:2261-73. [Crossref] [PubMed]
- Adhoute X, Penaranda G, Naude S, et al. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol 2015;62:855-62. [Crossref] [PubMed]
- Kim BK, Shim JH, Kim SU, et al. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: development of a prediction model. Liver Int 2016;36:92-9. [Crossref] [PubMed]
- Han G, Berhane S, Toyoda H, et al. Prediction of Survival Among Patients Receiving Transarterial Chemoembolization for Hepatocellular Carcinoma: A Response-Based Approach. 2020;72:198-212.
- Kotsifa E, Vergadis C, Vailas M, et al. Transarterial Chemoembolization for Hepatocellular Carcinoma: Why, When, How? J Pers Med 2022;12:436. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Zhao SM, Qiu LW, Zhao H, et al. Prognostic nomogram for hepatocellular carcinoma patients after transarterial chemoembolization based on des-γ-carboxy prothrombin reactivity and modified Response Evaluation Criteria in Solid Tumors. J Cancer Res Ther 2021;17:707-14. [Crossref] [PubMed]
- Lin J, Li X, Shi X, et al. Nomogram for predicting pathologic complete response after transarterial chemoembolization in patients with hepatocellular carcinoma. Ann Transl Med 2021;9:1130. [Crossref] [PubMed]
- Ogasawara S, Ooka Y, Koroki K, et al. Switching to systemic therapy after locoregional treatment failure: Definition and best timing. Clin Mol Hepatol 2020;26:155-62. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238-iv55. [Crossref] [PubMed]
- Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288-306. [Crossref] [PubMed]
- Hu X, Chen R, Wei Q, et al. The Landscape Of Alpha Fetoprotein In Hepatocellular Carcinoma: Where Are We? Int J Biol Sci 2022;18:536-51. [Crossref] [PubMed]
- Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36. [Crossref] [PubMed]
- Zhu S, Huang X, Zhang K, et al. Low expression of long noncoding RNA CTC-297N7.9 predicts poor prognosis in patients with hepatocellular carcinoma. Cancer Med 2019;8:7679-92. [Crossref] [PubMed]
- Chen W, Ou M, Tang D, et al. Identification and Validation of Immune-Related Gene Prognostic Signature for Hepatocellular Carcinoma. J Immunol Res 2020;2020:5494858. [Crossref] [PubMed]
- Peng J, Kang S, Ning Z, et al. Residual convolutional neural network for predicting response of transarterial chemoembolization in hepatocellular carcinoma from CT imaging. Eur Radiol 2020;30:413-24. [Crossref] [PubMed]
- Liu D, Liu F, Xie X, et al. Accurate prediction of responses to transarterial chemoembolization for patients with hepatocellular carcinoma by using artificial intelligence in contrast-enhanced ultrasound. Eur Radiol 2020;30:2365-76. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis 2016;48:571-7. [Crossref] [PubMed]
(English Language Editor: J. Jones)


