
Mechanism of Aspirin oxidative stress regulating interleukin-induced apoptosis in nucleus pulposus cells in a rat model of intervertebral disc degeneration
Highlight box
Key findings
• Increase of reactive oxygen species (ROS), overexpression of inflammatory factors, and loss of extracellular matrix are important factors in the pathological changes of IDD.
What is known and what is new?
• Intervertebral disc degeneration (IDD) is an important cause of low back pain.
• LPS can be used as a drug to alleviate oxidative stress and intervene in the IDD process.
What is the implication, and what should change now?
• Our results clarified the important role of oxidative stress in IDD and proved that LPS can be used as a drug to alleviate oxidative stress and intervene in the IDD process.
Introduction
With the increasing incidence of intervertebral disc degeneration (IDD), low back pain will become impossible to ignore, and result in a serious impact on the physiology and psychology of patients as well as the broader economy (1). According to research, more than 50% of motor dysfunctions are caused by degenerative disc disease. At present, the relevant clinical therapeutic interventions mainly include surgical and non-surgical conservative treatments, which primarily aim to achieve the relief of clinical symptoms rather than to alleviate the progression of IDD (2). In addition, surgical treatment inevitably involves a certain degree of trauma, and thus, how to effectively prevent and treat the occurrence and development of IDD has become a research hotspot among orthopedic surgeons and other related researchers (3-5).
Structurally, the intervertebral disc is mainly composed of the superior vertebral endplate with a lower vertebral endplate composed of the following: a concentric arrangement of dense fibrous ring and a center filled with half cellophane tape frozen samples of nucleus pulposus tissue. Functionally, the upper and lower endplates can provide nutrient exchange; the dense annulus fibrosus can effectively resist the horizontal stretching force generated by vertebral body movement; and the jelly-like nucleus pulposus tissue, which is rich in water, can absorb the vertical compression force generated by the movement of the vertebral body (6).
At present, the specific mechanism of IDD is unclear, but most scholars believe that the nucleus pulposus is the main functional component of the intervertebral disc, and pathological changes in the nucleus pulposus may be an important inducing or promoting factor of IDD (7). Due to the special anatomical structure of intervertebral discs, NPCs are in a relatively low oxygen physiological state for prolonged periods. Cell energy metabolism disorder can occur easily under the influence of various pathological factors, such as mechanical instability, inflammation, and lack of nutrition (8). Thus, cytotoxic reactive oxygen species (ROS), oxygen free radicals, and other products can be produced to affect the normal physiological function of the nucleus pulposus and induce the release of inflammatory factors, such as tumor necrosis factor-a (TNF-a). Interleukin (IL)-1β and IL-6 will eventually lead to the degradation of the extracellular matrix, which is dominated by collagen-II (Col-2) and polyglycoprotein (Aggrecan), in the nucleus pulposus, resulting in the degeneration of the nucleus pulposus (9).
Therefore, effectively preventing and reducing oxidative stress in the disc nucleus pulposus is a new strategy to alleviate and treat IDD (10). Acetylsalicylic acid (Aspirin), as a classical acetylsalicylic acid, belongs to a class of drugs that have been widely used in clinical applications since the 1890s for the treatment of various inflammatory diseases. In clinical practice, they can effectively inhibit platelet aggregation and reduce the risk of cardiovascular disease. Anti-inflammatory drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs), are also used to treat rheumatic diseases and osteoarthritis. Recent studies have shown that these medications can alleviate the excessive expression of ROS by microglia in an abnormal energy metabolism pathological state. Adenosine monophosphate (AMP) -activated protein kinase (AMPK) is an important regulator of cellular energy; AMPK activation helps to correct energy metabolism disorders. In response to cellular stress, AMPK can inhibit the expression of ROS by inducing endogenous antioxidant substances (11-13). Considering that ROS can directly participate in inflammation, autophagy, apoptosis, and other pathophysiological processes, AMPK activation may help to alleviate the above pathological processes. Aspirin can directly reduce ROS expression in the nucleus pulposus cells by activating AMPK. However, it is not clear whether it exerts a protective anti-oxidation function and alleviates degeneration, which would reduce the oxidative stress and lessen the occurrence and development of IDD (14).
Therefore, the present study used a nucleus pulposus cell (NPC) oxidative stress model and a rat caudal disc degeneration model to verify whether they can diminish the oxidative stress of NPCs and reduce the expression of ROS and other substances to abate nucleus pulposus degeneration and ultimately hinder the progression of IDD. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5700/rc).
Methods
Extraction of nucleus pulposus cells
- Five male Sprague Dawley rats were randomly selected and euthanized by excessive pentobarbital sodium. Their tail hairs were then shaved and sterilized repeatedly with 75% medical alcohol. Animal experiments were performed under a project license (No. 202217865) granted by ethics board of Changchun University of Chinese Medicine, in compliance with Guide for the Care and Use of laboratory Animals, 8th edition. A protocol was prepared before the study without registration.
- The following operations were carried out on the sterilization cell table. The tissues were rinsed repeatedly with 37 ℃ Phosphate Buffered Saline (PBS), and the epidermis and muscle were removed sequentially and washed with 37 ℃ PBS solution.
- The intervertebral space was dissected close to the cartilage endplate of the upper vertebral body under a stereomic microscope, and the boundary between the nucleus pulposus tissue and the annulus fibrosus was carefully observed. The nucleus pulposus tissue in the center was carefully removed and immediately place into F12 complete medium at 37 ℃. The nucleus pulposus 7–8, 8–9, and 9–10 of the caudal vertebra were successively removed and finally collected into a 15 mL sterile centrifuge tube.
- Centrifugation was performed at 1×103 rpm for 3 min, and the bottom tissue was digested with 0.25% trypsin for 3 min, followed by centrifugation at 1×103 rpm for 3 min, and then digested with 0.5% collagenase type II (containing 1% Fetal Bovine Serum, FBS) at 37 ℃ for 1 h.
- Centrifugation was then performed at 1×103 rpm for 3 min, followed by the addition of F12 (containing 15% FBS) complete medium at 37 ℃, which was blown evenly and then transferred to a Petri dish for culturing.
Cell culture
- The cells were incubated at 37 ℃ and 5% carbon dioxide (CO2) in an incubator under constant temperature and humidity.
- When the cell density was about 80%, the culture medium was removed, washed three times with PBS at 37 ℃, and digested in the incubator for 3 min with 1 mL trypsin (0.25%).
- 2 mL of preheated F12 medium was dropped to blow the cells and stop digestion, followed by centrifugation at 1×103 rpm for 3 min.
- The supernatant was then discarded, and the cells were blown and resuspended in 1 mL preheated F12 medium.
- P2–P5 NPCs were used for the experiment, and the density of the cell seed plate was selected according to the experimental requirements.
Detection of the light-burning probe
NPCs were planted in 24-well plates in advance, and there were two plates of cells with a density of 1×104 wells. The NPCs in each group were successively treated with different concentrations of LPS and then washed three times with PBS at 37 ℃. The serum-free F12 medium was selected to prepare DCFH-DA at a ratio of 1:1,000, and 1 mL of DCFH-DA probe reagent was added to the samples in each group, which were then incubated at 37 ℃ for 20 min under a light.
Reverse transcription-polymerase chain reaction (RT-PCR)
The NPCs were seeded in six-well plates based on a cell density of 2×105 wells. Following successive treatment of the corresponding groups with different concentrations of LPS, the cells were lysed with Trizol, and the RNA was extracted. RT-PCR was used to assay Col-2, Aggrecan, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX2), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression levels, respectively, with GAPDH as an internal reference.
- The corresponding primer sequences were obtained from Jin Weizhi Biotechnology Co., LTD. (Suzhou, China) according to the relevant literature. The primers in the experiment were used at a concentration of 10 µM.
- Total RNA extraction:
- Step 1: the NPCs were lysed, the supernatant was discarded, and the cells were washed with PBS three times. Next, 1 mL of Trizol was added to each well and lysed for 10 min, and then blown repeatedly until the bottom of the well plate was clean. Thereafter, the cells were moved to the corresponding labeled 1.5 mL Eppendorf Tubes (EP) tube and kept at room temperature for 10 min.
- Step 2: for RNA extraction, 0.2 mL of chloroform was added to the EP tube of each group using a dropper, which was then shaken was 30 seconds. It was then allowed to stand for 10 min in the fume hood and 10 min on ice. Centrifugation was subsequently performed at 1.2×103 rpm for 10 min at 4 ℃. The supernatant was then discarded and 1 mL of precooled 75% ethanol was added to each EP tube.
- Step 3: to determine the RNA concentration, the absorbance values of RNA at 260 nm and 280 nm were detected by NanoDrop2000 using a 1 µL RNA solution.
- Step 4: for calculation, a ratio between 260 nm and 280 nm of each group of samples of between 1.8 and 2.0 was considered to indicate that the sample RNA purity was high; a ratio <1.8 indicated protein contamination; and a ratio >2.0 denoted DNA contamination.
- Step 5: reverse transcription was applied to obtain cDNA. The specific parameters were 42 ℃, 60 min, 70 ℃, 15 min, and rapid ice precooling to obtain the cDNA samples.
Immunofluorescence staining
NPCs were seeded into 24-well plates at a density of 2×104 wells, and each group was successively treated with LPS.
- The medium in each well was absorbed and washed with PBS three times. 0.5 mL of 4% paraformaldehyde was added to each well and fixed on ice at 4 ℃ for 20 min.
- Next, this was washed with PBS three times (5 min each time), and 0.5 mL of TritonX-100 was added to each well and drilled for 10 min at room temperature.
- Subsequently, this was washed with PBS three times (5 min each time), and 0.5 mL of Quicktime block blocking solution was added to each well for 1 h at room temperature.
- The primary antibody was recovered and washed with PBS three times (3 min each time).
- Next, a diluent of secondary antibody was used to avoid light as required, 0.5 mL of secondary antibody was added to each well, and the cells were completely covered and incubated for 1 h with a shaking table at room temperature under a light.
- The secondary antibody was then absorbed and washed with PBS three times (3 min each time).
- 0.5 mL of 4',6-Diamidino-2'-phenylindole staining solution was added to each well and incubated for 15 min in the dark.
- DAPI was then absorbed, washed with PBS three times (3 min each time), and the samples were observed under an inverted fluorescence microscope (EVOS, America).
Establishment of the IDD model in rats
All animals included in the experiment were purchased from the Laboratory Animal Center of Changchun University of Chinese Medicine. The animals were fasted for 12 h and deprived of water for 4 hours before the operation and anesthetized by intraperitoneal injection of 10% chloral hydrate (3.5 mL/kg). According to the experimental basis of our laboratory, the 7–8 caudal vertebrae (Co7–8), 8–9 caudal vertebrae (Co8–9), and 9–10 caudal vertebrae (Co9–10) of the rats were located and marked before the procedure. A 21G needle was vertically inserted into the intervertebral space of Co8–9 and Co9–10 and penetrated through. The needle was rotated for 5 s, held in place for 30 s, and then pulled out, and subsequently placed in the cage upon completion of the operation.
Method and dosage of experimental drug in rats
After modeling, 40 SD rats were randomly divided into high-dose and low-dose groups (n=20/group) to receive Aspirin local injection within the interdisk on the day of modeling (Aspirin concentration 100× μg/mL in the high-dose group and Aspirin in the low-dose group with 10 pg/mL). To avoid individual differences, we selected C07-8 as the normal group, C08 and 9 as the treatment group (Subject to local Aspirin injection after modeling), and C09 and 10 as the regressive group (Subject to the same amount of PBS injection after modeling).
Sampling and preparation of intervertebral disc specimens from rats
After imaging examination, the rats were sacrificed under excessive anesthesia (10% chloral hydrate 10 mL/kg). The rat tails were cut off from Co6–7 and Co10–11 successively to obtain the experimental specimens. The skin was then removed, leaving the disc, vertebra, and attached muscles and ligaments intact. The specimens were fixed with 10% formalin for 48 h, rinsed repeatedly with running water for 12 h, and then immersed in 10% neutral Ethylene Diamine Tetraacetic Acid (EDTA) for decalcification. The decalcification solution was changed once every 2 days, and the decalcification was stopped when the needle specimen exhibited no obvious resistance.
Statistical analysis
The data were analyzed using SPSS 25.0 statistical software (America). The one-way ANOVA test was used for multiple group comparison, and LSD and Dunnett’s t method were used for pairwise comparison under the condition of homogeneity of the population variance. *P<0.05 indicated statistical significance.
Results
Effect of LPS on the proliferation of NPCs
To detect whether LPS affected the proliferation of NPCs, this experiment adopted the Cell Counting Kit-8 (CCK-8) experimental method for cell proliferation toxicity tests. The NPCs used in the experiment exhibited spindle growth morphology under the inverted phase-contrast microscope (Figure 1A). The cell proliferation toxicity assay results showed that cell proliferation gradually accelerated with the increase of time (Figure 1B). Compared with the control group, an LPS concentration lower than 10 µg/mL had no adverse effects on the proliferation of NPCs. At concentrations of 10 or 100 µg/mL, the proliferation of NPCs was significantly inhibited compared with the control group, but still showed a trend of proliferation with time (Figure 2).
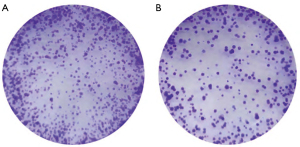

LPS induced oxidative stress in NPCs
Following the above experiments, we determined the reasonable concentration range of LPS. NPCs were treated with different concentrations of LPS (0, 0.01, 0.1, 1 µg/mL) in two 24-well plates. After 24 h of LPS treatment, the ROS were labeled with a fluorescent probe. NPCs in the first 24-well plate were observed under an inverted fluorescence microscope.
When the concentration of LPS increased, the labeled fluorescence intensity and the number of labeled cells showed an increasing trend. The fluorescence intensity of NPCs in the second 24-well plate was quantified by flow cytometry, and we found that the fluorescence intensity increased with the increasing LPS concentration. Subsequently, NPCs were treated with 1 µg/mL of LPS at different times (0, 0.25, 0.5, 1, 3, 6, 12, and 24 h), and the intensity of oxidative stress was assessed and quantified by flow cytometry. The results showed that LPS-induced oxidative stress exhibited a significant time-dependent effect and reached a maximum value at 12 h (Figure 3).

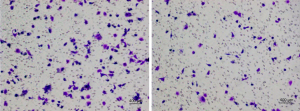
Effects of Aspirin on NPC proliferation
The CCK-8 reagent was used to detect the proliferative toxicity of NPCs to eliminate the Aspirin effect on cell proliferation. The results showed that cell proliferation accelerated gradually with the increase of time. Compared with the control group, an Aspirin concentration ≤100 µg/mL significantly inhibited cell proliferation. Notably, although the Aspirin concentration was at 5 µg/mL, the optical density (OD) value increased compared with other Aspirin concentrations (P<0.05) (Figure 4).
Aspirin alleviated LPS-induced oxidative stress
We selected high-dose and low-dose Aspirin (5 µg/mL, 25 µg/mL) to observe its therapeutic effect. After 2 h of Aspirin, 1 µg/mL LPS was added for 24 h. The first group of NPCs was observed under a microscope. Compared with the control group, the number of marker cells increased significantly after LPS treatment. However, a decrease in the number of cell marker cells was observed in all Aspirin treatment groups, and this effect was more pronounced in the high-dose group than in the low-dose group (Figure 5).
Imaging findings (MRI)
In groups A and B, the lumbar spine was arranged in an orderly manner, and there was no abnormal change in height. The color of the intervertebral disc was normal, and no obvious gray was observed. The intervertebral disc showed hyperintensity in the T2 signal on MRI, and no decreased intervertebral disc signal was observed. The intervertebral disc signal values of the four groups decreased progressively with time, and the T2 signal values of group D decreased most significantly. Also, the degree of intervertebral disc degeneration was the most severe, with statistically significant differences (P<0.05) (Figure 6).

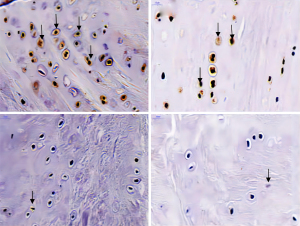
Immunohistochemical results
The expression of the hypoxia-inducible factor-1α (HIF-1α) protein in groups C and D was markedly lower than that in group A, and the mean number of positive cells per high-power field and the number of brown positive staining cells was significantly lower (P<0.05). Compared with group C, the mean number of positive cells per high-power field in group D was substantially lower (P<0.05). However, there was no significant difference between groups B and A (P>0.05) (Figure 7).
Aspirin can ameliorate the degeneration of NPCs
RT-PCR results showed that LPS also promoted the expression of MMP-13 gene and upstream genes of the MMPs family (P<0.05), while Aspirin treatment group could decrease the expression of these genes (Figure 8).
Discussion
Oxidative stress is one of the most important pathological changes in the body (15). The occurrence of oxidative stress is often accompanied by inflammatory infiltration of immune cells and the production of a large number of oxidative products, which affect the normal physiological function of cells and tissues. Existing studies have shown that oxidative stress can cause diabetes, cardiovascular disease, osteoarthritis, rheumatoid arthritis, and other diseases (16-18). Therefore, improving and relieving oxidative stress in cells or tissues is a key condition of reducing the incidence of related diseases. Due to the special anatomical structure of intervertebral discs, the nucleus pulposus is under hypoxic physiological conditions for prolonged periods (19). Compared with other human tissues, the nucleus pulposus tissue is more prone to oxidative stress when the physiological environment changes. In this study, LPS was applied to establish an NPC oxidative stress model. The effect of oxidative stress on NPCs was evaluated by observing the expressions of ROS and related inflammatory factors (20).
IDD is one of the main causes of clinical low back pain (21). At present, conservative treatment mainly involves conventional physical therapy and oral drugs to relieve symptoms. However, due to the special anatomical structure of the intervertebral disc, the effect of physical therapy has certain limitations. Also, oral drugs are often accompanied by inevitable side effects, and there is a lack of relevant drugs for etiological treatment (22). With the aging of the body and the progression of tissue degeneration, most patients will eventually choose surgical treatment to relieve the clinical symptoms caused by IDD, such as neuralgia (23). At present, there is a lack of effective early intervention measures for IDD in clinical practice, and thus, it is necessary to further explore the causes of IDD.
The specific causative factors of IDD are still not fully understood; however, a large number of studies have shown that aging, mechanical stress changes, inflammation, and other pathological factors (such as smoking, trauma, and personal genetic factors) can induce disc degeneration. With the progression of IDD, NPCs undergo degeneration or death, and a large number of extracellular matrix components, such as Col-2 and Aggrecan, are lost (24-26). At the same time, the high expression of MMPs in the metalloproteinase family will further aggravate the degradation of extracellular matrix and eventually lead to IDD. It is worth noting that oxidative stress, a pathological change, plays an important role in the above process of degeneration. Owing to its organizational structure, the intervertebral disc is in a relatively low oxygen state and lacks adequate blood supply; thus, the NPCs cannot directly obtain nutrients through the capillaries, and rather must rely on infiltration into the upper and lower vertebral cartilage endplates for the exchange of nutrients and waste products. A previous study has shown that NPCs are in a physiological environment, hypoxia, and an acidic state, in which pathological changes and oxidative stress responses are easily induced (27).
Oxidative stress is often accompanied by the expression of active oxygen excess material and antioxidant system dysfunction, which eventually result in pathological changes. ROS are mostly composed of bioactive components such as superoxide anion, hydrogen peroxide, and hydroxyl radicals (28); they are mainly produced by cell metabolism and participate in various physiological functions such as cell self-defense and signal transduction. However, in the case of pathological overexpression, ROS react with intracellular proteins, lipids, and nucleic acids and affect the normal physiological function of cells and tissues. In our experiment, we used LPS to induce oxidative stress in NPCs based on the relevant literature and previous research. In the experiment, we detected the expression of a large number of ROS, indicating that the NPC oxidative stress model was successfully established. At the same time, we demonstrated in our experiments that with the occurrence of oxidative stress, the expression of the antioxidant Nrf-2 protein in cells decreased, while those of iNOS and COX2 increased, indicating that the balance between oxidation and antioxidant in the cells was broken at this time. This subsequently led to the loss of Col-2 and other components, and finally resulted in the degeneration of NPCs. However, the loss of extracellular matrix components will cause the entire nucleus pulposus to lose water as well as the function of absorbing stress and “shock” during bodily movement (29-31).
IDD is a pathological change caused by multiple factors, and its pathogenesis is still unknown (32). During the process of IDD, pathological changes such as the degeneration and death of NPCs, degradation of extracellular matrix, and destruction of the peripheral annulus fibrosus can be observed. In this part of the experiment, we observed that Aspirin could not only effectively reduce oxidative stress but also alleviate the expression of inflammatory factors. An existing study has demonstrated that inflammation is an important cause of IDD, especially the expressions of the interleukin family and TNF-a, which are closely related to the degeneration of NPCs (33). IL-1β accelerates the degradation of extracellular matrix by increasing the biological activities of MMPs and polysaccharide proteases. In addition, there is a close interaction between inflammatory factors and oxidative stress, which mediate each other in the pathological process, thereby aggravating the pathological changes of cells and tissues. It is worth noting that NO, as an active substance that is produced downstream of NOS mediation, is an important inflammatory regulator in osteoarthritis. In our experiment, a positive correlation between iNOS and NO was observed. A previous study has shown that reducing NO production by inhibiting iNOS can significantly reduce the production of MMPs and IL-1β (34).
Additionally, in this part of the experiment, we added HIF-1α indicators to observe the role of Aspirin in relieving NPC degeneration. HIF-1α is a type of interstitial lysin that acts mainly on the core proteins of polyglycoproteins (elastic fibers, fibronectin, laminin, etc.). Metalloproteinases are among the most important enzymes in the regulation of extracellular matrix homeostasis. It is now widely accepted that the degeneration and degradation of extracellular matrix in disease states such as IDD or osteoarthritis are associated with the physiological dysfunction of metalloproteinases. Another important zinc-dependent secretory metalloproteinase family, ADAMTS, plays a similar role to MMPs and is also involved in matrix degradation. Its most important members, ADAMTS4 and ADAMTS5, are proteoglycan-degrading enzymes and are involved in Aggrecan degradation.
Conclusions
At present, scholars are committed to studying the diagnostic value of various biological indicators for different diseases in order to predict the prognosis of patients and guide treatment (35-39). In this study, we observed that oxidative stress is one of the important causes of IDD and that the representative drug, Aspirin could effectively inhibit or improve the gene expressions of the above indicators (40), thereby improving the development of the IDD process.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5700/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5700/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5700/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. 202217865) granted by ethics board of Changchun University of Chinese Medicine, in compliance with Guide for the Care and Use of laboratory Animals, 8th edition.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 2021;20:689-709. [Crossref] [PubMed]
- Poblete-Aro C, Russell-Guzmán J, Parra P, et al. Exercise and oxidative stress in type 2 diabetes mellitus. Rev Med Chil 2018;146:362-72. [Crossref] [PubMed]
- Khatri N, Thakur M, Pareek V, et al. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol Disord Drug Targets 2018;17:689-95. [Crossref] [PubMed]
- Senoner T, Dichtl W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019;11:2090. [Crossref] [PubMed]
- van der Pol A, van Gilst WH, Voors AA, et al. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 2019;21:425-35. [Crossref] [PubMed]
- Wu L, Zheng Y, Liu J, et al. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY) 2021;13:10833-52. [Crossref] [PubMed]
- Wu Z, Wang H, Fang S, et al. Roles of endoplasmic reticulum stress and autophagy on H2O2-induced oxidative stress injury in HepG2 cells. Mol Med Rep 2018;18:4163-74. [Crossref] [PubMed]
- Hauck AK, Huang Y, Hertzel AV, et al. Adipose oxidative stress and protein carbonylation. J Biol Chem 2019;294:1083-8. [Crossref] [PubMed]
- Luo J, Mills K, le Cessie S, et al. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res Rev 2020;57:100982. [Crossref] [PubMed]
- Beyfuss K, Hood DA. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep 2018;23:100-17. [Crossref] [PubMed]
- Stanek A, Brożyna-Tkaczyk K, Myśliński W. Oxidative Stress Markers among Obstructive Sleep Apnea Patients. Oxid Med Cell Longev 2021;2021:9681595. [Crossref] [PubMed]
- Pierzynowska K, Gaffke L, Cyske Z, et al. Oxidative Stress in Mucopolysaccharidoses: Pharmacological Implications. Molecules 2021;26:5616. [Crossref] [PubMed]
- Steven S, Frenis K, Oelze M, et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid Med Cell Longev 2019;2019:7092151. [Crossref] [PubMed]
- Wu L, Zhong Y, Wu D, et al. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines 2022;10:2248. [Crossref] [PubMed]
- Cazzanelli P, Wuertz-Kozak K. MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology. Int J Mol Sci 2020;21:3601. [Crossref] [PubMed]
- Wang Y, Che M, Xin J, et al. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother 2020;131:110660. [Crossref] [PubMed]
- Krut Z, Pelled G, Gazit D, et al. Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration. Cells 2021;10:2241. [Crossref] [PubMed]
- Wu L, Zheng Y, Ruan X, et al. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs 2022;33:e590-603. [Crossref] [PubMed]
- Liao Z, Liu H, Ma L, et al. Engineering Extracellular Vesicles Restore the Impaired Cellular Uptake and Attenuate Intervertebral Disc Degeneration. ACS Nano 2021;15:14709-24. [Crossref] [PubMed]
- Das UN. Bioactive lipids in intervertebral disc degeneration and its therapeutic implications. Biosci Rep 2019;39:BSR20192117. [Crossref] [PubMed]
- Cheng Z, Xiang Q, Wang J, et al. The potential role of melatonin in retarding intervertebral disc ageing and degeneration: A systematic review. Ageing Res Rev 2021;70:101394. [Crossref] [PubMed]
- Yang RZ, Xu WN, Zheng HL, et al. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J Cell Physiol 2021;236:2725-39. [Crossref] [PubMed]
- Liao Z, Li S, Lu S, et al. Metformin facilitates mesenchymal stem cell-derived extracellular nanovesicles release and optimizes therapeutic efficacy in intervertebral disc degeneration. Biomaterials 2021;274:120850. [Crossref] [PubMed]
- Cannata F, Vadalà G, Ambrosio L, et al. Intervertebral disc degeneration: A focus on obesity and type 2 diabetes. Diabetes Metab Res Rev 2020;36:e3224. [Crossref] [PubMed]
- Kang L, Liu S, Li J, et al. The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif 2020;53:e12779. [Crossref] [PubMed]
- Ji ML, Jiang H, Zhang XJ, et al. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun 2018;9:5051. [Crossref] [PubMed]
- Wei H, Li B, Sun A, et al. Interleukin-10 Family Cytokines Immunobiology and Structure. Adv Exp Med Biol 2019;1172:79-96. [Crossref] [PubMed]
- Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the Interleukin-36 Pathway for the Treatment of Generalized Pustular Psoriasis. N Engl J Med 2019;380:981-3. [Crossref] [PubMed]
- Ye C, Yano H, Workman CJ, et al. Interleukin-35: Structure, Function and Its Impact on Immune-Related Diseases. J Interferon Cytokine Res 2021;41:391-406. [Crossref] [PubMed]
- Muñoz-Garcia J, Cochonneau D, Télétchéa S, et al. The twin cytokines interleukin-34 and CSF-1: masterful conductors of macrophage homeostasis. Theranostics 2021;11:1568-93. [Crossref] [PubMed]
- Shohan M, Dehghani R, Khodadadi A, et al. Interleukin-22 and intestinal homeostasis: Protective or destructive? IUBMB Life 2020;72:1585-602. [Crossref] [PubMed]
- Wu L, Zhong Y, Yu X, et al. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs 2022;33:943-59. [Crossref] [PubMed]
- Iznardo H, Puig L. The interleukin-1 family cytokines in psoriasis: pathogenetic role and therapeutic perspectives. Expert Rev Clin Immunol 2021;17:187-99. [Crossref] [PubMed]
- Jafarizade M, Kahe F, Sharfaei S, et al. The Role of Interleukin-27 in Atherosclerosis: A Contemporary Review. Cardiology 2021;146:517-30. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Qi A, Li Y, Sun H, et al. Incidence and risk factors of sexual dysfunction in young breast cancer survivors. Ann Palliat Med 2021;10:4428-34. [Crossref] [PubMed]
- He XC, Chen HY, Qiu Y, et al. Associations of iron status with breast cancer risk factors in adult women: Findings from National Health and Nutrition Examination Survey 2017-2018. J Trace Elem Med Biol 2021;68:126867. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Melton E, Qiu H. Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis. Int J Mol Sci 2020;21:6458. [Crossref] [PubMed]
(English Language Editor: A. Kassem)




