
Diagnostic value of the fractional excretion of urine potassium for primary aldosteronism
Highlight box
Key findings
• The fractional excretion of potassium (FEK) is significantly related to renal potassium loss. The spot FEK and 24-hr FEK have some value for assessing primary aldosteronism (PA).
What is known and what is new?
• PA is regarded as the most common type of secondary hypertension. FEK has been proposed as a useful tool for the measurement of urinary potassium excretion.
• Spot FEK and 24-hr FEK performed a certain diagnostic value for PA.
What is the implication, and what should change now?
• Spot FEK may be an effective and convenient method of diagnosing PA, which might help clinicians to screen at-risk individuals earlier.
Introduction
Primary aldosteronism (PA), the most common type of secondary hypertension, is defined as the excessive secretion of the steroid hormone aldosterone by the adrenal glands, which leads to hypokalemia and hypernatremia (1). The prevalence of PA in patients with stage 1, 2, and 3 hypertension is 1.99%, 8.02%, and 13.2%, respectively (2), which increases with the severity of hypertension, reaching up to 29.1% in patients with resistant hypertension (3). Several studies have found that excessive aldosterone is inextricably linked to myocardial hypertrophy, heart failure, and renal function damage. Compared to patients with essential hypertension (EH), PA patients tend to have more severe damage to the target organs, including the heart and kidneys (4,5). PA can be traced to severe glucose metabolism disorders (6). Early diagnosis of PA is necessary to enable clinicians to implement interventions to improve the prognosis of patients.
To data, several diagnostic indicators of PA have been reported. The aldosterone-to-renin ratio (ARR) is recommended as an important screening tool for PA (7-10). However, this method requires reliable laboratory testing conditions, higher costs, and a longer period. In addition, hormonal screening may result in false positives, which can lead to unnecessary diagnostic procedures (11). Willenberg et al. founded that serum sodium to urinary sodium to (serum potassium)2 to urinary potassium (SUSPPUP) ratio and serum sodium to urinary sodium to serum potassium to urinary potassium (SUSPUP) ratio performed high sensitivity and specificity in the diagnosis of PA (12), which calculated the amount of sodium retention relative to potassium loss in exchange for sodium to reflect mineralocorticoid action. Considering the difficulties of collecting and measuring daily sodium retention, an easy and quick screening method for PA should be mentioned. Urinary potassium excretion is a readily available indicator measured by the fractional excretion of urine potassium (FEK) (13,14). To the best of our knowledge, the role of the FEK for screening PA remains unclear.
Herein, we investigated the diagnostic value of the spot FEK and 24-h FEK in PA, and further compared their diagnostic performance with SUSPUP, SUSPPUP, ARR and urine potassium-creatinine ratio, which may be a significant screening tool for PA to provide reference for clinical diagnosis. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6410/rc).
Methods
Study participants
This was a cross-sectional study involving a total of 155 patients who were diagnosed with hypertension in the wards of the endocrinology department at Zhongshan Hospital Affiliated to Fudan University, Shanghai, China, from April 2021 to August 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Zhongshan Hospital, Fudan University (No. 2022-341) and individual consent for this retrospective analysis was waived.
Hypertension was diagnosed after multiple consecutive repeated measurements in the outpatient department with blood pressure (BP) values above 140/90 mmHg. According to the inclusion criteria, the patients were divided into a PA group and an EH group. The inclusion criteria for the PA group were as follows: (I) a positive screening test, represented by an upright ARR >30 ng/dL per ng/mL/h with a plasma renin activity (PRA) <1 ng/(mL·h), or an upright ARR >30 ng/dL per ng/mL/h with a plasma aldosterone concentration (PAC) >15 ng/dL; and (II) one or more positive confirmatory tests, such as the captopril challenge test (15). The inclusion criteria for the EH group were as follows: (I) systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg in the absence of antihypertensive medication; and (II) a negative PA screening test or a positive PA screening test but negative confirmatory test. Patients with other types of secondary hypertension, such as renal artery stenosis, pheochromocytoma, or Cushing's syndrome, were excluded. In addition, patients whose urine biochemistry data were not available and those who had developed chronic kidney disease were excluded from the study.
General clinical information collection and laboratory examination
On admission, all patients were consulted regarding their clinical data (age, gender, and drug utilization). Patients who were diagnosed with hypertension discontinued spironolactone for at least 4 weeks and loop diuretics for at least 2 weeks before testing. Certain antihypertensive drugs that could affect blood test results, such as angiotensin-converting enzyme inhibitors, were paused for at least 1 week.
After getting up in the early morning, the patients were requested to keep sitting still for 30 minutes, and then their blood samples were collected. The spot urine sample was obtained from the second miction. On the same day, the patients were asked to retain their urine for 24 hours, and their creatinine, potassium, and sodium concentrations were assessed in the serum and urine. The total urinary potassium and sodium amount were identified based on the 24-hr urine collection.
The PAC (Waters Corporation, Massachusetts, USA) and PRA (SCIEX, Framingham, Massachusetts, USA) screening parameters were collected from every patient. Serum aldosterone and renin levels were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS).
The related formulas were calculated as follows:
where PAC = plasma aldosterone concentration; PRA = plasma renin activity; ARR = aldosterone-to-renin ratio; KS = serum potassium concentration; NaS = serum sodium concentration; CrS = serum creatinine concentration; CrU = urine creatinine concentration from a spot urine sample; Cr24hU = urine creatinine concentration from a 24-hr urine sample; KU = urine potassium concentration from a spot urine sample; K24hU = urine potassium concentration from a 24-hr urine sample; spot FEK = fractional excretion of urine potassium from a spot urine sample; 24-hr FEK = fractional excretion of urine potassium from a 24-hr urine sample; SUSPUP = serum sodium to urinary sodium to serum potassium to urinary potassium; and SUSPPUP = serum sodium to urinary sodium to (serum potassium)2 to urinary potassium.
Statistical analysis
In this study, SPSS (v.20.0, IBM, USA) and MedCalc (v.19.0, MedCalc Software Ltd., Belgium) were used for data analysis and processing. The homogeneity of variance of the data was calculated using Levene’s test. The normality of the data was evaluated using the Shapiro-Wilks test. Clinical data of normal distribution were expressed as the mean ± standard deviation (SD). Non-normally distributed data were presented as the median and quartile [M (Q1, Q3)]. For group comparisons of continuous variables, normally distributed data were compared by the independent sample t-test. For data showing a non-normal distribution, group comparisons were performed using the Mann-Whitney U-test. The Bland-Altman plot was used to calculate differences between the spot FEK and other indexes. The area under the curve (AUC) with 95% confidential intervals (CIs), sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR−) of the related diagnostic indexes were analyzed using receiver operating characteristic (ROC) curves, and the optimal cut-point value of the diagnostic index was determined according to the Youden index (YI) (sensitivity + specificity − 1). Correlation analysis was performed between the spot FEK and 24-hr FEK using Pearson’s correlation coefficient. Two-sided P<0.05 was considered statistically significant.
Results
The characteristics of patients with hypertension
A total of 155 hypertensive patients were enrolled in this study, including 62 patients with PA and 93 patients with EH. Table 1 illustrates the basic characteristics of the patient cohorts. The baseline sex, age, serum creatinine, 24-hr urine creatinine excretion, 24-hr urine potassium excretion, 24-hr urine sodium excretion, and spot urine potassium concentration were not significantly different between the PA and EH patients. However, the spot urine creatinine concentrations in the PA patients were markedly lower than those in the EH patients [7,476.5 (4,045.5–9,659.5) vs. 8,615.0 (5,237.0–12,646.5), P=0.042]. Also, patients with PA had notably lower serum potassium concentrations and PRA levels than those with EH [3.3±0.5 vs. 3.9±0.3, P<0.001; 0.1 (0.1–0.2) vs. 0.7 (0.3–1.7), P<0.001]. The serum sodium concentrations (143.9±2.3 vs. 141.7±2.5, P<0.001), PAC [126.5 (90.7–174.0) vs. 64.0 (41.7–94.7), P<0.001], ARR [94.9 (53.0–227.5) vs. 10.1 (2.9–19.8), P<0.001], spot FEK [7.3 (5.5–11.4) vs. 5.9 (4.0–7.1), P<0.001], 24-hr FEK [9.3 (7.1–13.5) vs. 8.0 (6.3–10.7), P=0.015], SUSPUP ratio [14.1 (11.0–20.4) vs. 10.3 (7.9–13.7), P<0.001], SUSPPUP ratio [4.2 (3.0–5.9) vs. 2.6 (2.0–3.8), P<0.001], and urine potassium-creatinine ratio [3.6 (2.6–4.8) vs. 3.0 (2.2–4.3), P=0.011], were significantly higher in the PA patients than in the EH patient group
Table 1
| Variables | Primary aldosteronism (n=62) | Essential hypertension (n=93) | P |
|---|---|---|---|
| Cases of renal potassium loss (n) | 34 | 0 | – |
| Male, n (%) | 35 (56.0) | 53 (57.0) | 0.947 |
| Female, n (%) | 27 (44.0) | 40 (43.0) | 0.947 |
| Age, years, mean ± SD | 54.5±13.7 | 57.9±14.9 | 0.163 |
| PAC, pg/mL, M (Q1, Q3) | 126.5 (90.7, 174.0) | 64.0 (41.7, 94.7) | <0.001 |
| PRA, ng/mL/h, M (Q1, Q3) | 0.1 (0.1, 0.2) | 0.7 (0.3, 1.7) | <0.001 |
| ARR, pg/mL per ng/mL/h, M (Q1, Q3) | 94.9 (53.0, 227.5) | 10.1 (2.9, 19.8) | <0.001 |
| KS, mmol/L, mean ± SD | 3.3±0.5 | 3.9±0.3 | <0.001 |
| NaS, mmol/L, mean ± SD | 143.9±2.3 | 141.7±2.5 | <0.001 |
| CrS, μmol/L, M (Q1, Q3) | 71.0 (59.8, 84.3) | 75.0 (65.0, 86.5) | 0.108 |
| 24-hr urine creatinine excretion, mmol/24 h, M (Q1, Q3) | 9,046.6±3,556.6 | 8,456.7±3,888.2 | 0.340 |
| 24-hr urine potassium excretion, mmol/24 h, M (Q1, Q3) | 36.6 (29.5, 53.2) | 33.6 (25.4, 47.8) | 0.111 |
| 24-hr urine sodium excretion, mmol/24 h, M (Q1, Q3) | 116.5 (73.0, 174.3) | 118.0 (92.5, 162.5) | 0.841 |
| CrU, μmol/L, M (Q1, Q3) | 7,476.5 (4,045.5, 9,659.5) | 8,615.0 (5,237.0, 12,646.5) | 0.042 |
| KU, mmol/L, M (Q1, Q3) | 25.1 (19.4, 32.1) | 25.1 (16.6, 33.3) | 0.834 |
| Spot FEK (%), M (Q1, Q3) | 7.3 (5.5, 11.4) | 5.9 (4.0, 7.1) | <0.001 |
| 24-hr FEK (%), M (Q1, Q3) | 9.3 (7.1, 13.5) | 8.0 (6.3, 10.7) | 0.015 |
| SUSPUP ratio, M (Q1, Q3) | 14.1 (11.0, 20.4) | 10.3 (7.9, 13.7) | <0.001 |
| SUSPPUP ratio, (mmol-1)-1, M (Q1, Q3) | 4.2 (3.0, 5.9) | 2.6 (2.0, 3.8) | <0.001 |
| Urine potassium-creatinine ratio, M (Q1, Q3) | 3.6 (2.6, 4.8) | 3.0 (2.2, 4.3) | 0.011 |
SD, standard deviation; PAC, plasma aldosterone concentration; M (Q1, Q3), median (quartile 1, quartile 3); PRA, plasma renin activity; ARR, aldosterone-to-renin ratio; KS, serum potassium concentration; NaS, serum sodium concentration; CrS, serum creatinine concentration; CrU, urine creatinine concentration; KU, urine potassium concentration; spot FEK, fractional excretion of urine potassium from a spot urine sample; 24-hr FEK, fractional excretion of urine potassium from a 24-hour urine sample; SUSPUP, serum sodium to urinary sodium to serum potassium to urinary potassium; SUSPPUP, serum sodium to urinary sodium to (serum potassium)2 to urinary potassium.
Diagnostic performance for renal potassium loss
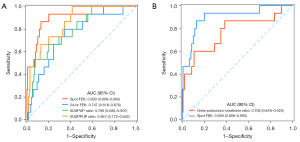
We found that hypokalemia was noted in 56.5% of PA patients and 0% of EH patients when the serum potassium level was lower than 3.5 mEq/L (3.5 mmol/L). Among the PA patients with hypokalemia, 97.1% were renal potassium loss according to the diagnostic criteria. The AUCs of the spot FEK, 24-hr FEK, SUSPUP ratio, SUSPPUP ratio, and urine potassium-creatinine ratio were 0.900, 0.747, 0.796, 0.857, and 0.768 in the screening for the renal potassium loss (Table 2), respectively. The AUC of spot FEK (threshold at 9.8%) was better than that of the other indices (Figure 1), with a sensitivity of 86.7% and a specificity of 87.1% (Table 2). Using 24-hr FEK to differentiate patients with renal potassium loss, we obtained a sensitivity of 80.0% and a specificity of 65.7%. The sensitivity and specificity of the urine potassium-creatinine ratio were 60% and 89.3%, respectively.
Table 2
| Variables | AUC (95% CI) | Cut-off | Sensitivity | Specificity | LR+ | LR− |
|---|---|---|---|---|---|---|
| Spot FEK | 0.900 (0.808–0.993) | 9.8 | 0.867 | 0.871 | 6.72 | 0.15 |
| 24-hr FEK | 0.747 (0.618–0.876) | 9.5 | 0.800 | 0.657 | 2.33 | 0.30 |
| SUSPUP ratio | 0.796 (0.685–0.907) | 16.3 | 0.667 | 0.800 | 3.33 | 0.42 |
| SUSPPUP ratio | 0.857 (0.772–0.942) | 3.2 | 1.000 | 0.586 | 2.42 | 0.00 |
| Urine potassium-creatinine ratio | 0.768 (0.616–0.920) | 5.5 | 0.600 | 0.893 | 5.61 | 0.45 |
AUC, area under curve; CI, confidential interval; LR+, positive likelihood ratio; LR−, negative likelihood ratio; SUSPUP, serum sodium to urinary sodium to serum potassium to urinary potassium; SUSPPUP, serum sodium to urinary sodium to (serum potassium)2 to urinary potassium; spot FEK, fractional excretion of urine potassium from a spot urine sample; 24-hr FEK, fractional excretion of urine potassium from 24-hour urine sample.
Diagnostic performance for PA
Table 3 presents the characteristics of the cut-off points for the screening of PA in patients with hypertension. The optimal cut-off value for ARR was 30.3, with a sensitivity of 93.5% and a specificity of 91.4% (Table 3). The sensitivity and specificity of the 24-hr FEK with the cut-off point (8.1%) were 66.1% and 52.7%, respectively. With the threshold at 7.2%, the spot FEK had a sensitivity of 51.6% and a specificity of 76.3%. At this threshold, the specificity of the spot FEK was better than that of the SUSPPUP ratio but the sensitivity was worse. The urine potassium-creatinine ratio (AUC =0.964) has a good diagnostic ability for PA, with a superior specificity (92.5%) (Table 3). The ROC curves of these parameters for diagnosing the PA were shown in Figure 2.
Table 3
| Variables | AUC (95% CI) | Cut-off | Sensitivity | Specificity | LR+ | LR− |
|---|---|---|---|---|---|---|
| Spot FEK | 0.669 (0.582–0.756) | 7.2 | 0.516 | 0.763 | 2.18 | 0.63 |
| 24-hr FEK | 0.616 (0.526–0.706) | 8.1 | 0.661 | 0.527 | 1.40 | 0.64 |
| SUSPUP ratio | 0.711 (0.629–0.794) | 11.0 | 0.774 | 0.624 | 2.06 | 0.36 |
| SUSPPUP ratio | 0.768 (0.693–0.844) | 2.8 | 0.855 | 0.624 | 2.27 | 0.23 |
| ARR | 0.959 (0.931–0.988) | 30.3 | 0.935 | 0.914 | 10.87 | 0.07 |
| Urine potassium-creatinine ratio | 0.964 (0.938–0.989) | 36.2 | 0.935 | 0.925 | 12.47 | 0.07 |
PA, primary aldosteronism; AUC, area under the curve; CI, confidential interval; LR+, positive likelihood ratio; LR−, negative likelihood ratio; ARR, aldosterone-to-renin ratio; SUSPUP, serum sodium to urinary sodium to serum potassium to urinary potassium; SUSPPUP, serum sodium to urinary sodium to (serum potassium)2 to urinary potassium; spot FEK, fractional excretion of urine potassium from a spot urine sample; 24-hr FEK, fractional excretion of urine potassium from 24-hour urine sample.

Correlation between these diagnostic factors
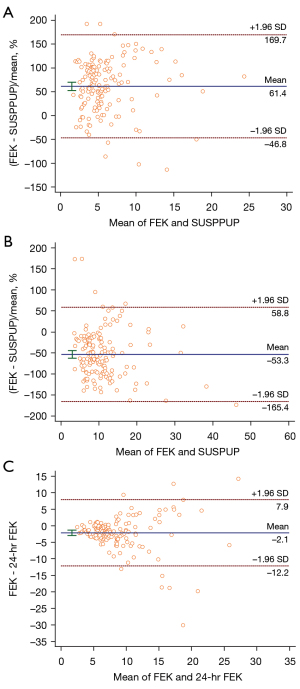
The Bland-Altman analysis showed that only 5.8% (9/155) of patients were beyond ± 2 SD from the mean between the spot FEK and 24-hr FEK, 3.2% (5/155) between the pot FEK and SUSPUP ratio, and 4.5% (7/155) between the spot FEK and SUSPPUP ratio (Figure 3). There was good agreement among each of these parameters.
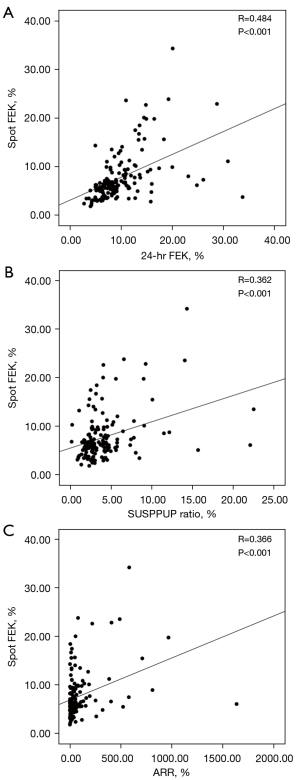
These parameters also correlated very well with each other, as shown by the linear regression analysis: r=0.48, P<0.001, r²=0.23 (spot FEK vs. 24-hr FEK), and r=0.36, P<0.001, r²=0.13 (spot FEK vs. SUSPPUP ratio). Pearson’s coefficient of the correlation between the spot FEK and ARR was 0.36 (P<0.001) (Figure 4).
Discussion
The clinical diagnosis of PA has always been difficult, and screening is a critical step. Finding accurate, sensitive, and easy screening indicators has always been an important research area. The combination of high blood pressure and low potassium levels is observed in many cases of PA. In this study, patients with PA had significantly lower serum potassium concentrations, and renal potassium loss was noted in 24.2% of PA patients and 0% of EH patients. It suggests that if a patient has hypertension, hypokalemia caused by renal potassium loss may also indicate the presence of PA.
Hypokalemia can result from poor potassium intake, increased translocation into the cells, or excessive potassium losses caused by diuretics or gastrointestinal disorders. As with osmotic diuresis, the mechanisms leading to increased urinary losses include increased sodium delivery to the distal nephron, mineralocorticoid excess, and increased urine flow (16,17). Various disorders can increase renal potassium excretion. The excess mineralocorticoid (i.e., aldosterone) effect can directly increase potassium secretion by the distal nephrons.
Traditional diagnostic criteria for renal potassium loss are based on an estimate of urine potassium excretion in a 24-hr urine collection (18). When the situation is urgent, patients cannot wait for a prolonged period of urine collection. Due to the difficulty and complexity of retaining 24-hr urine, a spot urine collection reflecting potassium excretion is the optimum sample to evaluate hypokalemia. In this study, we sought to evaluate the diagnostic value of spot urine tests in patients with hypertension.
We have found that the spot FEK can serve as a helpful tool for the measurement of urinary potassium excretion. This finding was also reported by Elisaf et al. (14). It has been stated that the determination of FEK is helpful in the differential diagnosis of hypokalemia. In comparison to this previous study, we compared the screening value of various parameters for PA by plotting ROC curves and determined the cut-point values corresponding to each index according to the Youden index. Spot FEK had the highest AUC at a cut-off of 9.8% in the screening for renal potassium loss, with a sensitivity of 86.7% and a specificity of 87.1%. The agreement between the spot FEK and the 24-hr FEK suggests that FEK in a spot urine sample is highly accurate for predicting renal potassium loss. Our results confirm that the spot FEK is a relatively reliable marker of potassium excretion. Furthermore, the detection of the spot FEK requires simple equipment, which costs less money and takes less time.
We also noticed that the spot FEK is of value to the diagnosis of PA. The sensitivity and specificity of the spot FEK in the screening of PA were 51.6% and 76.3%, respectively. The spot FEK and 24-hr FEK were less accurate than ARR in distinguishing PA from EH in this population depending on the choice of the threshold values. The distribution of ARR and the spot FEK suggests that combining these two tests may potentially be valuable. Spot FEK combined with ARR increased the sensitivity of PA diagnosis compared to ARR alone. Patients with EH were enrolled in the control group, which may have helped increase the specificity of the FEK.
Interestingly, we observed that the spot urine creatinine concentration was significantly lower in PA patients than in EH patients. Long-term massive potassium loss causes vacuolar degeneration of renal tubular epithelial cells. Reduced renal concentrating function leads to polyuria and increased nocturia, followed by low urine specific gravity and high urine creatinine concentration.
There were several limitations in this study that should be noted. Firstly, the sample size was small. Secondly, we did not conduct the study in a normal cohort but only in the population of patients with hypertension. Further studies on FEK should be conducted to verify our findings.
Conclusions
Our study indicates that spot FEK is a highly useful tool in the diagnosis of renal potassium loss. Our results suggest a possibility of using the spot FEK, which is more convenient, to help clinicians to easily diagnose PA.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6410/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6410/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6410/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Zhongshan Hospital, Fudan University (No. 2022-341) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol 2021;9:876-92. [Crossref] [PubMed]
- Mosso L, Carvajal C, González A, et al. Primary aldosteronism and hypertensive disease. Hypertension 2003;42:161-5. [Crossref] [PubMed]
- Bioletto F, Bollati M, Lopez C, et al. Primary Aldosteronism and Resistant Hypertension: A Pathophysiological Insight. Int J Mol Sci 2022;23:4803. [Crossref] [PubMed]
- Haze T, Hirawa N, Yano Y, et al. Association of aldosterone and blood pressure with the risk for cardiovascular events after treatments in primary aldosteronism. Atherosclerosis 2021;324:84-90. [Crossref] [PubMed]
- Katsuragawa S, Tsurutani Y, Takiguchi T, et al. Impact of primary aldosteronism on renal function in patients with type 2 diabetes. J Diabetes Investig 2021;12:217-25. [Crossref] [PubMed]
- Grewal S, Fosam A, Chalk L, et al. Insulin sensitivity and pancreatic β-cell function in patients with primary aldosteronism. Endocrine 2021;72:96-103. [Crossref] [PubMed]
- Ng E, Gwini SM, Libianto R, et al. Aldosterone, Renin, and Aldosterone-to-Renin Ratio Variability in Screening for Primary Aldosteronism. J Clin Endocrinol Metab 2022;108:33-41. [Crossref] [PubMed]
- Ariens J, Horvath AR, Yang J, et al. Performance of the aldosterone-to-renin ratio as a screening test for primary aldosteronism in primary care. Endocrine 2022;77:11-20. [Crossref] [PubMed]
- Hung A, Ahmed S, Gupta A, et al. Performance of the aldosterone to renin ratio as a screening test for primary aldosteronism. J Clin Endocrinol Metab 2021;106:2423-35. [Crossref] [PubMed]
- Gao H, Luo R, Li J, et al. Aldosterone/direct renin concentration ratio as a screening test for primary aldosteronism: a systematic review and meta-analysis. Ann Transl Med 2022;10:679. [Crossref] [PubMed]
- Stowasser M, Ahmed AH, Pimenta E, et al. Factors affecting the aldosterone/renin ratio. Horm Metab Res 2012;44:170-6. [Crossref] [PubMed]
- Willenberg HS, Kolentini C, Quinkler M, et al. The serum sodium to urinary sodium to (serum potassium)2 to urinary potassium (SUSPPUP) ratio in patients with primary aldosteronism. Eur J Clin Invest 2009;39:43-50. [Crossref] [PubMed]
- Batlle DC, Arruda JA, Kurtzman NA. Hyperkalemic distal renal tubular acidosis associated with obstructive uropathy. N Engl J Med 1981;304:373-80. [Crossref] [PubMed]
- Elisaf M, Siamopoulos KC. Fractional excretion of potassium in normal subjects and in patients with hypokalaemia. Postgrad Med J 1995;71:211-2. [Crossref] [PubMed]
- Hundemer GL, Vaidya A. Primary Aldosteronism Diagnosis and Management: A Clinical Approach. Endocrinol Metab Clin North Am 2019;48:681-700. [Crossref] [PubMed]
- Gennari FJ. Hypokalemia. N Engl J Med 1998;339:451-8. [Crossref] [PubMed]
- Stewart PM. Mineralocorticoid hypertension. Lancet 1999;353:1341-7. [Crossref] [PubMed]
- Narins RG, Jones ER, Stom MC, et al. Diagnostic strategies in disorders of fluid, electrolyte and acid-base homeostasis. Am J Med 1982;72:496-520. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


