Accuracy of stroke volume variation and pulse pressure variation in predicting fluid responsiveness undergoing one-lung ventilation during thoracic surgery: a systematic review and meta-analysis
Highlight box
Key findings
• SVV and PPV are not suitable for guiding intraoperative fluid therapy due to their poor ability to predict fluid responsiveness in patients undergoing OLV.
What is known and what is new?
• Dynamic preload variables, such as SVV and PPV, are based on heart-lung interactions for guiding volume administration in mechanically ventilated patients. However, the debate continues as to whether SVV and PPV can accurately predict fluid responsiveness in patients undergoing OLV during thoracic surgery;
• This study retrieved the relevant studies on the accuracy of SVV and PPV in OLV and performed a systematic review and meta-analysis in an attempt to reach a conclusion on this issue.
What is the implication, and what should change now?
• In the future, a multicenter and large sample research needs to be conducted and analyzed.
Introduction
Goal-directed hemodynamic therapy (GDHT) can affect the prognosis of critical and major surgery patients (1). The real-time and accurate monitoring of stroke volume variation (SVV) and pulse pressure variation (PPV) during anesthesia play an important role in clinical decision making. The FloTrac-Vigileo system is a minimally invasive hemodynamic monitoring method based on arterial pressure waveform analysis, which has many advantages, including that it is sensitive, convenient, and can be applied in real-time (2,3). SVV and PPV are based on the interaction between the heart and lungs under mechanical ventilation, and periodic changes in stroke volume (SV) and pulse pressure (PP) are caused by different changes in lung volume in the inspiratory phase and expiratory phase (4). However, the one-lung ventilation (OLV) condition alters the basic assumption of the SVV and PPV physiological rationale. The receiver operating characteristic (ROC) curve is usually to judge the accuracy, which is considered that the parameter is accurate if the area under the curve (AUC) is greater than 0.7. Fu et al. reported that both SVV had a poor ability (the AUC is 0.57) to predict fluid responsiveness during OLV (5). Conversely, Suehiro et al. did not find significant changes of SVV after OLV (the AUC is 0.90) and proved that both SVV and PPV have a good ability to predict fluid responsiveness during OLV (6). The debate continues as to whether SVV and PPV can accurately predict fluid responsiveness in patients during OLV. To our knowledge, one systematic review was carried out 5 years ago that investigated the diagnostic accuracy of SVV and PPV during cardiac and thoracic surgery (3). However, they did not discuss the impact of OLV on SVV and PPV, nor did they use meta-analysis to combine the parameters in the literature. More recently, many additional new studies have been published, and the body of evidence requires updating. Thus, we retrieved the relevant studies on the accuracy of SVV and PPV in OLV and performed a systematic review and meta-analysis in an attempt to reach a conclusion on this issue. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6030/rc).
Methods
This study was registered on PROSPERO (registration No. CRD 42022343057).
Data sources and searching strategy
We followed the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) guidelines on conducting meta-analyses of observational cohort studies (7). We retrieved the full texts of the relevant studies, published from 2010 to 2021, from the MEDLINE, EMBASE, WANFANG, and CENTRAL databases, and we conducted our last search on July 4, 2022. The keywords included SVV, PPV, fluid therapy, fluid responsiveness, OLV, and thoracic surgery. There was no language restriction in this search. All the references of the relevant studies were also read and reviewed to identify any additional relevant studies for inclusion in the final analysis.
Study selection and inclusion criteria
The study selection process was independently performed by 2 reviewers. The selection of studies was conducted systematically based on the prespecified PICOS (participants, interventions, comparisons, outcome, and study design) eligibility criteria: (I) participants: mechanically ventilated adult patients (≥18 years) with OLV during thoracic surgery; (II) interventions: fluid responsiveness test after OLV; (III) comparisons: parameters of patients who were responsive and non-responsive to fluid challenge; (IV) outcomes: AUCs and the threshold values of SVV and PPV; (V) study design: randomized clinical trials. Quality assessment was performed for each included study according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) document.
Studies conducted with patients with arrhythmia or spontaneous breathing were excluded, as were studies with patients who did not undergo OLV, animal experiment articles, reviews, and editorials.
Statistical analysis
The qualities of the selected studies on SVV and PPV were evaluated based on the QUADAS-2 tool before the analysis, which consists of 4 key domains (i.e., patient selection, the index test, the reference standard, and the flow of patients through the study), and the timing of the index test(s) and reference standard (8). We used Stata SE16 and MedCalc 19.0.4 for the meta-analysis. Heterogeneity was assessed using I2 statistics. The I2 results were divided using the boundary values of 25%, 50% and 75%, which represented low, medium and high heterogeneity, respectively (9). The criteria for determining heterogeneity in the tunnel diagram analysis were symmetry and a P value <0.05. Next, the pooled sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, diagnostic odds ratios (DORs), and relevant 95% confidence intervals (95% CIs) were obtained. The effect sizes were converted into standard normal metrics, and a weighted average of these transformed scores was then calculated. The AUC greater than 0.7 was considered to have high accuracy. The AUC was pooled by a weighted average of areas from individual studies, and they were combined using MedCalc 19.0.4 based on the weighted average of each study (10). The combined sensitivity, specificity and the summary ROC (SROC) curve were calculated by StataSE16, and the accuracy of the SVV and PPV was comprehensively determined by the area under the combined SROC curves (11,12).
Results
Search results and study characteristics
A total of 47 studies were identified after an initial search. After repeated screening, and the exclusion of articles that related to animal experiments, did not use OLV during the operation, did not have the full text available, did not use the fluid infusion test, or did not include a ROC curve analysis of the OLV, 9 eligible articles were ultimately included in the meta-analysis (5,6,13-19) (Figure 1).

The nine studies included a total of 452 patients, including 217 (48%) responders and 235 (52%) nonresponders to intravascular volume expansion. The operations included the radical resection of esophageal cancer and lobectomy/the segmental resection of the lung, and the methods of lung surgery included thoracotomy and thoracoscopy. The methods of OLV included double-lumen endotracheal intubation and single-lumen endotracheal intubation combined with a bronchial occlude. The tidal volume of the patients during OLV was 6–8 mL/kg, and the positive end expiratory pressure (PEEP) was 0–5 cmH2O, except for one study, in which the tidal volume was 10 mL/kg (15). SV, cardiac output (CO), cardiac index (CI) and SVV were monitored by the FloTrac-Vigileo system monitor (Edwards Lifesciences, Irvine, CA, USA). PPV was monitored by the Philips IntelliVue MP70 or MP50 system (Phillips, Suresnes, France). However, in one study, a hemosonic esophageal Doppler probe (Arrow International, Everett, Washington, USA) was used to monitor SV, CO, CI, and Adobe Photoshop CS2 (Adobe Systems Inc., San Jose, CA, USA) Software was used to calculate PPV (16). Among the nine selected studies, the accuracy of SVV was counted in eight studies (5-6,13,15-19), and the accuracy of PPV was counted in five studies (13,14,16-18). Hydroxyethyl starch (HES) was adopted for the fluid responsiveness test in each study, at a dose of 7–10 mL/kg in six studies (5,13-17), a dose of 500 mL in two studies (6,19), and a dose of 250 mL in one study (16). The standards of fluid responsiveness were as follows: Δ[stroke volume index (SVI)] was used in five studies [≥10% in one study (18), ≥15% in two studies (13,17), >25% in two studies (6,15)], ΔCI was used in three studies [≥10% in one study (11), >15% in two studies (14,19)], and ΔSV ≥10% was used in one study (16). The characteristics of the individual studies are presented in Table 1.
Table 1
| Study | Year | Sample size (lung/esophagus) | Type of operation (thoracotomy/thoracoscopy) | Tidal volume (mL/kg) | PEEP (cmH2O) | SVV | PPV | Infusion volume | Distinguish between responders and non-responders |
|---|---|---|---|---|---|---|---|---|---|
| Fu et al. (5) | 2014 | 30 (30/0) | 30/0 | 8 | 0 | Yes | No | 8 mL/kg HES | ΔCI >10% |
| Suehiro et al. (6) | 2010 | 30 (30/0) | 0/30 | 8 | 5 | Yes | No | 500 mL HES | ΔSVI >25% |
| Fu et al. (13) | 2015 | 45 (0/45) | 0/45 | 6/8 | 5/0 | Yes | Yes | 7 mL/kg HES | ΔSVI >15% |
| Lee et al. (14) | 2011 | 49 (49/0) | 8/41 | 6/10 | 5/0 | Yes | Yes | 7 mL/kg HES | ΔCI ≥15% |
| Kang et al. (15) | 2016 | 76 (76/0) | 17/59 | 7 | 0 | Yes | No | 10 mL/kg HES | ΔSVI >25% |
| Kimura et al. (16) | 2021 | 30 (19/11) | 0/30 | 6 | 5 | Yes | Yes | 250 mL, HES | ΔSV >10% |
| Choi et al. (17) | 2021 | 40 (40/0) | 0/40 | 6 | 5 | Yes | Yes | 7 mL/kg HES | ΔSVI ≥15% |
| Jeong et al. (18) | 2017 | 79 (79/0) | 39/40 | 6 | 5 | Yes | Yes | 7 mL/kg HES | ΔSVI ≥10% |
| Suehiro et al. (19) | 2011 | 73 (73/0) | 0/73 | 6/8 | 5 | Yes | No | 500 mL HES | ΔCI ≥15% |
PEEP, positive end expiratory pressure; SVV, stroke volume variation; PPV, pulse pressure variation; HES, hydroxyethyl starch; CI, cardiac index; SV, stroke volume; SVI, stroke volume index.
Quality assessment
The quality of the included studies was assessed using the QUADAS-2 tool, and the results are set out in Table 2. Two studies included patients undergoing esophageal surgery (13,16), while patients in the other studies underwent lung surgery. Eligibility criteria were sufficiently described in all selected studies. One out of 9 (11%) papers were scored as unclear risk of bias on reference standard (16), and another 1 out of 9 (11%) papers were scored as unclear risk of bias on flow and timing (15). The reference standard employed in the recognized standard in distinguishing between responders and non-responders is the increase in CI or SVI, whose variation shall be at least 10%. However, it was unclear whether the standard for fluid responsiveness used in one study was sufficiently accurate, which selected the increase in SV as the reference standard (16). The researchers reported there were an appropriate time interval between the fluid challenge test and reference standard, except for the study by Kang et al. (15). Because the measurement of cardiac performance was taken immediately after fluid challenge, there was no disease progression bias for all studies. There was no partial or differential verification bias in all studies. No studies reported uninterpretable test results and missing or incomplete data. All other papers were scored as low risk of bias.
Table 2
| Study | Risk of bias | Applicability concerns | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | ||
| Fu et al. (5) | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | |
| Suehiro et al. (6) | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | |
| Fu et al. (13) | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | |
| Lee et al. (14) | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | |
| Kang et al. (15) | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ | |
| Kimura et al. (16) | ☺ | ☺ | ? | ☺ | ☺ | ☺ | ☺ | |
| Choi et al. (17) | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | |
| Jeong et al. (18) | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | |
| Suehiro et al. (19) | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | |
☺, low risk; ?, unclear risk. QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies-2.
Synthesis
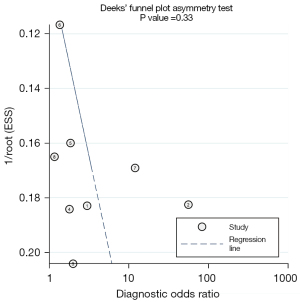
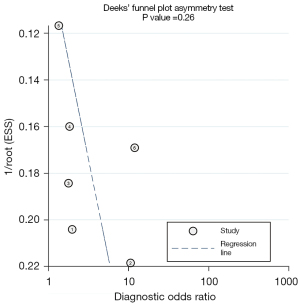
After strict screening applying the inclusion and exclusion criteria, a total of nine studies were included in the final analysis. After combining the correlation coefficients, we found that there was a slight heterogeneity between the SVV and PPV in the selected studies, and the I2 values of SVV and PPV were 19.7% (16.2–21.5%) and 15.3% (14.7–18.1%). The funnel diagram analysis also showed that the P values of SVV and PPV were 0.33 and 0.26 (Figures 2,3), and P<0.05 represented statistically significant difference. Heterogeneity and the funnel diagram analysis showed that heterogeneity, publication bias had little influence on the pooled results. Thus, the fixed-effects model was selected to combine and comprehensively analyze the effect size of each study.
The data obtained directly from the original literature or indirectly through our calculations are set out in the table, including the number of true positive results, false positive results, false negative results, true negative results, and AUCs for SVV and PPV in each study (Tables 3,4). The results of the comprehensive analysis showed that the sensitivity of SVV and PPV in predicting fluid responsiveness was 0.66 (SVV, 95% CI: 0.56–0.74) and 0.61 (PPV, 95% CI: 0.52–0.69), respectively, the specificity was 0.62 (SVV, 95% CI: 0.52–0.71) and 0.53 (PPV, 95% CI: 0.42–0.63), the positive likelihood ratios were 1.7 (SVV, 95% CI: 1.3–2.4) and 1.3 (PPV, 95% CI: 1.0–1.7), respectively, the negative likelihood ratios were 0.55 (SVV, 95% CI: 0.39–0.78) and 0.74 (PPV, 95% CI: 0.55–1.00), respectively, and the DORs were 3 (SVV, 95% CI: 2–6) and 2 (PPV, 95% CI: 1–3), respectively (Table 5, and Figures 4,5). The areas under the SROC curve of SVV and PPV were 0.68 (SVV, 95% CI: 0.64–0.72) and 0.60 (PPV, 95% CI: 0.56–0.64), respectively, according to STATA SE16 software (Figures 6,7), and the combined AUCs of SVV and PPV were 0.681 (SVV, 95% CI: 0.665–0.698) and 0.604 (PPV, 95% CI: 0.578–0.640), respectively, according to MedCalc19.0.4 software (Tables 6,7).
Table 3
| Reference | No. of fluid challenges | Sensitivity (%) | Specificity (%) | AUC-ROC (95% CI) | |||
|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | ||||
| Fu et al. (5) | 10 | 5 | 6 | 9 | 62.5 | 64.2 | 0.507 (0.294–0.720) |
| Suehiro et al. (6) | 12 | 1 | 3 | 14 | 82.4 | 92.3 | 0.900 (0.809–0.991) |
| Fu et al. (tidal volume 6 mL/kg) (13) | 8 | 6 | 4 | 6 | 66.7 | 50.0 | 0.767 |
| Fu et al. (tidal volume 8 mL/kg) (13) | 8 | 3 | 2 | 8 | 80 | 70.0 | 0.885 |
| Kang et al. (15) | 33 | 13 | 5 | 25 | 86.8 | 65.8 | 0.820 (0.724–0.915) |
| Kimura et al. (16) | 9 | 5 | 8 | 8 | 52.9 | 61.5 | 0.65 (0.45–0.81) |
| Choi et al. (17) | 10 | 10 | 7 | 13 | 58.8 | 56.5 | 0.64 (0.45–0.82) |
| Jeong et al. (18) | 15 | 22 | 14 | 28 | 51.7 | 56.0 | 0.53 (0.42–0.65) |
| Suehiro et al. (tidal volume 8 mL/kg) (19) | 18 | 5 | 3 | 10 | 85.7 | 66.7 | 0.776 (0.630–0.922) |
| Suehiro et al. (tidal volume 6 mL/kg) (19) | 10 | 11 | 7 | 9 | 69.5 | 64.3 | 0.648 (0.495–0.802) |
SVV, stroke volume variation; TP, true positive; FP, false positive; TN, true negative; FN, false negative; AUC-ROC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval.
Table 4
| Reference | No. of fluid challenges | Sensitivity (%) | Specificity (%) | AUC-ROC (95% CI) | |||
|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | ||||
| Fu et al. (tidal volume 6 mL/kg) (13) | 13 | 1 | 5 | 5 | 72.2 | 83.3 | 0.778 |
| Fu et al. (tidal volume 8 mL/kg) (13) | 9 | 2 | 1 | 9 | 90.0 | 81.8 | 0.890 |
| Lee et al. (tidal volume 6 mL/kg) (14) | 11 | 2 | 2 | 10 | 84.6 | 83.3 | 0.857 (0.712–1.003) |
| Lee et al. (tidal volume 10 mL/kg) (14) | 7 | 6 | 5 | 6 | 58.3 | 50.0 | 0.524 (0.283–0.766) |
| Kimura et al. (16) | 12 | 2 | 12 | 4 | 50.0 | 66.7 | 0.608 (0.37–0.74) |
| Choi et al. (17) | 10 | 10 | 7 | 13 | 58.8 | 56.5 | 0.630 (0.47–0.83) |
| Jeong et al. (18) | 18 | 20 | 11 | 30 | 62.1 | 60.0 | 0.550 (0.52–0.74) |
PPV, pulse pressure variation; TP, true positive; FP, false positive; TN, true negative; FN, false negative; AUC-ROC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval.
Table 5
| Parameter | SVV estimate (95% CI) | PPV estimate (95% CI) |
|---|---|---|
| Sensitivity | 0.66 (0.56 to 0.74) | 0.61 (0.52 to 0.69) |
| Specificity | 0.62 (0.52 to 0.71) | 0.53 (0.42 to 0.63) |
| Positive likelihood ratio | 1.7 (1.3 to 2.4) | 1.3 (1.0 to 1.7) |
| Negative likelihood ratio | 0.55 (0.39 to 0.78) | 0.74 (0.55 to 1.00) |
| Diagnostic odds ratio | 3 (2 to 6) | 2 (1 to 3) |
SVV, stroke volume variation; PPV, pulse pressure variation; 95% CI, 95% confidence interval.




Table 6
| Study | ROC area | 95% CI | Weight (%) |
|---|---|---|---|
| Fu et al. (5) | 0.507 | 0.448 to 0.566 | 7.62 |
| Suehiro et al. (6) | 0.900 | 0.841 to 0.959 | 7.62 |
| Fu et al. (tidal volume of 6 mL/kg) (13) | 0.767 | 0.694 to 0.840 | 5.01 |
| Fu et al. (tidal volume of 8 mL/kg) (13) | 0.885 | 0.816 to 0.954 | 5.59 |
| Kang et al. (15) | 0.820 | 0.781 to 0.859 | 17.13 |
| Kimura et al. (16) | 0.650 | 0.591 to 0.709 | 7.62 |
| Choi et al. (17) | 0.640 | 0.601 to 0.679 | 17.13 |
| Jeong et al. (18) | 0.530 | 0.497 to 0.563 | 23.72 |
| Suehiro et al. (tidal volume 8 mL/kg) (19) | 0.776 | 0.698 to 0.854 | 4.28 |
| Suehiro et al. (tidal volume 6 mL/kg) (19) | 0.648 | 0.570 to 0.726 | 4.28 |
| Total (fixed-effects) | 0.681 | 0.665 to 0.698 | 100.00 |
AUC, area under the curve; ROC, receiver operating characteristic; 95% CI, 95% confidence interval; SVV, stroke volume variation.
Table 7
| Study | ROC area | 95% CI | Weight (%) |
|---|---|---|---|
| Fu et al. (tidal volume of 6 mL/kg) (13) | 0.778 | 0.705 to 0.851 | 4.59 |
| Fu et al. (tidal volume of 8 mL/kg) (13) | 0.890 | 0.821 to 0.959 | 5.12 |
| Lee et al. (tidal volume 6 mL/kg) (14) | 0.857 | 0.753 to 0.961 | 2.23 |
| Lee et al. (tidal volume 10 mL/kg) (14) | 0.524 | 0.428 to 0.620 | 2.61 |
| Kimura et al. (16) | 0.608 | 0.549 to 0.667 | 6.97 |
| Choi et al. (17) | 0.650 | 0.611 to 0.689 | 15.69 |
| Jeong et al. (18) | 0.630 | 0.610 to 0.650 | 62.77 |
| Total (fixed-effects) | 0.604 | 0.578 to 0.640 | 100.00 |
AUC, area under the curve; ROC, receiver operating characteristic; 95% CI, 95% confidence interval; PPV, pulse pressure variation.
Discussion
SVV and PPV are common, dynamic hemodynamic monitoring indicators used to predict fluid responsiveness in patients undergoing mechanical ventilation during general anesthesia and usually have good sensitivity and specificity (20). However, in some special cases, such as OLV in thoracic surgery, the accuracy of SVV and PPV is still controversial. Some studies have reported that the destruction of the integrity of the pleural cavity on the side of thoracotomy or video-assisted thoracoscopic surgery was not enough to affect the periodic changes in the venous return, which leads to the reduction and false negative results of SVV (5,16-18) and PPV (14,16-18).
This is the first meta-analysis in which researchers tested the predictive response of SVV in patients undergoing OLV during thoracic surgery. We examined nine studies comprising a total of 452 patients. Our meta-analysis results showed that the combined sensitivity, combined specificity, and area under the SROC curve of SVV were 0.66, 0.62, and 0.68, respectively, and the combined sensitivity, combined specificity, and area under the SROC curve of PPV were 0.61, 0.53, and 0.60, respectively. Additionally, neither SVV nor PPV had good diagnostic accuracy and neither were suitable for guiding intraoperative fluid therapy due to their poor prediction of fluid responsiveness in patients undergoing OLV.
Some of the included studies reached contradictory conclusions. However, the following reasons may account for this: (I) insufficient sample sizes (the sample size of some studies was only 30, which may have affected the judgment of results); (II) different methods and judgment standards of liquid reaction tests may have led to conclusion errors; and (III) differences in surgical types and procedures. For example, esophageal surgery requires more enhancement in the field of visual surgery than pulmonary surgery. Different degrees of lung traction by different operators during surgery may lead to variations in the detected parameter values, which may have affected the accuracy of the results.
SVV and PPV are dependent on cardiopulmonary interactions, which are periodic changes in venous return and trans pulmonary pressure caused by mechanical ventilation (21). They are important volume indexes in the GDHT and are also important components of enhanced recovery after surgery. However, the accuracy of SVV and PPV in predicting fluid responsiveness is affected by many factors, such as tidal volume, respiratory rate, arrhythmia, pleural integrity, and cardiac function during mechanical ventilation (22).
During OLV, the patients are usually placed in the lateral position, and changes in body position have been shown to have no statistically significant effect on the monitoring results of SVV (23). Additionally, research has found no statistically significant difference in the effects of left and right lateral positions on circulatory function and blood volume status (24). Low PEEP is often used in lung protection ventilation strategies, and relevant studies have also shown that low PEEP will not affect the accuracy of SVV (25).
During OLV, the integrity of the pleural cavity and the periodicity of pulmonary respiration on the operative side are destroyed, regardless of whether a thoracotomy or thoracoscopic surgery were performed (26). The following questions arise: (I) does the periodicity of intrapleural pressure caused by mechanical ventilation that relies only on the positive pressure of the other side change the venous return blood volume and lead to a change in SV? (II) Does the accuracy of SVV and PPV be affected during OLV? According to our meta-analysis results, the accuracy of SVV and PPV were greatly affected by OLV.
First, low tidal volume (6–8 mL/kg) is widely known to be a major factor that may lead to prediction failure. Many studies have shown that the sensitivity and specificity of SVV and PPV in predicting fluid responsiveness are related to tidal volume, and their accuracy is greatly reduced when tidal volume is <8 mL/kg (27). However, during OLV, the tidal volume is usually 6–8 mL/kg. Excessive tidal volume will increase airway pressure and cause lung injury, and also limit surgical vision (28). If the tidal volume is too small, the trans-pulmonary pressure will also decrease, and its periodic changes will not be enough to cause changes in the blood flow of the vena cava, pulmonary artery, and aorta. The Frank-Starling curve moves to the left. Thus, patients occupying the flat part of the Frank-Starling curve may theoretically occupy the steep part of the curve during low tidal volume ventilation, which will result in decreased accuracy (29).
Second, the increase in the intrapulmonary shunt rate (Qs/Qt) during OLV leads to an increase in intrapulmonary shunts due to the doping of intrapulmonary vein blood on the non-ventilated side. At this time, the airway pressure increases significantly, which increases the vascular resistance. Part of the blood flow will transfer to the collapsed opposite lung, which easily results in hypoxia. Hypoxic pulmonary vasoconstriction (HPV) during OLV is considered an important mechanism for preventing hypoxemia, but HPV and autonomic regulation are weakened due to the influence of anesthesia methods, drugs and other factors during surgery. Studies have shown that the intrapulmonary shunt rate and hypoxic vasoconstriction may be important factors affecting the accuracy of CI, which suggests that they may affect the accuracy of dynamic hemodynamic indicators, such as SVV and PPV, but the specific mechanism remains unclear (30,31).
Third, dynamic indicators distinguish the steep part of the Frank-Starling curve according to the periodic change in cardiopulmonary interaction, which not only depends on the change in venous return but also on the change in right ventricular afterload and left ventricular transmural pressure. OLV will cause an increase in intrapulmonary shunt and dead space ventilation due to the collapse of the affected side and atelectasis of the healthy side, resulting in hypercapnia. Hypercapnia defined as the arterial carbon dioxide pressure (PaCO2) >48 mmHg is an independent predictor of right ventricular dysfunction in patients with chronic obstructive pulmonary disease receiving low tidal volume mechanical ventilation, which can cause pulmonary vasoconstriction, increase the afterload of the right heart, decrease the function of the right heart, and affect the accuracy of SVV and PPV (31,32).
Limitations and strengths
Our meta-analysis had some limitations. First, as heterogeneity was found in the whole data set, the conclusions should be interpreted carefully. Second, the effects of vasoactive drugs on SVV and PPV could not be discussed due to a lack of relevant data. Third, as the types of surgery included mainly involved the lung and esophagus, our conclusions are not applicable to all patients undergoing OLV surgery. In the future, multicenter and large sample research needs to be conducted.
Our meta-analysis also had several strengths. First, this is the first diagnostic meta-analysis in which the reliability of SVV and PPV was studied in terms of predicting fluid responsiveness in patients under OLV. Second, the quality of the included articles was good. Third, we used two different software programs to compare the combined ROC curves of SVV and PPV, and the results of the two software analyses were consistent, which increases the reliability of our results.
Conclusions
This was the first meta-analysis to examine the accuracy of SVV and PPV in terms of predicting fluid responsiveness in patients undergoing OLV. Our results indicate that SVV and PPV are not suitable for guiding intraoperative fluid therapy due to their poor prediction of fluid responsiveness in patients undergoing OLV, and we need a better indicator instead.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6030/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6030/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- French WB, Scott M. Fluid and Hemodynamics. Anesthesiol Clin 2022;40:59-71. [Crossref] [PubMed]
- Argueta E, Berdine G, Pena C, et al. FloTrac® monitoring system: what are its uses in critically ill medical patients? Am J Med Sci 2015;349:352-6. [Crossref] [PubMed]
- Piccioni F, Bernasconi F, Tramontano GTA, et al. A systematic review of pulse pressure variation and stroke volume variation to predict fluid responsiveness during cardiac and thoracic surgery. J Clin Monit Comput 2017;31:677-84. [Crossref] [PubMed]
- Pinsky MR. Functional haemodynamic monitoring. Curr Opin Crit Care 2014;20:288-93. [Crossref] [PubMed]
- Fu Q, Zhao F, Mi W, et al. Stroke volume variation fail to predict fluid responsiveness in patients undergoing pulmonary lobectomy with one-lung ventilation using thoracotomy. Biosci Trends 2014;8:59-63. [Crossref] [PubMed]
- Suehiro K, Okutani R. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing one-lung ventilation. J Cardiothorac Vasc Anesth 2010;24:772-5. [Crossref] [PubMed]
- McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Menten J, Lesaffre E. A general framework for comparative Bayesian meta-analysis of diagnostic studies. BMC Med Res Methodol 2015;15:70. [Crossref] [PubMed]
- McClish DK. Combining and comparing area estimates across studies or strata. Med Decis Making 1992;12:274-9. [Crossref] [PubMed]
- Harbord RM, Whiting P. Metandi: meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009;9:211-29. [Crossref]
- Xu H, Qian J, Paynter NP, et al. Estimating the receiver operating characteristic curve in matched case control studies. Stat Med 2019;38:437-51. [Crossref] [PubMed]
- Fu Q, Duan M, Zhao F, et al. Evaluation of stroke volume variation and pulse pressure variation as predictors of fluid responsiveness in patients undergoing protective one-lung ventilation. Drug Discov Ther 2015;9:296-302. [Crossref] [PubMed]
- Lee JH, Jeon Y, Bahk JH, et al. Pulse-pressure variation predicts fluid responsiveness during heart displacement for off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2011;25:1056-62. [Crossref] [PubMed]
- Kang WS, Oh CS, Park C, et al. Diagnosis Accuracy of Mean Arterial Pressure Variation during a Lung Recruitment Maneuver to Predict Fluid Responsiveness in Thoracic Surgery with One-Lung Ventilation. Biomed Res Int 2016;2016:3623710. [Crossref] [PubMed]
- Kimura A, Suehiro K, Juri T, et al. Hemodynamic Changes via the Lung Recruitment Maneuver Can Predict Fluid Responsiveness in Stroke Volume and Arterial Pressure During One-Lung Ventilation. Anesth Analg 2021;133:44-52. [Crossref] [PubMed]
- Choi KH, Shim JK, Kim DW, et al. Dynamic Indices Fail to Predict Fluid Responsiveness in Patients Undergoing One-Lung Ventilation for Thoracoscopic Surgery. J Clin Med 2021;10:2335. [Crossref] [PubMed]
- Jeong DM, Ahn HJ, Park HW, et al. Stroke Volume Variation and Pulse Pressure Variation Are Not Useful for Predicting Fluid Responsiveness in Thoracic Surgery. Anesth Analg 2017;125:1158-65. [Crossref] [PubMed]
- Suehiro K, Okutani R. Influence of tidal volume for stroke volume variation to predict fluid responsiveness in patients undergoing one-lung ventilation. J Anesth 2011;25:777-80. [Crossref] [PubMed]
- Rathore A, Singh S, Lamsal R, et al. Validity of Pulse Pressure Variation (PPV) Compared with Stroke Volume Variation (SVV) in Predicting Fluid Responsiveness. Turk J Anaesthesiol Reanim 2017;45:210-7. [Crossref] [PubMed]
- Chen Y, Guo X, Fu J, et al. Accuracy of stroke volume variation and pulse pressure variation to predict fluid responsiveness in patients with thoracic kyphosis. Ann Palliat Med 2021;10:7571-8. [Crossref] [PubMed]
- Bennett VA, Aya HD, Cecconi M. Evaluation of cardiac function using heart-lung interactions. Ann Transl Med 2018;6:356. [Crossref] [PubMed]
- Daihua Y, Wei C, Xude S, et al. The effect of body position changes on stroke volume variation in 66 mechanically ventilated patients with sepsis. J Crit Care 2012;27:416.e7-12. [Crossref] [PubMed]
- Xiang SW, Wang L, Wang T, et al. The effect of lateral decubitus position on the abilities of stroke volume variation to predict fluid responsiveness. Journal of Clinical Anesthesiology 2014;3:873-76.
- Chen Y, Fu Q, Mi WD. Effects of stroke volume variation, pulse pressure variation, and pleth variability index in predicting fluid responsiveness during different positive end expiratory pressure in prone position. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2015;37:179-84. [PubMed]
- Jun IJ, Chung MH, Kim JE, et al. The influence of positive end-expiratory pressure (PEEP) in predicting fluid responsiveness in patients undergoing one-lung ventilation. Int J Med Sci 2021;18:2589-98. [Crossref] [PubMed]
- Renner J, Cavus E, Meybohm P, et al. Stroke volume variation during hemorrhage and after fluid loading: impact of different tidal volumes. Acta Anaesthesiol Scand 2007;51:538-44. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- Bernasconi F, Piccioni F. One-lung ventilation for thoracic surgery: current perspectives. Tumori 2017;103:495-503. [Crossref] [PubMed]
- Sentürk M. New concepts of the management of one-lung ventilation. Curr Opin Anaesthesiol 2006;19:1-4. [Crossref] [PubMed]
- Lesser T, Wolfram F, Braun C, et al. Effects of one-lung flooding on porcine haemodynamics and gas exchange. Int J Med Sci 2020;17:3165-73. [Crossref] [PubMed]
- Berlin DA, Bakker J. Starling curves and central venous pressure. Crit Care 2015;19:55. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)