Prevalence and associated factors of drug-drug interactions in elderly outpatients in a tertiary care hospital: a cross-sectional study based on three databases
Highlight box
Key findings
• The prevalence of potential csDDIs in elderly outpatients ranged from 4.56% to 14.53%.
What is known and what is new?
• Fair consistency of Lexicomp® and Micromedex® in other populations and some drug classes and the prevalence of potential DDIs in single prescriptions for outpatient geriatric patients is known.
• This manuscript adds: (I) the prevalence of potential csDDIs and the consistency of Lexicomp® and Micromedex® in elderly outpatients; (II) the factors associated with potential csDDIs identified in multiple databases; (III) the trends of DDIs in outpatients under different combination cycles
What is the implication, and what should change now?
• More research on the risk and actual adverse outcomes of csDDIs is needed.
Introduction
A drug-drug interaction (DDI) can be defined as 1 drug’s effect on another (1). The interaction mechanism may be roughly classified into pharmacokinetic and pharmacodynamic. DDIs may increase efficacy, decrease efficacy, or increase toxicity. As a primary type of drug-related problems (DRPs), some clinically significant DDIs (csDDIs) are associated with clinically adverse outcomes, such as adverse drug reactions (ADRs), readmission, and death (2-6). The elderly population is more vulnerable to the adverse effects of DDIs due to the coexistence of multiple diseases, the prevalence of polypharmacy, and age-related pharmacokinetic and pharmacodynamic changes (1,7). Epidemiological study of DDIs shows that older patients are more likely to be exposed to csDDIs (8). DDIs are somewhat predictable when clinical evidence is available and pharmacological effects are known. Identifying and preventing csDDIs is necessary to optimize pharmacotherapeutic outcomes in the elderly.
The prevalence of potential DDIs in elderly with multimorbidity in primary care varies 20–100% (9). A system review shows that the weighted mean prevalence of severe DDI was in rank order: hospital 28.9%, primary care 4.4%, and nursing home 3.3% (10). In prescriptions containing ≥2 medications belonging to elderly outpatients at least one clinically relevant DDI was detected in 61.7% (11). There is less data on potential DDIs and csDDIs in multiple prescriptions for elderly outpatients. Factors contributing to the occurrence of DDIs in populations are varied in different researches, such as age, comorbidities, polypharmacy, nutritional status, and genetic constitution of an individual (8,12,13), comparison between studies is not straightforward as a result of differences DDI detections. Potential DDIs in clinical practice can be identified via drug instructions, pharmacy guidebooks, consensus lists, clinical decision support systems (CDSS), and electronic databases. CDSS can implement pre-event risk identification for prescriptions instead of after-the-fact reviews. However, balancing the burdens and benefits of risk alerts has always been an essential issue of CDSS (14-16). For csDDIs alerts in outpatients, choosing an appropriate drug combination cycle is important. There is a lack of research in this area.
This study aimed to investigate the prevalence and factors associated with potential DDIs, especially csDDIs, detected by Lexicomp®, Micromedex®, and DDInter in elderly outpatient prescriptions. Further, we aimed to assess the consistency of the 3 databases for rating DDIs and study the appropriate assessment cycle for potential csDDIs risk identification in CDSS for outpatients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5463/rc) (17).
Methods
Study design and settings
This was a descriptive, observational, cross-sectional study conducted in PLA General Hospital in Beijing, which integrated medical service, education, and research. All prescriptions for elderly patients (≥65 years of age) were collected consecutively between January and March 2022. Patient-related information was retrieved from the hospital information system. The collection process ensured patient anonymity and data confidentiality. The study was conducted by the Declaration of Helsinki (as revised in 2013) and approved by PLA General Hospital Ethics Committee (No. S2022-497-01). Individual consent for this observational analysis was waived.
Participants
The target population was outpatients ≥65 years of age who had been prescribed at least 1 systemic drug (intravenous administration, gastrointestinal administration, gastrointestinal administration, respiratory administration, rectal mucosal administration).
Data collection
The prescription inclusion criteria were as follows: Outpatient prescriptions for patients aged ≥65 years.
The prescription exclusion criteria were as follows: (I) prescriptions that do not include systemic medication; (II) prescriptions for traditional Chinese medicine (TCM) decoction preparations.
All systemic medications except Chinese patent medicines were involved in the DDI analysis. Chinese patent medicines were only counted in the patient’s total number of drugs.
Different brands of medications with the same route of administration and the same generic name were considered 1 drug. Medications were classified according to the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) classification system.
Medications for each patient were combined into pairs under different assessment cycles: (I) single prescription; (II) single day; (III) single period (prescriptions prescribed with intervals less than 7 days were counted as 1 period). Combinations were conducted after removing duplicate medications in a single assessment cycle.
This study had no interaction or direct contact with any patient. Age, gender, number of departments visited, number of medications, number of diagnoses, and the cost of medications for each patient were collected.
Evaluation of DDIs
All medication combinations were evaluated by 3 electronic databases: Lexicomp® Drug Interactions, Micromedex® Drug-Reax, and DDInter. Lexicomp® and Micromedex® were accessed through the hospital library. Drug combinations were classified according to the interaction risk of the different databases in Table 1. For compounded formulations, the corresponding drug was entered directly if it was available in the database, and the components included in the formulation were entered individually if it was not available in the database. All the drug combinations were entered independently by 2 trained pharmacists, with a third pharmacist participating in the case of any discrepancies in the results. The cut-off date for all database searches was 31 July 2022.
Table 1
| Database | Rating |
|---|---|
| Lexicomp | X (avoid combination) |
| D (consider therapy modification) | |
| C (monitor Therapy) | |
| B (no Action Needed) | |
| A (no Known Interaction) | |
| Micromedex | Contraindicated (the drugs are contraindicated for concurrent use) |
| Major (interaction might be life-threatening and/or require medical intervention) | |
| Moderate (interaction might result in exacerbation of the patient’s condition and/or requires an alternative therapy) | |
| Minor (interaction has limited clinical effects) | |
| Unknown (no known drug interactions) | |
| DDInter | Major (interaction was highly clinically significant and the drug combinations should be strictly avoided) |
| Moderate (interaction may result in exacerbation of the disease of the patient and/or change in therapy) | |
| Minor (interactions were minimally clinically significant and usually they do not require changes in therapy) | |
| Unknown (interaction description was unavailable or incomplete) |
DDI, drug-drug interaction.
We defined the different rating DDIs into two classes. Potential DDIs including drug combinations: (I) X, D, C, B in Lexicomp®; (II) Contraindicated, Major, Moderate, Minor in Micromedex®; (III) Major, Moderate, and Minor in DDInter. Potential csDDIs including drug combinations: (I) X and D in Lexicomp®; (II) Contraindicated and Major in Micromedex®; (III) Major in DDInter.
Statistical analysis
Data were analyzed with the statistical program SPSS 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables with normal distribution were presented as mean ± standard deviation (SD); non-normal variables were reported as the median and interquartile range (IQR).
The prevalence of different rating DDIs was expressed as a percentage. The prevalence of potential DDIs and csDDIs was expressed as the proportion of patients with at least 1 potential DDI or csDDI.
Univariate analysis was performed to assess the effect of covariates on the occurrence of csDDIs. To control for confounding variables, variables showing association in univariate analysis (P<0.2) were included in logistic regression analysis by forward procedures to identify variables that may be associated with potential csDDIs. Logistic regression analyses were presented with odds ratios (ORs) and 95% confidence intervals (95% CIs).
Database coverage of drugs refers to the proportion of all drugs involved in the interaction that could be retrieved in the database.
The consistencies were analyzed using weighted Cohen’s kappa. Drug combinations that could be retrieved simultaneously from both databases were included in the analysis. Weighted kappa values of 0–0.2 indicated poor concordance; 0.21–0.40 indicated fair concordance; 0.41–0. 60 indicated moderate concordance; 0.61–0.80 indicated strong concordance; and 0.81–1.0 indicated perfect concordance. The overlap was analyzed by jevenn (an interactive Venn diagram viewer) (18). A P value <0.05 was considered to indicate statistical significance.
Results
Participants
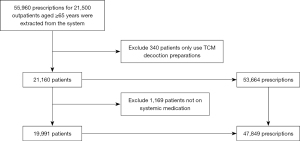
From January to March 2022, a total of 19,991 eligible patients were included. Figure 1 describes the specific inclusion and exclusion process.
Characteristics of the study population
Of the 19,991 patients included, 45.8% were women. The median age of the patients was 71 years (IQR 67–69). Patients were prescribed 5–9 types of systemic drugs in 25.2% of cases, and 10 or more types in 7.9% of cases. There were 6,135 (30.7%) patients with more than 2 department visits, 3.2% with more than 5 visit days, and 34.5% with more than 2 visit periods. At different assessment cycles, 19,991 patients had different numbers of medications to be combined into pairs. When assessed with a single prescription, 12,252 patients had at least 1 drug combination; 13,439 patients when assessed within a single day, and 13,551 patients when assessed within a single period. Table 2 shows the specific patient characteristics.
Table 2
| Characteristics | Data |
|---|---|
| Female, n (%) | 9,149 (45.8) |
| Age, median [IQR] | 71 [67–79] |
| 65–69, n (%) | 7,794 (39.0) |
| 70–74, n (%) | 4,514 (22.6) |
| 75–79, n (%) | 2,842 (14.2) |
| 80–84, n (%) | 2,314 (11.6) |
| 85–89, n (%) | 1,609 (8.0) |
| 90–94, n (%) | 823 (4.1) |
| ≥95, n (%) | 95 (0.5) |
| Types of departments patient visited, median [IQR] | 1 [1–2] |
| 1, n (%) | 13,856 (69.3) |
| 2–3, n (%) | 4,860 (24.3) |
| 4–6, n (%) | 1,171 (5.9) |
| ≥7, n (%) | 104 (0.5) |
| Numbers of medications, median [IQR] | 3 [2–5] |
| 1–4, n (%) | 13,375 (66.9) |
| 5–9, n (%) | 5,038 (25.2) |
| ≥10, n (%) | 1,578 (7.9) |
| Costs for medications (CNY), median [IQR] | 835 [272–2,064] |
| Costs ≤500, n (%) | 7,495 (37.5) |
| 500< costs ≤1,000, n (%) | 3,419 (17.1) |
| 1,000< costs ≤2,000, n (%) | 3,920 (19.6) |
| 2,000< costs ≤5,000, n (%) | 3,639 (18.2) |
| 5,000< costs ≤10,000, n (%) | 1,022 (5.1) |
| costs >10,000, n (%) | 496 (2.5) |
| Numbers of prescriptions, median [IQR] | 2 [1–3] |
| 1–2, n (%) | 14,055 (70.3) |
| 3–5, n (%) | 4,382 (21.9) |
| 6–9, n (%) | 1,154 (5.8) |
| ≥10, n (%) | 400 (2.0) |
| Numbers of visit days, median [IQR] | 1 [1–2] |
| 1, n (%) | 12,394 (62.0) |
| 2–4, n (%) | 6,960 (34.8) |
| 5–9, n (%) | 615 (3.1) |
| ≥10, n (%) | 22 (0.1) |
| Numbers of consecutive visit periods, median [IQR] | 1 [1–2] |
| 1, n (%) | 13,083 (65.4) |
| 2–4, n (%) | 6,661 (33.3) |
| ≥5, n (%) | 247 (1.2) |
IQR, interquartile range.
Frequency and prevalence of potential DDIs and csDDIs
A total of 21,527 drug combinations were identified in patients. Table 3 shows the number of different ratings of drug combinations. Lexicomp® detected 2,604 potential DDIs and 366 potential csDDIs. Micromedex® detected 1,411 potential DDIs and 740 potential csDDIs. DDInter detected 2,676 potential DDIs and 245 potential csDDIs.
Table 3
| Lexicomp | Micromedex | DDInter | |||||
|---|---|---|---|---|---|---|---|
| Rating | n | Rating | n | Rating | n | ||
| X | 79 | Contraindicated | 4 | Major | 245 | ||
| D | 287 | Major | 736 | Moderate | 2,203 | ||
| C | 1,872 | Moderate | 645 | Minor | 228 | ||
| B | 366 | Minor | 26 | Unknown | 3,752 | ||
| A | 4 | Unknown | 0 | None | 5,158 | ||
| None | 14,835 | None | 13,497 | – | 9,941 | ||
| – | 4,084 | – | 6,619 | ||||
No interaction between the two drugs according to the feedback of the corre-sponding database. –, cannot be retrieved in the corresponding database. DDIs, drug-drug interactions.
Figure 2 demonstrates the frequency of potential DDIs and csDDIs during different assessment cycles. There are 214,344, and 128 csDDIs were identified during single prescription cycles by Lexicomp®, Micromedex®, and DDInter, respectively. When the assessment cycles extended to a single day, another 105, 298, and 95 csDDIs were detected, respectively. The frequency of csDDIs happened increased by 41.38% (1,798 to 2,542 in Lexicomp®), 53.86% (3,316 to 5,102 in Micromedex®), and 50.12% (812 to 1,219 in DDInter). When the assessment cycles expanded from a single day to a single period, the frequency of csDDIs increased by 5.9%, 6.0%, and 6.4%, respectively.

When DDIs were assessed with a single prescription, the prevalence of potential csDDIs was 2.93–10.59%. The prevalence increased by 2.28% (Lexicomp®), 3.37% (Micromedex®), and 1.41% (DDInter), when the assessment cycle was extended to 1 day. This further increased by 0.42%, 0.57%, and 0.22% when the assessment cycle was extended to 1 period. Figure 3 demonstrates the prevalence of potential csDDIs and DDIs under different assessment cycles.

Characteristics of medications and departments patients visited
A total of 486 medications (excluding Chinese patent medicines) were prescribed for 19,991 patients, to a total of 67,177 times of patient use. The most used were ATC Class C (cardiovascular system class, 27.93%), Class A (digestive and metabolic tract, 24.53%), Class B (blood and hematopoietic organs, 11.61%), and Class N (nervous system class, 11.50%). Among the 366 csDDIs evaluated by Lexicomp®, drugs used in diabetes (A10) participated in the most csDDIs (41.26%), followed by psycholeptics (N05, 18.85%) and anti-inflammatory and antirheumatic products (M01, 10.38%). Among the 740 csDDIs evaluated by Micromedex®, the most involved drug classes in the csDDIs were psychoanaleptics (N06, 30.68%), drugs used in diabetes (A10, 25.41%), and psycholeptics (N05, 17.16%). The top 3 drug classes involved in the 245 csDDIs evaluated by DDInter were psychoanaleptics (N06, 9.46%), immunosuppressants (L04, 7.7%), and agents acting on the renin-angiotensin system (C09, 6.62%). Table S1 shows the numbers of participating csDDIs and usage frequencies of each ATC category.
Studied outpatients visited a total of 40 types of departments. The department with the highest number of patient visits was Cardiology (60.40%), followed by Neurology (29.88%), and Gastroenterology (22.73%).
The factors of potential csDDIs
In the multifactorial analysis, regardless of the database used for DDIs assessment, polypharmacy and Neurology Department visits were risk factors for potential csDDIs. Visiting the Gastroenterology or TCM Department was a protective factor for the prevalence of potential csDDIs. Table S2 shows the results of univariate and multifactorial analyses of potential csDDIs when assessed within a single period.
For patients aged 70–74 years (OR 1.184, 95% CI: 1.075–1.304), ≥7 department visits (OR 10.357, 95% CI: 3.894–27.546), on multiple medications (5–9 medications, OR 9.267, 95% CI: 8.482–10.125; ≥10 medications, OR 44.859, 95% CI: 36.591–54.995), visiting the Cardiology (OR 1.536, 95% CI: 1.406–1.667), Neurology (OR 1.946, 95% CI: 1.754–2.160), Orthopedics (OR 1.396, 95% CI: 1.235–1.579), or Endocrinology (OR 2.634, 95% CI: 2.303–3.014) departments were at higher risk of having potential csDDIs under Lexicomp® criteria.
For those aged 70–74 years (OR 1.138, 95% CI: 1.012–1.279); with multiple medications (5–9 medications, OR 6.992, 95% CI: 6.261–7.808; ≥10 medications, OR 19.466, 95% CI: 16.328–23.208), visiting the Cardiology (OR 1.685, 95% CI: 1.521–1.866), Neurology (OR 2.305, 95% CI: 2.057–2.582), and Endocrinology departments (OR 2.415, 95% CI: 2.107–2.768) had a higher risk of having potential csDDIs under Micromedex® criteria.
For patients on multiple medications (5–9 medications, OR 4.479, 95% CI: 3.767–5.326; ≥10 medications, OR 12.64, 95% CI: 9.866–16.195), those seen in the Neurology department (OR 1.278, 95% CI: 1.079–1.513) were at higher risk of potential csDDIs under the DDInter criteria.
Consistency evaluation of the three databases
For 486 drugs, the coverage in Lexicomp® and Micromedex®, and DDInter databases was 427 (87.86%), 398 (81.89%), and 356 (73.25%), respectively. A total of 53 drugs were not included in any of the 3 databases.
Lexicomp® and Micromedex® retrieved 14,593 DDIs together, 11,489 for Lexicomp® and DDInter, and 11,432 for Micromedex® and DDInter. Weighted Kappa analysis of the 3 databases for the risk ratings of drug combinations showed moderate consistency for Lexicomp® and Micromedex® (weighted kappa =0.473) and fair consistency for both DDInter with the other 2 databases (0.364 with Lexicomp® and 0.303 with Micromedex®). Figure 4 shows the overlap of csDDIs detected by the 3 databases. Sixty-six drug combinations were identified as csDDIs by all databases simultaneously.

Potential csDDIs detected by Lexicomp® and Micromedex® together
A total of 149 drug combinations were detected as csDDIs both in Lexicomp® and Micromedex®. These could be classified into 68 categories (Table S3). The most common (22/68) mechanism of drug interactions is that 1 drug enhances the pharmacological effects of another.
In a single prescription, only 83 combinations were detected. The most frequent combinations were aspirin and Ginkgo Biloba (113 times), aspirin and ticagrelor (72 times), and leflunomide and methotrexate (46 times).
There was an exponential increase in 18 categories (including 60 combinations) when the assessment cycles were extended to a single day. This was particularly noticeable for the combination of central nervous system (CNS) depressants and oxycodone, which increased from 2 to 107.
Only 2 combinations (atorvastatin and clarithromycin, citalopram and omeprazole) exponentially increased when the assessment period was extended from a single day to a single period.
Under any assessment cycle, the combination of aspirin and Ginkgo biloba was the most common combination. Ginkgo biloba may enhance the anticoagulant effect of aspirin and increase the bleeding risk. If the combination is used, it needs to be monitored for signs and symptoms of bleeding (especially intracranial bleeding).
Discussion
This study evaluated the consistencies of 2 classical commercial databases and 1 recently developed free database with 21,527 drug combinations in geriatric outpatients. Only the consistency between Lexicomp® and Micromedex® was moderate. The prevalence of potential csDDIs detected by the 3 databases ranged from 2.93% to 14.53% at different assessment cycles. Polypharmacy and Neurology Department visits were risk factors for potential csDDIs detected by all 3 databases. When the drug combination assessment cycle was expanded from single prescription to single visit day, the prevalence of csDDIs increased by 1.32–1.48 times.
DDIs in elderly outpatients showed widely heterogeneous results in previous studies. The prevalence of potential DDIs ranged from 20% to 100% (9). This heterogeneity was not only present in outpatient settings, but also in relevant studies in community and inpatient settings (19-21). These differences were partly due to study populations and regional differences; more importantly, the reasons included differences in the definition the DDIs (potential or clinically significant, especially for csDDIs) and the criteria evaluating DDIs (different databases, consensuses, and reference books). In this study, the prevalence can differ to about 10% (9.97% for potential csDDIs and 10.31% for potential DDIs) in the same population with different criteria. We defined the potential DDIs as drug combinations with interactions that can be retrieved from the database. Therefore, the types of “A” in Lexicomp® and “Unknown” in Micromedex® in DDInter® were not included. In some studies, csDDIs covered more combinations (22). Since DDIs in elderly patients are common, focusing on the DDIs with severe risks (such as life-threatening) that require avoiding combination or medical intervention is more practical. The consensuses related to csDDIs in elderly patients are also focused on this type of DDIs (23-27). The “C” risk rating in Lexicomp® requires monitoring the risk, and the DDIs rating “moderate” in Micromedex® indicates risk but is not life-threatening, so they were not included in potential csDDIs in our study.
The comparison research among different databases had already revealed that Micromedex® is the most commonly used software (28). Both Lexicomp® and Micromedex® showed the best performances (29,30). There were no studies of consistency analysis between Lexicomp® and Micromedex® in elderly patients. In other population research, the drug combinations evaluated for consistency were fewer and showed fair or moderate consistency of Lexicomp® and Micromedex® (31-35). In our study, a total of 21,527 drug combinations in elderly outpatient pharmaceuticals were examined. Some 17,758 drug combinations could be retrieved in at least 1 database. Lexicomp® and Micromedex® showed moderate consistency with a weighted kappa of 0.473. DDInter® is a newly developed open-access drug interaction database (36) for which there has been no comparative study with other databases. Prescribing behavior of elderly patients is common due to the high prevalence of chronic diseases. In China, the accessibility of commercial databases for community pharmacists when reviewing medications for older patients is poor. It is necessary to find a reliable alternative tool. In this study, DDInter® showed fair consistency with the 2 classic commercial databases and had the lowest drug coverage. As in the other 2 databases, DDInter® describes risk rating, clinical effect, management advice, and references of each DDIs. However, there is no reliability rating in DDInter®. The investigator must retrieve drug components individually because DDInter® does not cover compounded formulations. Only a maximum of 5 drug components can be entered in 1 search, which reduced the efficiency of identification. However, at the same time, DDInter® has also demonstrated some advantages during the research process. It has a user-friendly interface; all drugs were ATC coded; each interaction was annotated with mechanisms, and alternative medications were provided based on the ATC code. We advise that DDInter® can be used as a simple alternative when commercial databases are not accessible to assess DDIs in elderly patients. Its recommendations can be used as a reference, but its reliability needs further strengthening.
Due to the variability in the risk ratings of DDIs, the factors associated with different databases are different. Polypharmacy was confirmed again in this study as a recognized risk factor for DDIs (19,34). For elderly outpatients, the risk increased by 4.479–9.267 times when using 5–9 medications and 12.64–44.859 times when using ≥10 medications. Notably, the risk of potential csDDIs did not increase with age. Multifactorial analysis under Lexicomp® and Micromedex® criteria showed that the risk of csDDIs was significant in patients aged 70–74 years in different age groups. However, the risk of potential csDDIs at age 80–89 years was lower in a multifactorial analysis of Micromedex®. This may be because physicians are more cautious in their prescribing practices for patients of advanced-age. The risk of potential csDDIs was only significantly elevated when the number of department visits was ≥7. Due to the disordered diagnosis in the outpatient prescription, it is hard to accurately evaluate the patient’s disease distribution. Therefore, we counted the distribution of the departments visited by patients for substitution. Among the top 10 departments that patients visited, Neurology Department visits were a risk factor for potential csDDIs, regardless of the assessment criteria. This result was further confirmed by the fact that neurological drugs were ranked in the top 3 in the participation of csDDIs detected in all 3 databases.
In outpatient studies without direct contact with patients, researchers could not know all the information about the medications taken by patients. Therefore, an appropriate drug combination cycle is vital in evaluating DDIs and developing strategies for identifying outpatient DDIs in CDSS. Only evaluating the drug combinations within a single prescription will underestimate the DDIs. Extending the cycle unlimitedly will place more demands on the program, lead to oversensitivity, and increase the alert burden. Our study evaluated the changes in potential csDDIs in elderly outpatients with different drug combination cycles. Our findings demonstrated that the prevalence of potential csDDIs increased by 31.82–48.12%, the frequencies of potential csDDIs increased by 41.38–53.86%, the types of csDDIs detected were increased by 49–86% when the cycle was extended from a single prescription cycle to a single visit day. Meanwhile, when it was extended from a single day to a single visit period (with intervals less than 7 days), the prevalence increased by 4.08–5.07%, the frequencies increased by 5.9–6.4%, and the types increased by 9.87–15.26%. Therefore, identifying DDIs throughout the visit can avoid the omission of most csDDIs, especially for some csDDIs involved in drugs that need to be prescribed separately. For example, the interactions between oxycodone and CNS depressants were highly prevalent in our investigation. Since the increase in the prevalence and frequencies of potential csDDIs is not considerable and will increase the work burden of physicians (verifying the actual medication usage of patients), we propose only a targeted extension of the identification cycle for a small proportion of csDDIs with a very high risk involved in long-term medications for chronic diseases.
Although DDIs are predictable when clinical evidence and pharmacological effects are known (1), current CDSS for DDIs screening are often overly sensitive, with a high alert burden, and clinicians often override clinically significant and insignificant alerts (14). The factors of barriers in CDSS most often reported were related to (a lack of) usefulness and relevance of information (37). There have been few studies conducted related to the clinical outcomes of DDIs. Elderly patients are more susceptible to some of the risks for csDDIs identified in this study, such as myopathy and QTc-prolonging. There are many outpatients with csDDIs that receive a combination of more than 2 CNS depressants. In Beers criteria, any combination of 3 or more CNS-active drugs needs to be avoided (24). Not only 2 drug interactions but also multiple drug interactions need to be considered (38). The lack of clinical relevance of the detected DDI should be addressed in upcoming studies, as this would provide more relevant information for prescribers in clinical practice. The strategy development for DDIs in CDSS needs a combination of more than 1 database, literature reviews, and advice from both expert physicians and pharmacists.
Strengths and limitations
The main strength of this study is the use of 2 well-established and reliable databases recognized from previous studies (Lexicomp® and Micromedex®) with a completely new database (DDInter) introduced to evaluate the same sample of larger (19,991 outpatients) elderly population (65 years and older) for comprehensive screening of DDIs (including 21,527 drug combinations) and assess changes of DDIs in outpatients with different combination cycles. It is the first consistent study of DDIs in elderly patients, the first use of the DDInter in clinical practice, and the first description of trends of DDIs in outpatients under different combination cycles. Some limitations of this study should also be highlighted. First, the evaluation was of potential DDIs and csDDIs, no clinical outcomes data were collected from patients to determine if the risk of interactions occurred. Second, this was a single-center study that only evaluated interactions between medications prescribed to elderly patients at the center, and did not collect complete medication data from patients, which would differ from the actual situation of patients. Third, some of the drug interactions were route- or dose-related; although we included systemic medications, we did not distinguish between IV or oral administration and we did not assess for the dose. Fourth, the use of Chinese patent medicines is widespread in elderly Chinese patients, and drug interactions with Chinese patent medicines were not evaluated in this study, and TCM decoction preparations were also excluded from the study.
Conclusions
More research on the risk of csDDIs is needed due to the inconsistency of DDIs ratings. In clinical pharmacotherapy practice, using multiple reference tools to evaluate DDIs and optimize the strategies is necessary. The use of neurologic drugs appears to predispose the elderly to csDDIs. Identifying medications prescribed for a full day allows for more accurate and comprehensive detection of csDDIs. DDIs risk strategies of CDSS should be multiple and individualized to be more effective in avoiding serious risks and optimizing pharmacotherapeutic outcomes.
Acknowledgments
Funding: This study was supported by the National Key Research and Development Program of China (No. 2020YFC2005005).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5463/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5463/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5463/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by PLA General Hospital Ethics Committee (No. S2022-497-01). Individual consent for this observational analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007;370:S0140-6736(07)61092-7.
- Wang M, Zeraatkar D, Obeda M, et al. Drug-drug interactions with warfarin: A systematic review and meta-analysis. Br J Clin Pharmacol 2021;87:4051-100. [Crossref] [PubMed]
- Protzenko D, Nakache J, Lombardi M, et al. Pharmacist-led medication review unveiled more medication-related problems in possibly medication-related hospitalisations than in unlikely medication-related hospitalisations in elderly patients. Eur J Hosp Pharm 2022;29:A106.
- Leal Rodríguez C, Kaas-Hansen BS, Eriksson R, et al. Drug interactions in hospital prescriptions in Denmark: Prevalence and associations with adverse outcomes. Pharmacoepidemiol Drug Saf 2022;31:632-42. [Crossref] [PubMed]
- Gnjidic D, Johnell K. Clinical implications from drug-drug and drug-disease interactions in older people. Clin Exp Pharmacol Physiol 2013;40:320-5. [Crossref] [PubMed]
- Samardžić I, Marinović I, Kuča N, et al. Potential clinically significant drug-drug interactions in prescribed pharmacotherapy in an outpatient setting. Pharmazie 2021;76:390-5. [PubMed]
- Davies LE, Spiers G, Kingston A, et al. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J Am Med Dir Assoc 2020;21:S1525-8610(19)30774-1.
- Ren W, Liu Y, Zhang J, et al. Prevalence of potential drug-drug interactions in outpatients of a general hospital in China: a retrospective investigation. Int J Clin Pharm 2020;42:1190-6. [Crossref] [PubMed]
- Sánchez-Fidalgo S, Guzmán-Ramos MI, Galván-Banqueri M, et al. Prevalence of drug interactions in elderly patients with multimorbidity in primary care. Int J Clin Pharm 2017;39:343-53. [Crossref] [PubMed]
- Bories M, Bouzillé G, Cuggia M, et al. Drug-Drug Interactions in Elderly Patients with Potentially Inappropriate Medications in Primary Care, Nursing Home and Hospital Settings: A Systematic Review and a Preliminary Study. Pharmaceutics 2021;13:266. [Crossref] [PubMed]
-
Fatemeh A Fatemeh R Kazem H Drug-drug interactions and potentially inappropriate medications among elderly outpatients. Braz J Pharm Sci 2021 . doi: - Daggupati SJV, Saxena PUP, Kamath A, et al. Drug-drug interactions in patients undergoing chemoradiotherapy and the impact of an expert team intervention. Int J Clin Pharm 2020;42:132-40. [Crossref] [PubMed]
- Medina-Barajas F, Vázquez-Méndez E, Pérez-Guerrero EE, et al. Pilot study: Evaluation of potential drug-drug interactions in hospitalized pediatric patients. Pediatr Neonatol 2020;61:S1875-9572(19)30546-7.
- Muylle KM, Gentens K, Dupont AG, et al. Evaluation of an optimized context-aware clinical decision support system for drug-drug interaction screening. Int J Med Inform 2021;148:S1386-5056(21)00019-8.
- Knight AM, Maygers J, Foltz KA, et al. The Effect of Eliminating Intermediate Severity Drug-Drug Interaction Alerts on Overall Medication Alert Burden and Acceptance Rate. Appl Clin Inform 2019;10:927-34. [Crossref] [PubMed]
- Cho I, Lee Y, Lee JH, et al. Wide variation and patterns of physicians' responses to drug-drug interaction alerts. Int J Qual Health Care 2019;31:89-95. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. [Crossref] [PubMed]
- Bardou P, Mariette J, Escudié F, et al. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics 2014;15:293. [Crossref] [PubMed]
- Burato S, Leonardi L, Antonazzo IC, et al. Comparing the Prevalence of Polypharmacy and Potential Drug-Drug Interactions in Nursing Homes and in the Community Dwelling Elderly of Emilia Romagna Region. Front Pharmacol 2020;11:624888. [Crossref] [PubMed]
- de Oliveira LM, Diel JDAC, Nunes A, et al. Prevalence of drug interactions in hospitalised elderly patients: a systematic review. Eur J Hosp Pharm 2021;28:4-9. [Crossref] [PubMed]
- Hanlon JT, Perera S, Newman AB, et al. Potential drug-drug and drug-disease interactions in well-functioning community-dwelling older adults. J Clin Pharm Ther 2017;42:228-33. [Crossref] [PubMed]
- Wang H, Shi H, Wang N, et al. Prevalence of potential drug - drug interactions in the cardiothoracic intensive care unit patients in a Chinese tertiary care teaching hospital. BMC Pharmacol Toxicol 2022;23:39. [Crossref] [PubMed]
- O'Mahony D, O'Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213-8. [Crossref] [PubMed]
- American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019;67:674-94. [Crossref] [PubMed]
- Tommelein E, Petrovic M, Somers A, et al. Older patients' prescriptions screening in the community pharmacy: development of the Ghent Older People's Prescriptions community Pharmacy Screening (GheOP3S) tool. J Public Health (Oxf) 2016;38:e158-70. [Crossref] [PubMed]
- Nyborg G, Straand J, Klovning A, et al. The Norwegian General Practice--Nursing Home criteria (NORGEP-NH) for potentially inappropriate medication use: A web-based Delphi study. Scand J Prim Health Care 2015;33:134-41. [Crossref] [PubMed]
- Anrys P, Petit AE, Thevelin S, et al. An International Consensus List of Potentially Clinically Significant Drug-Drug Interactions in Older People. J Am Med Dir Assoc 2021;22:S1525-8610(21)00315-7.
- Roblek T, Vaupotic T, Mrhar A, et al. Drug-drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol 2015;71:131-42. [Crossref] [PubMed]
- Kheshti R, Aalipour M, Namazi S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract 2016;5:257-63. [Crossref] [PubMed]
- Patel RI, Beckett RD. Evaluation of resources for analyzing drug interactions. J Med Libr Assoc 2016;104:290-5. [Crossref] [PubMed]
- Monteith S, Glenn T. Comparison of potential psychiatric drug interactions in six drug interaction database programs: A replication study after 2 years of updates. Hum Psychopharmacol 2021;36:e2802. [Crossref] [PubMed]
- Bektay MY, Seker Z, Eke HK, et al. Comparison of different decision support software programs in perspective of potential drug-drug interactions in the oncology clinic. J Oncol Pharm Pract 2022; Epub ahead of print. [Crossref] [PubMed]
- Bossaer JB, Thomas CM. Drug Interaction Database Sensitivity With Oral Antineoplastics: An Exploratory Analysis. J Oncol Pract 2017;13:e217-22. [Crossref] [PubMed]
- Roca B, Roca M. Assessment of Drug Interactions with Online Electronic Checkers in Multi-Pathological Patients. Pharmacology 2022;107:111-5. [Crossref] [PubMed]
- Abbas A, Al-Shaibi S, Sankaralingam S, et al. Determination of potential drug-drug interactions in prescription orders dispensed in a community pharmacy setting using Micromedex(®) and Lexicomp(®): a retrospective observational study. Int J Clin Pharm 2022;44:348-56. [Crossref] [PubMed]
- Xiong G, Yang Z, Yi J, et al. DDInter: an online drug-drug interaction database towards improving clinical decision-making and patient safety. Nucleic Acids Res 2022;50:D1200-7. [Crossref] [PubMed]
- Westerbeek L, Ploegmakers KJ, de Bruijn GJ, et al. Barriers and facilitators influencing medication-related CDSS acceptance according to clinicians: A systematic review. Int J Med Inform 2021;152:S1386-5056(21)00132-5.
- Anand TV, Wallace BK, Chase HS. Prevalence of potentially harmful multidrug interactions on medication lists of elderly ambulatory patients. BMC Geriatr 2021;21:648. [Crossref] [PubMed]