Red blood cell distribution width independently predicts medium-term mortality and major adverse cardiac events after an acute coronary syndrome
Introduction
According to the recent 2016 Report Update published by the American Heart Association (AHA) (1), as many as 0.21% Americans experiment a new coronary event each year, and approximately 0.10% will have a recurrent event afterward. It is also estimated that 0.5% Americans will have a silent acute coronary syndrome (ACS) each year, thus raising the cumulative yearly rate of ACS up to 0.35%. Based on mortality data (1), ACS was found to be the underlying cause of death in approximately 14% of cases in the US, with an overall ACS death rate of 102.6 per 100,000 US residents. Even more importantly, approximately 34% of subjects who experience a new coronary event will die of it. Impressively, the mortality rate at 5 years after a prior ACS is 36% in men and 47% in women at the age of ≥45 years, but it dramatically increases up to 55% in men and 60% in women after the age of 75 years. Despite the mortality curve rate has considerably bent in the past decades, cardiovascular death remains the leading cause of mortality in the US, as well as in many other Countries worldwide (2). The likelihood of developing chronic complication later after an ACS is also considerably high, wherein heart failure may occur in 16% of men and 22% of women. Overall the estimated direct and indirect cost of ACS exceeds $200 billion in the US, but this expenditure is predicted to double in the next 15 years (1).
The concerning figures about the overall incidence and the death rate will make ACS the most relevant public health care issue in the forthcoming years, all around the globe. It is hence reasonable that major focus should be placed to identify reliable prediction models, which may help stratify the cardiovascular and overall risk of complications and death after an ACS. The diagnostic approach to patients with ACS encompasses the evaluation of clinical signs and symptoms, electrocardiographic assessment, combined with the measurement of circulating biomarkers which may provide reliable diagnostic and prognostic information (3-5). Despite the introduction of high-sensitivity immunoassays for the measurement of cardiac troponins has virtually revolutionized the diagnostic approach to patients with suspected ACS (6,7) providing also useful valuable information for the prognosis of these patients (8), the prediction of the future risk of major adverse cardiac events (MACE) still engages the minds of many emergency physicians and cardiologists. Many putative biomarkers have been proposed over the past decades, each of them targeting different pathways of ACS such as apoptosis, cardiomyocyte stress, cardiac fibrosis, inflammation and extra-cardiac involvement (9). Among these, interesting data emerged from the assessment of red blood cell distribution width (RDW), a simple measure of anisocytosis that can be routinely obtained from a simple and relatively inexpensive complete blood cell count (CBC) (10). Therefore, we planned a large retrospective analysis to verify whether the value of RDW may provide useful prognostic information on the medium-term risk in patients admitted to our facility for an episode of ACS.
Methods
Study population
This study was designed to retrospectively evaluate all patients admitted to Emergency Department of University Hospital of Verona from June 2014 to November 2014 for chest pain of proven cardiac origin. The baseline demographic and clinical characteristics, previous medical history regarding cardiovascular risk factors, past and concomitant medications and clinical characteristics of chest pain were collected. Patients with incomplete medical history were not originally included in the dataset. Obesity was defined as a body mass index (BMI) >25.
The primary endpoint in this study was the occurrence of MACE within 3-month of initial presentation. The definition of MACE (11) consisted in the development of acute myocardial infarction (AMI), need of percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) and coronary angiography, revealing procedurally correctable stenosis managed conservatively.
The diagnosis of ACS was made in accord with the ESC guidelines, as a rise and fall of cardiac troponin I values (Siemens Dimension Vista, Siemens Healthcare Diagnostics, Tarrytown, NY, USA) with at least one value above the 99th percentile of the upper reference limit (i.e., 45 ng/L) (12) along with evidence of myocardial ischemia. Within the diagnosis of AMI, distinction was made between either ST-elevation myocardial infarction (STEMI) or non ST-elevation myocardial infarction (NSTEMI) (13). PCI was defined as any therapeutic catheter intervention in the coronary arteries, whereas CABG was defined as any cardiac surgery in which coronary arteries were operated. Coronary angiography revealing critical coronary stenosis, not treated with coronary revascularization but only with conservative medical therapy for reasons of comorbidity, was also considered a MACE. The secondary endpoint of the study was death occurred at the end of follow-up, which lasted until June 2015 (i.e., approximately 1-year). Data were retrieved from digital and written patient records, including discharge letters, revascularization reports and any other relevant documentation. In a few cases where follow-up data were not available from hospital records, the patients or their general practitioner were contacted to obtain information on their condition, hospital admissions, ACS and revascularization.
The CBC, thus including the measurement of RDW and hemoglobin, was performed in all patients using Sysmex analyzer (Sysmex XE-2100, Sysmex Inc., Kobe, Japan). The quality of data was validated throughout the study period by regular internal quality control (IQC) procedures and participation to an External Quality Assessment (EQA) scheme.
Statistical analysis
Continuous variables were reported as median value and interquartile range. The difference between groups was evaluated using Kruskal-Wallis Test. Discrete variables were described as percentage and overall number of events, and were then analyzed by Chi-square test (or Fisher’s exact test). RDW was compared with clinical outcomes after ACS (MACE at 3 months from initial presentation or death at the end of follow-up) as continuous and categorical variable (quartiles). Logistic binomial regression was used to evaluate independent effects of RDW on clinical outcomes. All variables that were found to be significant associated with RDW were entered into a multivariate model. The predictive accuracy of RDW for MACE at 3 months after ACS and the most informative cut-off value were identified by receiver operating characteristics (ROC) curve analysis. A Kaplan Meier curve was also constructed to evaluate the survival difference at the end of follow-up according to the RDW cut-off value previously identified. Statistical analysis was performed using SPSS 22.0. The level of statistical significance was set at P<0.05. This retrospective observational study was carried in accordance with the Declaration of Helsinki and under the terms of relevant local legislation.
Results
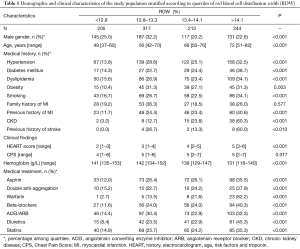
A total number of 979 patients were included from Jun 2014 to Nov 2014. The median and interquartile ranges of RDW were 13.4% and 12.8–14.1%, respectively. The baseline characteristics of patients stratified according to quartiles of RDW are shown in Table 1. Patients in the highest quartile of RDW displayed a higher frequency of cardiovascular risk factors, an older age and more concomitant medical treatments. The median value of the HEART (history, electrocardiogram, age, risk factors and troponin) score also increased in parallel with RDW values.
Full table
As regards the follow-up, the median RDW value in patients with 3-month MACE and in those who died was significantly higher than that of patients without 3-month MACE (14.0% vs. 13.3%; P<0.001) and in those who were still alive at the end of follow-up (14.4% vs. 13.4%; P<0.001). After stratifying the entire study population in quartiles of RDW, the incidence of death and 3-month MACE increased in parallel with RDW quartiles (P=0.001 for both) (Table 2).

Full table
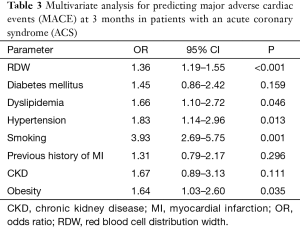
In univariate analysis, RDW was found to be a risk factor for 3-month MACE calculated both as continue [odds ratio (OR), 1.52; 95% CI, 1.35–1.72; P<0.001] and as a categorical variable (OR, 1.70; 95% CI, 1.44–2.00; P<0.001). This association was then confirmed in multivariate analysis (Table 3), which showed that RDW remained independently associated with 3-month MACE (adjusted OR, 1.36; 95% CI, 1.19–1.55; P<0.001) and death at the end of follow-up (adjusted OR, 1.34; 95% CI, 1.05–1.71; P=0.020).
Full table
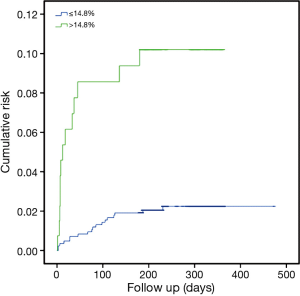
Dyslipidemia, hypertension, smoking status and obesity were found to be additional independent predictors of MACE at 3 months, whereas no significant association was noticed between 3-month MACE and diabetes mellitus, previous history of myocardial infarction (MI) and the presence of chronic kidney disease (Table 2). The accuracy of RDW for predicting 3-month MACE was characterized by an area under the curve (AUC) of 0.67 (95% CI, 0.66–0.72; P<0.001). The most efficient discriminatory RDW value was 14.8%, which was associated with a relative risk (RR) of 3.82 (2.55–5.70; P<0.001) for 3-month MACE. The median survival at the end of follow-up of patients stratified according to this cut-off of RDW is shown in Figure 1. Patients with RDW value >14.8% exhibited a significantly short survival period at the end of follow-up than those with RDW values ≤14.8% (331 vs. 465 days; P<0.001).
Discussion
Despite the establishment of many preventive strategies and the development of effective treatments have significantly contributed to reduce the frequency and complication of ACS, heart disease remains the leading cause of mortality and morbidity around the globe (1). Therefore, the assessment of reliable, rapid, easy and relatively inexpensive biomarkers for predicting clinical outcomes in these patients should be regarded as a highly valuable perspective for stratifying the future risk of MACE and death, so establishing the most appropriate therapeutic management in the individual patient (14). Anisocytosis is conventionally referred as the heterogeneity of erythrocyte volumes, which may be dependent upon many demographic and clinical variables. Briefly, the release of erythrocytes of different shape and volume from the bone marrow is not only influenced by the birth season (15), ageing (16) and eventually physical exercise (17), but also by a number of red blood cells disorders such as iron, vitamin B or folate deficiencies, genetic diseases (e.g., thalassemia, sickle cell anemia, hereditary spherocytosis), hemolytic anemia and transfusions. Several lines of evidence also attest that oxidative stress, inflammation, dyslipidemia, hypertension, poor nutritional status, impairment of erythropoietin synthesis and erythropoietin hyporesponsiveness may be concomitant causes of anisocytosis (18). Many of these metabolic abnormalities are commonly encountered in patients with heart disease, and may contribute to development of acute events such as an ACS. Therefore, the potential association between anisocytosis and ACS is supported by reliable biological mechanisms (19).
A number of studies previously investigated the role of RDW in predicting adverse outcomes after an ACS. Arbel et al. studied 3,222 patients undergoing coronary angiography and explored the association between RDW and 3-year outcome using Cox’s proportional hazards analysis (20). The overall MACE rate was found to be consistently higher for patients in the highest RDW quartile compared to those in the lowest quartile (18.2% vs. 7.7%; P<0.001). In the fully adjusted model, the RDW value remained independently associated with worse outcome [hazard ratio (HR) for 1% increase, 1.12; 95% CI, 1.07–1.18; P<0.001]. In a subsequent study, Bekler et al. retrospectively studied 202 patients with ACS, who were followed up for a median period of 3 months (21). The rate of both cardiovascular death and MACE was found to be higher in patients with increased RDW values than in those with normal values. In multiple regression analysis, a RDW value >14.0% was found to be an independent predictor of cardiovascular mortality (OR: 3.0; 95% CI, 1.0–8.9; P=0.039). In another large population study, including as many as 1,760 coronary angiography patients, Gijsberts et al. (22) reported that each one standard deviation (SD) increase of RDW was associated with a 19% higher risk of MACE in multivariable, fully adjusted analysis (HR, 1.19; 95% CI; 1.08–1.32; P<0.001). The relationship between RDW and 4-year cardiovascular events after PCI was also investigated in 96 consecutive patients with ACS by Isik et al. (23), who finally reported that an increased RDW value was an independent predictor of long-term MACE (HR 5.26; 95% CI, 1.71–16.10; P=0.004). More recently, Ghaffari et al. studied 312 patients undergoing thrombolysis for ACS (24), and reported that RDW was an independent predictor of in-hospital occurrence of MACE (RR, 3.17; 95% CI, 1.23–8.46; P=0.017). Important evidence also emerged from the recent study of Huang and Hu, who analyzed 3,304 subjects admitted to the intensive care unit after an ACS (25). Interestingly, in univariate analysis the RDW was found to be significantly associated with both in-hospital mortality (OR for 1% increase, 1.21; 95% CI, 1.15–1.28; P<0.001) and 1-year mortality (OR per 1% increase, 1.21; 95% CI; 1.18–1.25; P<0.001). The association between RDW and 1-year mortality remained significant also in multivariate analysis (HR for 1% increase, 1.06; 95% CI, 1.02–1.11; P=0.005).
Taken together, the results of our study corroborate and extend previous findings relative to short-term (i.e., in-hospital) and long-term (i.e., >1-year) mortality in patients with increased RDW values, by confirming that anisocytosis should be regarded as a significant predictor of medium-term outcomes (i.e., 3-month MACE and 1-year mortality). More specifically, a RDW higher than 14.8% was found to be associated with a more than 3-fold enhanced risk of 3-month MACE after an ACS, and patients with increased RDW values also exhibited a 29% reduced survival compared to those with RDW within the normal range (Figure 1). Notably, the association between RDW, 3-month MACE and mortality was confirmed to be independent from other known risk factors of adverse outcome after cardiac ischemia, thus highlighting the valuable role of measuring RDW in this clinical setting.
The intriguing association between RDW and outcomes of ACS adds further evidence about the clinical usefulness of investigating anisocytosis in health and disease. Enhanced RDW values have been found to be associated with death and complications in many other chronic and highly prevalent conditions such as cancer (26), diabetes (27), venous thromboembolism (28) and severe allergic reactions (29). Indeed, major derangements of many common biological pathways are known to occur in these conditions, which are mostly characterized by apoptosis, malnutrition, chronic inflammatory response and fibrosis.
Conclusions
It presently remains to be definitely evaluated whether anisocytosis and MACE are both due to common metabolic abnormalities, and therefore increased RDW values should only be considered an epiphenomenon, or rather the heterogeneity of erythrocyte volumes directly interplays with the pathogenesis of ACS and its complications. Nevertheless, the results of this large retrospective study confirm that RDW may be regarded as a valuable, easy and inexpensive parameter for stratifying the medium-term risk of MACE and death in patients with ACS. We hence suggest that its measurement should be routinely implemented in current models for stratifying the risk of adverse events after an episode of myocardial ischemia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective observational study was carried in accordance with the Declaration of Helsinki and under the terms of relevant local legislation.
References
- Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38-60. [Crossref] [PubMed]
- Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016. [Epub ahead of print].
- Cervellin G, Lippi G. Of MIs and men--a historical perspective on the diagnostics of acute myocardial infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref] [PubMed]
- Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med 2016;4:194. [Crossref] [PubMed]
- Cervellin G, Rastelli G. The clinics of acute coronary syndrome. Ann Transl Med 2016;4:191. [Crossref] [PubMed]
- Lippi G. Biomarkers: Novel troponin immunoassay for early ACS rule-out. Nat Rev Cardiol 2016;13:9-10. [Crossref] [PubMed]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
- Gualandro DM, Puelacher C, Mueller C. High-sensitivity cardiac troponin in acute conditions. Curr Opin Crit Care 2014;20:472-7. [Crossref] [PubMed]
- Lippi G, Cervellin G. Risk assessment of post-infarction heart failure. Systematic review on the role of emerging biomarkers. Crit Rev Clin Lab Sci 2014;51:13-29. [Crossref] [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol 2010;9:164-9. [Crossref] [PubMed]
- Cardinaels EP, Mingels AM, Jacobs LH, et al. A comprehensive review of upper reference limits reported for (high-) sensitivity cardiac troponin assays: the challenges that lie ahead. Clin Chem Lab Med 2012;50:791-806. [Crossref] [PubMed]
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619.
- Lippi G, Mattiuzzi C. The biomarker paradigm: between diagnostic efficiency and clinical efficacy. Pol Arch Med Wewn 2015;125:282-8. [PubMed]
- Lippi G, Salvagno GL, Montagnana M, et al. Birth season predicts the values of red blood cell distribution width (RDW) in adulthood. Clin Chem Lab Med 2016;54:667-71. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med 2014;52:e197-9. [PubMed]
- Lippi G, Salvagno GL, Danese E, et al. Variation of red blood cell distribution width and mean platelet volume after moderate endurance exercise. Adv Hematol 2014;2014:192173.
- Lippi G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width and cardiovascular disorders. Does it really matter which comes first, the chicken or the egg? Int J Cardiol 2016;206:129-30. [Crossref] [PubMed]
- Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis 2015;7:E402-11. [PubMed]
- Arbel Y, Birati EY, Finkelstein A, et al. Red blood cell distribution width and 3-year outcome in patients undergoing cardiac catheterization. J Thromb Thrombolysis 2014;37:469-74. [Crossref] [PubMed]
- Bekler A, Tenekecioğlu E, Erbağ G, et al. Relationship between red cell distribution width and long-term mortality in patients with non-ST elevation acute coronary syndrome. Anatol J Cardiol 2015;15:634-9. [Crossref] [PubMed]
- Gijsberts CM, den Ruijter HM, de Kleijn DP, et al. Hematological Parameters Improve Prediction of Mortality and Secondary Adverse Events in Coronary Angiography Patients: A Longitudinal Cohort Study. Medicine (Baltimore) 2015;94:e1992. [Crossref] [PubMed]
- Isik T, Kurt M, Tanboga IH, et al. The impact of admission red cell distribution width on long-term cardiovascular events after primary percutaneous intervention: A four-year prospective study. Cardiol J 2016;23:281-8. [Crossref] [PubMed]
- Ghaffari S, Pourafkari L, Sepehrvand N, et al. Red cell distribution width is a predictor of ST resolution and clinical outcome following thrombolysis in acute ST elevation myocardial infarction. Thromb Res 2016;140:1-6. [Crossref] [PubMed]
- Huang YL, Hu ZD. Lower mean corpuscular hemoglobin concentration is associated with poorer outcomes in intensive care unit admitted patients with acute myocardial infarction. Ann Transl Med 2016;4:190. [Crossref] [PubMed]
- Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci 2010;65:258-65. [Crossref] [PubMed]
- Lippi G, Targher G, Salvagno GL, et al. Increased red blood cell distribution width (RDW) is associated with higher glycosylated hemoglobin (HbA1c) in the elderly. Clin Lab 2014;60:2095-8. [PubMed]
- Lippi G, Buonocore R, Cervellin G. Value of Red Blood Cell Distribution Width on Emergency Department Admission in Patients With Venous Thrombosis. Am J Cardiol 2016;117:670-5. [Crossref] [PubMed]
- Lippi G, Buonocore R, Picanza A, et al. Red blood cell distribution width and haemoglobin are associated with hospital admission in patients with acute allergic reactions. Br J Biomed Sci 2016;73:21-4. [Crossref] [PubMed]


