High-sensitivity cardiac troponin testing for primary care: analytical assay considerations required before widespread implementation
For over 20 years, cardiac troponins T and I have been used as a biomarker for diagnosis of acute coronary syndromes (ACS) and risk stratification for future adverse cardiac events (1,2). Results of cardiac troponin testing produces higher clinical sensitivity and specificity than testing for the creatine kinase-MB (CK-MB) isoenzyme, and today, is recognized as the preferred marker for use in patients suspected of acute myocardial infarction (3). As with other clinical lab tests, the ones for cardiac troponin have undergone improvements over the years, especially with regards to analytical sensitivity and precision. This is reflected by the fact that using the first generation assay, it was not possible to reliably detect cardiac troponin in healthy subjects. As such, the cutoff was established at a troponin concentration that produced an acceptable assay precision, e.g., 10%, rather than the traditional approach of testing a reference population. With release of next generation assays, the 99th percentile upper limit of normal was used as the cutoff, as recommended by international cardiology guidelines. With each improvement in troponin assays, this cutoff for detecting myocardial injury has progressively been lowered. Today, with the commercial release of “high-sensitivity” (HS) assays, cardiac troponin can be detected in all subjects with precision below 10% (4), and the 99th percentile limit is typically between 10 and 20 ng/L. Use of HS troponins has enabled physicians to detect more patients who have myocardial injury, including those without ACS.
Given the critical role that the heart plays in normal human physiology, any myocardial injury could affect myocardial function. Moreover, increased concentrations of troponin are associated with short-term adverse cardiac events, irrespective to the underlying etiology, e.g., renal disease, heart failure, pulmonary embolism, cancer chemotherapy, and others (5-8). The value of measuring troponin in the general population has received interest in the last few years (9,10).
In the largest study of troponin testing among the general population published to date, Blankenberg et al. measured cardiac troponin I in over 74,000 patients and showed that when the traditional biomarkers of cardiovascular risk were included, cardiac troponin remained as an independent predictor, with hazard ratios of 1.37 for cardiovascular mortality and 1.23 for cardiovascular disease (11). Using the upper quintiles of results, they established a cross-sectional cardiac troponin cutoff of 6.0 ng/L. Furthermore, in patients using rosuvastatin therapy, cardiac troponin value >6 ng/L resulted in a higher absolute risk reduction compared to cardiac troponin values below this limit.
Are these results sufficiently compelling to incorporation into routine clinical practice? As summarized in Table 1, there are several analytical issues that may limit the value of troponin for use in risk stratification among the general public. Use of troponin for primary care requires implementation of HS immunoassays. Unfortunately, the performance criteria for defining what is a HS assay have not been established. Apple suggested assigning troponin assay generations based on the percentage of healthy subjects that each test can detect (12). An assay that can detect greater than 95% of healthy subjects was defined as a third-generation HS immunoassay. Using this definition, there are currently no commercial assays that are FDA cleared for use in the USA. If HS troponin is to be used for primary care, testing must be conducted at reference laboratories that have validated non-approved cardiac troponin assays for clinical purposes. Of the assays that are FDA-cleared, there is significant heterogeneity in the analytical performance (13). There are also significant differences in analytical sensitivity (>10-fold) between point-of-care testing devices that produce rapid turnaround times for results compared to main-frame immunochemistry analyzers.

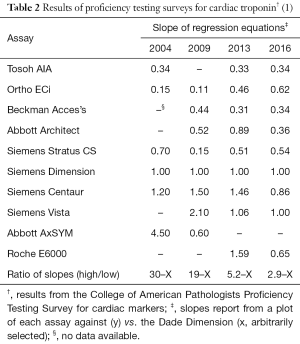
Full table
Unlike tests for cholesterol, hemoglobin A1c, or creatinine, there is poor standardization of assays between manufacturers of troponin reagents, which further limits the potential of measuring troponin as a screening test. Table 2 shows the proficiency test results of several commercial assays from 2004 to 2016. While results correlate from one assay to another (data not shown), the absolute difference between tests that produce the lowest and highest results vary by a factor of 30 (bottom row of Table 2). Over the years, manufacturers of troponin assays have attempted to harmonize test results to the extent that in 2016, the difference between the lowest and highest troponin producing assays now only vary by nearly three-fold. Nevertheless, this difference remains too high and a test result from one assay cannot be substituted for another. In the acute setting, only one assay is typically used for serial testing of blood from the emergency department. For primary care, different troponin tests could be used over time, if the sample is sent to different clinical or reference labs, and results may not be accurately interpreted. Even when the exactly same assay is used between labs, there may be differences in the 99th percentile cutoff that is assigned, according to the reference population used for defining this value. That limit is hence highly dependent on how the lab establishes its local 99th percentile limit. Collinson et al. showed that there are significant differences in the 99th percentile based on how stringency of the inclusion or exclusion criteria (14).
The utility of using a single population-based or outcome based cutoff, such as the 99th percentile, can also be challenged for primary care. Longitudinal studies have been conducted on healthy subjects to determine the biological variation of cardiac troponins (15). When blood is collected over several months, the intra-individual variation of results is significantly less than the between-individual results. Tests that have this attribute (termed the “index of individuality”) are best used for monitoring and not for diagnosis. For example, a patient could normally produce a result of 1 ng/L over time. If that individual were to exhibit a sudden increase to 10 ng/L, it would represent a 10-fold increase and likely be caused by some cardiac damage. For other individuals, however, a value of 10 ng/L is within the reference range. In this case, the detection of recent cardiac injury was only detectable if a baseline concentration was available, i.e., the value taken when the patient was healthy (16).
In the study by Blankenberg et al., only a single troponin result at a cutoff of 6 ng/L was used to risk stratify for adverse events (11). As acknowledged by the authors, this cutoff is below the 99th percentile limit determined for the cardiac troponin I immunoassay used in this study. It is likely that subjects at the highest risk would be those on whom troponin concentrations have increased over time. An unchanging value near the upper limit of normal may not necessarily impart the future cardiovascular risk. Therefore if serial results were available, an increase in troponin results would likely be a superior indicator than a single value judged against a reference population. In that case, a serial change cutoff is needed. At the present moment, this requires all testing to be conducted using the same methodology, as the lack of standardization, as stated above, would prohibit an accurate interpretation of results.
More analytical work is needed if troponin is to be used as an independent biomarker of cardiovascular disease risk. Perhaps, if a single manufacturer of a HS assay could obtain an FDA-cleared indication for its use in primary care, this could facilitate adoption within outpatient reference laboratories. However, there will be temptation for physicians to use a troponin assay that is not approved for this indication.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Executive Editor Zhi-De Hu (Department of Laboratory Medicine, General Hospital of Ji’nan Military Region, Ji’nan, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Katus HA, Remppis A, Looser S, et al. Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol 1989;21:1349-53. [Crossref] [PubMed]
- Bodor GS, Porter S, Landt Y, et al. Development of monoclonal antibodies for an assay of cardiac troponin-I and preliminary results in suspected cases of myocardial infarction. Clin Chem 1992;38:2203-14. [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:e344-426. [Crossref] [PubMed]
- Wu AH, Fukushima N, Puskas R, et al. Development and preliminary clinical validation of a high sensitivity assay for cardiac troponin using a capillary flow (single molecule) fluorescence detector. Clin Chem 2006;52:2157-9. [Crossref] [PubMed]
- Apple FS, Murakami MM, Pearce LA, et al. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002;106:2941-5. [Crossref] [PubMed]
- Peacock WF, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117-26. [Crossref] [PubMed]
- Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749-54. [Crossref] [PubMed]
- Konstantinides S, Geibel A, Olschewski M, et al. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation 2002;106:1263-8. [Crossref] [PubMed]
- Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123:1367-76. [Crossref] [PubMed]
- McKie PM, AbouEzzeddine OF, Scott CG, et al. High-sensitivity troponin I and amino-terminal pro--B-type natriuretic peptide predict heart failure and mortality in the general population. Clin Chem 2014;60:1225-33. [Crossref] [PubMed]
- Blankenberg S, Salomaa V, Makarova N, et al. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Apple FS. A new season for cardiac troponin assays: it's time to keep a scorecard. Clin Chem 2009;55:1303-6. [Crossref] [PubMed]
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574-81. [Crossref] [PubMed]
- Collinson PO, Heung YM, Gaze D, et al. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin Chem 2012;58:219-25. [Crossref] [PubMed]
- Wu AH, Lu QA, Todd J, et al. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem 2009;55:52-8. [Crossref] [PubMed]
- Wu AH, Christenson RH. Analytical and assay issues for use of cardiac troponin testing for risk stratification in primary care. Clin Biochem 2013;46:969-78. [Crossref] [PubMed]