
Chlamydia psittaci pneumonia: a clinical analysis of 12 patients
Highlight box
Key findings
• Compared with conventional etiological diagnosis, mNGS is a promising and vital tool for the early diagnosis of C. psittaci pneumonia.
What is known and what is new?
• Many patients with C. psittaci pneumonia present with high fever, cough, fatigue, dyspnea, hepatic and renal damage, and pulmonary infiltrative lesions with pleural effusion.
• We examined the dynamic chest CT images of C. psittaci pneumonia to better understand the recovery from this disease.
What is the implication, and what should change now?
• Not all patients have a definite history of poultry contact, suggesting the possibility of recessive transmission. If there is a history of poultry or bird contact, the detection of C. psittaci infection should be a part of the routine diagnosis of CAP. Rapid diagnostic methods including mNGS or other methods need to be applied in time.
Introduction
Psittacosis is a zoonotic disease that is caused by Chlamydia psittaci (C. psittaci) (1). Most cases of human psittacosis are associated with vectors such as parrots or ornamental birds. Although C. psittaci is the only pathogen that causes avian psittacosis, it can infect a wide range of animals, including poultry, pigeons, and other mammals—e.g., sheep, cattle, pigs, horses, and cats—and consequently induce zoonotic diseases (2). Globally, this pathogen can currently be detected in over 460 avian species (3). Close contact with infected birds spreads the infection in humans via the inhalation of respiratory secretions or dust in dried feces (4). In addition, this obligated intracellular pathogen is capable of cross-host transmission and causing systemic diseases in mammals, including humans (5,6).
C. psittaci infection initially manifests as influenza-like symptoms including fever, chills, headache, cough, and myalgia, and C. psittaci pneumonia primarily presents as atypical community-acquired pneumonia (CAP), which cannot be easily distinguished from common pneumonia. The disease may progress to severe pneumonia, respiratory failure, and even life-threatening conditions in some patients (6,7). Currently, C. psittaci pneumonia is considered a global epidemic (2), and the reported number of cases has been progressively increasing in China (8,9). Although the proportion of CAP cases caused by C. psittaci is unclear, a review shows that approximately 1% of annual CAP is caused by C. psittaci infection. C. psittaci pneumonia is frequently overlooked by physicians owing to its low proportion—among CAPs (10).
Traditional methods for confirming a diagnosis of this disease, including serological tests and pathogenic cultures, often do not provide a definitive result. Additionally, no specific clinical manifestations have been associated with C. psittaci pneumonia, which has resulted in a poor level of awareness and difficulty among clinicians in establishing an early definitive diagnosis. Consequently, C. psittaci pneumonia is frequently underdiagnosed or misdiagnosed (11). Untimely diagnosis of suspected cases of C. psittaci pneumonia is a key cause of delayed or inadequate treatment as well as prolonged hospitalization, rehospitalization, and increased mortality and morbidity.
The novel method of metagenomics next-generation sequencing (mNGS) of pathogenic microorganisms provides a reliable and important basis for the rapid diagnosis of unexplained infectious diseases (12). In this study, the clinical data of 12 patients with C. psittaci pneumonia confirmed by mNGS were retrospectively analyzed, and more importantly, we showed the characteristics of its imaging changes dynamically, providing a reference for the clinical diagnosis and treatment. Maybe this is a detailed article on C. psittaci pneumonia in Guangzhou of China. We present the following article in accordance with the AME Case Series and STROBE reporting checklists (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6624/rc).
Methods
Sample sources
Data were collected from 12 patients who were hospitalized in the Department of Pulmonary and Critical Care Medicine at The Third Affiliated Hospital of Sun Yat-sen University from December 2019 to June 2022. The patients were diagnosed with C. psittaci pneumonia by mNGS examination after obtaining bronchoalveolar lavage fluid (BALF). Patient data including demographic characteristics, comorbidities, epidemiological data, clinical manifestations, laboratory and imaging findings, and treatment schemes were collected.
All patients who participated in this study signed an informed consent form, and this study was approved by the institutional ethics board of The Third Affiliated Hospital of Sun Yat-sen University (No. II2023-001-01). All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Study methods
Clinical data of patients
The patients’ clinical data included age, sex, days of hospitalization, clinical manifestations, epidemiological data, comorbidities, laboratory tests and imaging findings, treatment schemes, and disease prognosis.
Laboratory tests and imaging findings
The following laboratory test indicators were examined: total white blood cell (WBC) count, neutrophil (NEUT) count, hemoglobin (HGB) concentration, platelet (PLT) count, C-reactive protein (CRP), procalcitonin (PCT), erythrocyte sedimentation rate (ESR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), globulin (GLB), total bilirubin (TBil), blood urea nitrogen (BUN), creatinine (Cr), lactate dehydrogenase (LDH), creatine kinase (CK), prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer, and oxygenation index (PO2/FiO2). Imaging examinations primarily included computed tomography (CT) scan or chest X-ray.
Pathogenic examinations
Routine pathogenic examinations included sputum culture, blood culture, and urine culture. BALF at the lung lesions was obtained through tracheoscopy and sent immediately under cryogenic refrigeration to Guangzhou Kingmed Diagnostics Group Co., Ltd. or Daan Gene Co., Ltd. (Guangzhou, China) for pathogenic microbial mNGS. The results were reported within 24–48 hours.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software [International Business Machines Corporation (IBM), USA]. Measurement data were expressed as the mean ± standard deviation (SD) or median, and the t-test or rank-sum test was used for comparison between the two groups. Enumeration data were expressed as the number of cases (percentage), and the χ2 test or Fisher’s exact test was used to perform intergroup comparisons. Statistical significance was indicated by P<0.05.
Results
Clinical information of patients
The 12 patients [7 men (58.3%), 5 women (41.7%)] included in the present study were aged 37.0–87.0 years (mean, 58.25±13.27 years). The primary clinical manifestations of the patients included fever (12/12), a body temperature of 39.0 ℃ (range, 37.7–39.7 ℃), cough (12/12), sputum (10/12), dyspnea (4/12), chills (4/12), malaise (7/12), headache (3/12), and hemoptysis manifested as coughing up bloody sputum (3/12). Furthermore, one patient had impaired consciousness. Other gastrointestinal symptoms were less common. In addition, the patients’ underlying diseases primarily included hypertension (3/12), coronary heart disease (1/12), chronic obstructive pulmonary disease (1/12), diabetes mellitus (1/12), and chronic hepatitis (2/12). Two patients developed severe pneumonia and received mechanical ventilation (Tables 1,2).
Table 1
| Manifestations | Cases (n=12) | Percentage (%) |
|---|---|---|
| Clinical manifestations | ||
| Fever | 12 | 100.0 |
| Cough | 12 | 100.0 |
| Hemoptysis | 3 | 25.0 |
| Chest distress | 2 | 16.67 |
| Myalgia | 1 | 8.33 |
| Chill | 4 | 33.33 |
| Dizzy | 3 | 25.0 |
| Headache | 5 | 41.67 |
| Nausea | 2 | 16.67 |
| Sputum | 10 | 83.33 |
| Dyspnea | 4 | 33.33 |
| Palpitation | 2 | 16.67 |
| Joint pain | 1 | 8.33 |
| Diarrhea | 1 | 8.33 |
| Malaise | 7 | 58.33 |
| Poor appetite | 2 | 16.67 |
| Vomiting | 1 | 8.33 |
| Disturbance of consciousness | 1 | 8.33 |
| General information | ||
| Smoking history | 4 | 33.3 |
| Chronic obstructive pulmonary disease | 1 | 8.33 |
| Coronary heart disease | 1 | 8.33 |
| Hypertension | 3 | 25.0 |
| Tracheal intubation | 2 | 16.67 |
| Chronic hepatitis | 2 | 16.67 |
| Diabetes mellitus | 1 | 8.33 |
| Old tuberculosis | 2 | 16.67 |
| Surgery history | 2 | 16.67 |
C. psittaci, Chlamydia psittaci.
Table 2
| Inspection Items | Proportion/number of cases of elevation or lowering, n (%) | Mean ± SD/median (range) |
|---|---|---|
| Age (years) | – | 58.25±13.27/57.5 (37.0–87.0) |
| Sex | Male: 7 (58.3); female: 5 (41.7) | – |
| Length of stay (d) | – | 12.5±8.50 |
| Tmax (℃) | >38.5, 10 (83.3) | 38.97±0.68 |
| WBC count [3.5–9.5]×109/L | Elevation, 3 (25.0) | 8.74±2.59 |
| HGB concentration [130–175] g/L | Lowering, 6 (50.0) | 120.33±26.93 |
| PLT count [100–350]×109/L | Lowering, 1 (8.3); elevation, 1 (8.3) | 176.17±117.93 |
| NEUT count [1.8–6.3]×109/L | Elevation, 8 (66.7) | 7.71±2.36 |
| LYM count [1.1–3.2]×109/L | Lowering, 10 (83.3) | 0.68±0.38 |
| CRP [0.0–6.0] mg/L | Elevation, 12 (100.0); among which >100, 11 (91.7) | 181.52 (82.60–310.80) |
| PCT [0–0.05] ng/mL | Elevation, 12 (100.0), among which >2 ng/mL, 5 (41.7) | 0.75 (0.144–15.39) |
| PT [11.0–14.5] sec | Elevation, 5 (41.7) | 14.70±1.64 |
| APTT [28.0–40.0] sec | Elevation, 10 (83.3) | 47.59±6.31 |
| D-dimer [0.0–0.5] µg/mL | Elevation, 11 (91.7) | 3.86 (2.08–20.0) |
| AST [15–40] U/L | Elevation, 9 (75.0) | 112.08±71.49 |
| ALT [3–35] U/L | Elevation, 11 (91.7) | 70.58±30.54 |
| ALB [36.0–51.0] g/L | Lowering, 10 (83.3); <30 g/L, 5 (41.7) | 32.02±4.06 |
| GLB [25.0–35.0] g/L | 29.35±3.74 | |
| TBIL [4.0–23.9] µmol/L | Elevation, 2 (16.7) | 13.41±8.99 |
| BUN [2.4–8.2] µmol/L | Elevation, 1 (8.3) | 7.35±6.97 |
| Cr [31.8–91.0] mmol/L | Elevation, 6 (50.0) | 95.83±47.04 |
| CK [24–184] U/L | Elevation, 8 (66.7) | 220.0 (61.0–7137.0) |
| PO2/FiO2 [400–500] | 290.85±83.12 | |
| 300–<400 | 11 (91.7) | |
| 250–<300 | 6 (50.0) | |
| 150–<250 | 4 (33.3) | |
| 0–<150 | 1 (8.33) | |
| Imaging characteristics | – | |
| Lesions in both lungs | 5 (41.7) | |
| Lesions in the left lung | 3 (25.0) | |
| Lesions in the right lung | 4 (33.3) | |
| Pleural effusion | 9 (75.0) | |
| Pericardial effusion | 1 (8.33) |
SD, standard deviation; Tmax, maximum body temperature; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; NEUT, neutrophil; LYM, lymphocyte; CRP, C-reactive protein; PCT, procalcitonin; PT, prothrombin time; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; GLB, globulin; TBIL, total bilirubin; BUN, blood urea nitrogen; Cr, creatinine; CK, creatine kinase; PO2/FiO2, oxygenation index.
Epidemiological data
Five of the 12 patients with C. psittaci pneumonia had a clear history of avian exposure (e.g., managing a farm, raising and slaughtering pigeons, farming, watching pigeons in the park, and keeping parrots, etc.). Meanwhile, the remaining patients did not show a relevant epidemiological history.
Analysis of laboratory and imaging data
Laboratory test findings
Respiratory failure was noted on admission in two patients; these patients received mechanical ventilation. The PO2/FiO2 was ≤400 in 11 patients, including six cases of <300 and one case of <150. Elevated WBC and NEUT counts were observed in three and eight patients, respectively. HGB count decreased in six patients, including one case of moderate-to-severe anemia (HGB 61.0 g/L). In addition, the PLT count was decreased in one patient (47.0×109/L). CRP levels were elevated in all patients. PCT levels were elevated to >0.05 ng/mL in all patients, among which five showed an elevation of >2 mg/L. Furthermore, 11, 9, 11, and 2 patients presented with various degrees of elevated D-dimer levels, elevated AST levels (mean, 112.08±71.49 U/L), elevated ALT levels (mean, 70.58±30.54 U/L), and mildly elevated TBil, respectively. Eight patients showed decreased levels of ALB, among whom five patients showed a significant degree of hypoproteinemia (<30.0 g/L). Moreover, six patients developed renal impairment, and eight patients had elevated CK levels (Table 2).
Analysis of imaging examination data
All patients included in the study underwent a chest CT scan, and their main imaging features revealed dense patchy, solid shadows with poorly defined borders, visible bronchial inflation signs, and a small amount of pleural effusion. The distribution of lesions was as follows: right lung (4/12), left lung (3/12), and both lungs (5/12). Nine patients had pleural effusion and one had pericardial effusion. Furthermore, some patients developed interstitial lung lesions including latticework and striae in the later stages, and two patients had old tuberculosis lesions (Table 2, Figure 1).
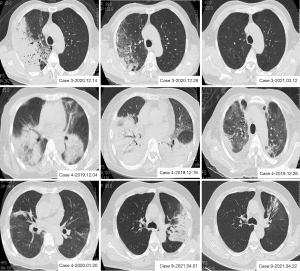
The chest CT findings in Case 3 were as follows: the findings on admission (12/14/2020) suggested multiple patchy high-density faint shadows in the right lung and a small amount of pleural effusion on the right side, emphysema, and multiple pulmonary bullae in both lungs. Re-examination after 2 weeks of treatment (12/28/2020) indicated that the inflammation in the right lung had been considerably absorbed. Follow-up chest CT (03/12/2021) suggested that the inflammation in the lungs had been absorbed completely.
The chest CT findings of Case 4 were as follows: initial (12/04/2019) findings revealed the presence of multiple patchy and solid shadows in both lungs with poorly defined sides, visible bronchial inflation signs, and a small amount of fluid in the bilateral chest cavities. Progressive inflammation in both lungs increased during treatment (12/18/2019); however, inflammation was absorbed following the administration of aggressive treatment, and some fibrous striae were observed. Follow-up (01/20/2020) continued to indicate the presence of a partial fibrous streak shadow.
The chest CT findings of Case 9 were as follows: the findings on admission (04/01/2021) suggested the presence of a large patchy solid shadow with blurred margins in the upper lobe of the left lung and a few streaks in the lower lobe of the right lung. The post-treatment re-examination (04/22/2021) suggested minor inflammation and a few streaks in the left lung.
Pathogenetic findings
The BALF of all patients had been collected via bronchoscopy for the detection of pathogens through the macrogenetic sequencing of pathogenic microorganisms. Pseudomonas aeruginosa was detected in one patient (sequence number 17742). Sputum cultures from this patient also identified carbapenem-resistant Pseudomonas aeruginosa (CRPA) and carbapenem-resistant Acinetobacter baumannii (CRAB). The above mentioned patient received mechanical ventilation and was diagnosed with a nosocomial infection secondary to a primary infection. The sputum culture results from one patient were suggestive of Acinetobacter ursingii infection; however, their blood cultures were not positive. Serologic testing for respiratory pathogens in Cases 2 and 6 suggested weak positivity for Q fever Rickettsia and positive immunoglobulin M (IgM) for Chlamydia pneumoniae, indicating the presence of co-infection or antibody cross-reactivity (Table 3).
Table 3
| Cases | Type of specimen | Sequence number of C. psittaci | Other major pathogens detected by mNGS | Sputum culture | Blood culture | Serology of respiratory pathogens |
|---|---|---|---|---|---|---|
| Case 1 | BALF | 149 | – | Negative | Negative | Negative |
| Case 2 | BALF | 271 | – | Negative | Negative | Weakly positive IgM for Q fever Rickettsiae |
| Case 3 | BALF | 4 | – | Negative | Negative | Negative |
| Case 4 | BALF | 161 | – | Negative | Negative | Negative |
| Case 5# | BALF | 10 | Pseudomonas aeruginosa [17742] | CRPA, CRAB | Negative | Negative |
| Case 6 | BALF | 412 | – | Negative | Negative | Chlamydia pneumoniae IgM (+) |
| Case 7 | BALF | 307 | – | Negative | Negative | Negative |
| Case 8 | BALF | 41 | – | Negative | Negative | Negative |
| Case 9 | BALF | 113 | – | Negative | Negative | Negative |
| Case 10 | BALF | 242 | – | Candida albicans | Negative | Negative |
| Case 11 | BALF | 108 | – | Acinetobacter ursingii | Negative | Negative |
| Case 12 | BALF | 104 | – | Negative | Negative | Negative |
#, transalveolar lavage fluid mNGS test performed at an outside hospital before admission to our hospital was suggestive of C. psittaci infection. mNGS, metagenomic next-generation sequencing; C. psittaci, Chlamydia psittaci; BALF, bronchoalveolar lavage fluid; IgM, immunoglobulin M; CRPA, carbapenem-resistant Pseudomonas aeruginosa; CRAB, carbapenem-resistant Acinetobacter baumannii.
Treatment and regression
All patients received empirical treatment after admission, which included cephalosporin antibiotics alone or in combination with quinolone or macrolide antibiotics until a clear etiological basis was obtained. The treatment regimens for each were promptly adjusted to targeted therapy after elucidating the pertinent etiologies. In addition, 2 of the 12 patients were critically ill and received mechanical ventilation. The condition of all patients subsequently improved and they were discharged after aggressive treatment (Table 4).
Table 4
| Cases | Initial treatment regimen | Adjusted treatment regimen after diagnosis | Regression |
|---|---|---|---|
| Case 1 | Cefoperazone-tazobactam + azithromycin | Cefoperazone-tazobactam + levofloxacin | Discharged after improvement |
| Case 2 | Cefoperazone-tazobactam + levofloxacin | Levofloxacin + doxycycline | Discharged after improvement |
| Case 3 | Cefoperazone-tazobactam + moxifloxacin | Cefoperazone-tazobactam + moxifloxacin | Discharged after improvement |
| Case 4 | Moxifloxacin, ganciclovir, ribavirin, azithromycin | Piperacillin tazobactam + doxycycline, azithromycin | Discharged after improvement |
| Case 5 | Moxifloxacin + doxycycline | Moxifloxacin + doxycycline, with complication treatment adjusted to polymyxin, voriconazole, tigecycline | Discharged after improvement |
| Case 6 | Cefoperazone-tazobactam + levofloxacin | Meropenem + moxifloxacin | Discharged after improvement |
| Case 7 | Cefoperazone-tazobactam | Cefoperazone-tazobactam + doxycycline | Discharged after improvement |
| Case 8 | Cefoperazone-tazobactam | Cefoperazone-tazobactam + moxifloxacin | Discharged after improvement |
| Case 9 | Cefoperazone-tazobactam + levofloxacin | Meropenem + doxycycline | Discharged after improvement |
| Case 10 | Meropenem + moxifloxacin/levofloxacin + oseltamivir | Piperacillin-tazobactam + levofloxacin | Discharged after improvement |
| Case 11 | Cefoperazone-tazobactam, moxifloxacin | Moxifloxacin + doxycycline | Discharged after improvement |
| Case 12 | Piperacillin-tazobactam | Piperacillin-tazobactam + doxycycline | Discharged after improvement |
Discussion
Psittacosis is a zoonotic disease caused by the obligated intracellular bacterium C. psittaci (13,14). C. psittaci can infect humans through the inhalation of aerosols formed by urine, feces, or respiratory secretions from birds infected with Chlamydophila psittaci (15). Furthermore, maintaining close contact when handling infected avian feathers and tissues and cleaning cages may also be a risk of infection (16). C. psittaci infection in birds and the subsequent transmission to humans has long been established (17). Previous studies have reported the rarity of human-to-human transmission of psittacosis (18,19). Wallensten et al. reported an epidemic of psittacosis outbreaks in Sweden in early 2013; during this epidemic, epidemiological and serological investigations suggested that human-to-human transmission was possible and that severely ill patients may be more infectious than previously thought (20). Family aggregation-associated infections have also been reported in China (21).
According to an epidemiological survey (22), the prevalence of C. psittaci infection in chickens, ducks, and pigeons sold on the market in Northwest China was 13.32%, 38.92%, and 31.09%, respectively. Globally, psittacosis reportedly occurs primarily among veterinary hospital staff, poultry slaughterhouses, or poultry breeders used for food production (17). Therefore, the possibility of a C. psittaci infection needs to be considered in patients with a history of bird and poultry contact as well as poultry slaughter and processing who present with high fever, respiratory symptoms, or progressive respiratory distress. A critical point to assist in establishing the clinical diagnosis is the patient’s relevant epidemiological history. Teng et al. reported that three of the five patients with psittacosis in their study had a history of exposure to birds or poultry (23). Moreover, Zhang et al. (24) reported the clinical features of 14 cases of C. psittaci pneumonia, of which 11 (79%) had a history of poultry contact. Additionally, five of the 12 patients in the present study demonstrated an epidemiological history of possible exposure to birds and parrots, which assisted in deciding the clinical diagnosis.
Most cases of C. psittaci pneumonia infections manifest as flu-like symptoms, including fever, cough, headache, myalgia, and fatigue, and the majority of patients present with a self-limiting course. However, some patients have reportedly experienced blood in the sputum, rash, abdominal pain, and diarrhea, and some may develop into atypical pneumonia (10,15,25). Some cases may develop severe pneumonia, acute respiratory distress syndrome, or even multi-organ failure including neurological, respiratory, renal, and hepatic failure. In rare cases, complications including myocarditis, endocarditis, and encephalitis can develop (26,27).
The incidence of C. psittaci pneumonia accounts for 0–2.1% of CAP. However, the actual incidence may be underestimated because the routine microbiologic diagnosis of CAP does not involve the detection of psittacosis. In their meta-analysis of 57 CAP studies (each with more than 100 cases), Hogerwerf et al. (10) concluded that C. psittaci pneumonia was estimated to account for approximately 1% of CAP cases. The remaining relevant studies suggested that a C. psittaci infection may be responsible for causing <5% of cases of CAP (28-31).
In their study, Chen et al. (28) noted that all patients with C. psittaci pneumonia (nine cases) presented with chills, fever (>39 ℃), cough, malaise, and dyspnea; seven patients presented with concurrent headache, cough, sputum, and myalgia at presentation, followed by progressive dyspnea; five patients presented with drowsiness or more severe disturbance of consciousness; and two patients were in a coma on admission. The patients had a mean WBC count of 11.9×109/L, a NEUT percentage of 82.4%, a CRP level of 175 mg/L, and a PCT level of 0.85 ng/mL on admission. Also, six, four, three and three patients presented with elevated levels of LDH, CK, AST, and ALT, respectively. All 12 patients with C. psittaci pneumonia in the present study presented with fever and cough, some cases with dyspnea, chills, and headache, and a small number had gastrointestinal symptoms. In addition, one patient developed impaired consciousness during the course of the disease; however, its association with C. psittaci infection could not be determined.
Longbottom et al. (32) reported that WBC counts were usually normal or slightly decreased during the acute phase of C. psittaci pneumonia; furthermore, a mean of 25% appeared to progress to leukopenia. In addition, anemia secondary to hemolysis was observed. Further, rare disseminated intravascular coagulation may occur in patients with a large number of infections. Three of the 12 patients in this study had varying degrees of elevated total leukocyte count; however, eight patients had significantly elevated total NEUT count and one patient had significant anemia. Coagulation tests suggested a significant prolongation of PT and APTT in 5 patients (41.7%) and a markedly elevated D-dimer level in 11 patients (91.7%), suggesting a possible effect of C. psittaci on the coagulation system.
In their study, Branley et al. (25) reported that 24 (60%) of 40 patients with C. psittaci pneumonia who underwent liver function testing presented with several abnormalities. They noted that liver function tests in 17 (35%) patients showed that these patients had less than twice the upper limit of normal (ULN) and 6 (12.5%) had tests between 2 and 59 the ULN; the predominant abnormal marker was transaminases (n=21, 44%). Elevated levels of ALT, AST, and CK were observed in 11 (91.7%), 9 (75.0%), and 8 (66.7%) patients in the present study, respectively. However, the elevation of these indicators did not appear to exhibit a significant correlation with disease severity.
Teng et al. reported that invasive ventilatory support and extracorporeal membrane oxygenation (ECMO) support was provided to two of five patients with C. psittaci pneumonia. CRP, PCT, and ESR were higher than normal at admission and follow-up; also, two patients had WBC counts of >10×109/L and three patients had PCT levels of >10 ng/mL. Furthermore, chest imaging primarily revealed the presence of solid changes. However, bacterial co-infection could not be excluded, despite the unidentified bacteria in the culture (23). In the present study, all 12 patients showed significantly elevated CRP levels, and 11 patients (91.7%) had CRP levels of >100 mg/L. In addition, 12 patients had varying degrees of elevated PCT levels, with 5 patients (41.7%) having PCT levels of >2 ng/mL; this suggested co-infection with bacteria, with more pronounced elevation in severely ill patients. In addition, routine blood cultures did not appear to be positive. Sputum culture examination revealed concurrent Pseudomonas aeruginosa and Acinetobacter baumannii infection in one patient. Additionally, the mechanical ventilation that this patient received in the intensive care unit (ICU) may have triggered ventilator-associated pneumonia.
Chest radiographic abnormalities are visualized in up to 90% of hospitalized patients with C. psittaci infection. The most common imaging abnormality reported in the past was unilateral dense solid changes in the lower lobes (33), followed by bilateral, nodular, cornified, or interstitial infiltrates (32). Chest radiographs indicating the presence of infiltrative plaques showed that heterogenous density can severely affect all lung lobes. Furthermore, CT images of the lungs have demonstrated the presence of solid or ground glass changes in the lower lungs in particular, with pleural involvement and pleural effusion (23,25). The main manifestations of chest CT in the 12 confirmed patients observed in this study were multiple patchy, solid shadows with poorly defined borders and visible bronchial inflation signs. The distribution of lesions was as follows: the right lung in four patients, the left lung in three patients, and both lungs in five patients. However, nine patients presented with a small amount of concurrent pleural effusion, and one case presented with a concurrent pericardial effusion.
Isolation of the disease to the protozoa during the acute phase of infection and before the use of antimicrobial drugs is the most reliable method of demonstrating the presence of viable organisms in human (or avian) cases of psittacosis. However, the pathogenic diagnosis of C. psittaci pneumonia is difficult in clinical settings. A pathogen culture needs to be performed in a biosafety laboratory (BSL-3) under harsh conditions (34). Therefore, serological methods are primarily employed globally to establish the diagnosis of psittacosis, including micro immunofluorescence and paired sera (10,14). Polymerase chain reaction (PCR) gene amplification assays have become an adjunct to molecular biological diagnosis owing to their high sensitivity and specificity (35). PCR—a faster and more specific diagnostic test—can be performed in specialized diagnostic laboratories (36). Psittacosis is easily missed and misdiagnosed owing to its non-specific symptoms and the limitations of current tests (37). Wolff et al. (38) reported the first use of real-time multiplex PCR for the detection and differentiation of C. psittaci from Chlamydia pneumoniae, along with an internal control used for human clinical specimens. This analysis is easier, faster, and more reliable than traditional diagnostic methods including culture and serology. The multiplex assay has considerable applications for surveillance and ensuring rapid response to outbreaks of important respiratory pathogens. However, the application of PCR and related techniques for the detection of C. psittaci in healthcare facilities is currently very limited.
Novel pathogenic microbial mNGS allows for the rapid and accurate identification of potential pathogens without a priori selection of the target pathogens, independent of genomic diversity, mutations, or organism culture, regardless of whether they are viruses, bacteria, fungi, or parasites (39,40). mNGS can be used for universal pathogen detection in a 24–48-hour time frame and allows for antibiotic resistance characterization (41). It is likely the most promising method for the comprehensive diagnosis of infections, especially severe pneumonia in the ICU setting (42). A previous study (43) diagnosed pneumonia in eight patients with C. psittaci detected by BALF or lung tissue puncture specimens and found that lung tissue puncture specimens used for mNGS testing were less confounded by background bacteria. Lung puncture specimens for mNGS testing are less likely to experience interference from background bacteria. However, this invasive test has led to patient noncompliance. mNGS is capable of detecting almost all known pathogens simultaneously from clinical samples (12,44-46). In this study, all 12 patients with pneumonia were diagnosed definitively by obtaining pathogenic results via mNGS testing of BALF, and the serological positivity rate was very low. In this study, PCR could not be performed due to the limitations of the medical institution and the considerable requirements for the diagnosis of psittacosis. Compared with conventional etiological diagnosis, mNGS is a promising and vital tool for the early diagnosis of C. psittaci pneumonia. In view of the excellent performance of mNGS in rapid diagnosis, this diagnostic method should be actively promoted for cases suspected of atypical pathogen infection.
The preferred therapeutic agents for C. psittaci pneumonia include tetracycline, macrolides, and quinolones (47). In the past, patients with C. psittaci pneumonia were often not treated appropriately and promptly because of diagnostic difficulties. The empirical treatment of patients in this study before diagnosis involved cefoperazone-tazobactam or piperacillin-tazobactam, with levofloxacin and azithromycin adjusted or added depending on the condition. The treatment strategy was adjusted after obtaining clear pathogenic results, in which good results were achieved using moxifloxacin or doxycycline as the main therapeutic agents. All cases, including two patients admitted to the ICU, were discharged in good condition. Therefore, the early acquisition of pathogenic information by mNGS and adjustment of treatment is very important for patient prognosis. The absence of appropriate treatment leads to a mortality rate of 15–20%. However, C. psittaci pneumonia is rarely fatal in the presence of appropriate treatment; several outbreaks of human psittacosis infection have occurred in different countries over the last 20 years or so, with a mortality rate of <1% (48).
Conclusions
In summary, the clinical presentation of C. psittaci pneumonia is not distinguishable from typical pneumonia features, leading to poor efficacy of conventional treatment. Pathogenic mNGS allows for rapid pathogenic diagnosis and immediate implementation of precise treatment strategies. Therefore, it has a positive impact on reducing the length of hospital stay and avoiding serious complications caused as a result of delayed treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series and STROBE reporting checklists. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6624/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6624/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6624/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients who participated in this study signed an informed consent form, and this study was approved by the institutional ethics board of The Third Affiliated Hospital of Sun Yat-sen University (No. II2023-001-01). All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kohn M, Lanfermann C, Laudeley R, et al. Complement and Chlamydia psittaci: Early Complement-Dependent Events Are Important for DC Migration and Protection During Mouse Lung Infection. Front Immunol 2021;12:580594. [Crossref] [PubMed]
- Hogerwerf L, Roof I, de Jong MJK, et al. Animal sources for zoonotic transmission of psittacosis: a systematic review. BMC Infect Dis 2020;20:192. [Crossref] [PubMed]
- Kasimov V, Dong Y, Shao R, et al. Emerging and well-characterized chlamydial infections detected in a wide range of wild Australian birds. Transbound Emerg Dis 2022;69:e3154-70. [Crossref] [PubMed]
- Vanrompay D, Harkinezhad T, van de Walle M, et al. Chlamydophila psittaci transmission from pet birds to humans. Emerg Infect Dis 2007;13:1108-10. [Crossref] [PubMed]
- Harkinezhad T, Geens T, Vanrompay D. Chlamydophila psittaci infections in birds: a review with emphasis on zoonotic consequences. Vet Microbiol 2009;135:68-77. [Crossref] [PubMed]
- Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis 2015;73:1-15. [Crossref] [PubMed]
- Su S, Su X, Zhou L, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med 2021;10:8051-60. [Crossref] [PubMed]
- Yang F, Li J, Qi B, et al. Clinical Symptoms and Outcomes of Severe Pneumonia Caused by Chlamydia psittaci in Southwest China. Front Cell Infect Microbiol 2022;11:727594. [Crossref] [PubMed]
- Xiao Q, Shen W, Zou Y, et al. Sixteen cases of severe pneumonia caused by Chlamydia psittaci in South China investigated via metagenomic next-generation sequencing. J Med Microbiol 2021; [Crossref] [PubMed]
- Hogerwerf L, DE, Gier B, Baan B, et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect 2017;145:3096-105. [Crossref] [PubMed]
- Nieuwenhuizen AA, Dijkstra F, Notermans DW, et al. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis 2018;18:442. [Crossref] [PubMed]
- Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol 2019;14:319-38. [Crossref] [PubMed]
- Blomqvist M, Christerson L, Waldenström J, et al. Chlamydia psittaci in birds of prey, Sweden. Infect Ecol Epidemiol 2012.
- Balsamo G, Maxted AM, Midla JW, et al. Compendium of Measures to Control Chlamydia psittaci Infection Among Humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2017. J Avian Med Surg 2017;31:262-82. [Crossref] [PubMed]
- Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect 2009;15:11-7. [Crossref] [PubMed]
- DE Boeck C. Managing a cluster outbreak of psittacosis in Belgium linked to a pet shop visit in The Netherlands. Epidemiol Infect 2016;144:1710-6. [Crossref] [PubMed]
- Vorimore F, Thébault A, Poisson S, et al. Chlamydia psittaci in ducks: a hidden health risk for poultry workers. Pathog Dis 2015;73:1-9. [Crossref] [PubMed]
- Hughes C, Maharg P, Rosario P, et al. Possible nosocomial transmission of psittacosis. Infect Control Hosp Epidemiol 1997;18:165-8. [Crossref] [PubMed]
- Ito I, Ishida T, Mishima M, et al. Familial cases of psittacosis: possible person-to-person transmission. Intern Med 2002;41:580-3. [Crossref] [PubMed]
- Wallensten A, Fredlund H, Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January-February 2013. Euro Surveill 2014;19:20937. [Crossref] [PubMed]
- Li N, Li S, Tan W, et al. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under COVID-19. Emerg Microbes Infect 2021;10:1418-28. [Crossref] [PubMed]
- Cong W, Huang SY, Zhang XY, et al. Seroprevalence of Chlamydia psittaci infection in market-sold adult chickens, ducks and pigeons in north-western China. J Med Microbiol 2013;62:1211-4. [Crossref] [PubMed]
- Teng XQ, Gong WC, Qi TT, et al. Clinical Analysis of Metagenomic Next-Generation Sequencing Confirmed Chlamydia psittaci Pneumonia: A Case Series and Literature Review. Infect Drug Resist 2021;14:1481-92. [Crossref] [PubMed]
- Zhang A, Xia X, Yuan X, et al. Clinical characteristics of 14 cases of severe Chlamydia psittaci pneumonia diagnosed by metagenomic next-generation sequencing: A case series. Medicine (Baltimore) 2022;101:e29238. [Crossref] [PubMed]
- Branley JM, Weston KM, England J, et al. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect 2014;2:7-12. [Crossref] [PubMed]
- Heddema ER, van Hannen EJ, Duim B, et al. An outbreak of psittacosis due to Chlamydophila psittaci genotype A in a veterinary teaching hospital. J Med Microbiol 2006;55:1571-5. [Crossref] [PubMed]
- Dickx V, Vanrompay D. Zoonotic transmission of Chlamydia psittaci in a chicken and turkey hatchery. J Med Microbiol 2011;60:775-9. [Crossref] [PubMed]
- Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection 2020;48:535-42. [Crossref] [PubMed]
- Petrovay F, Balla E. Two fatal cases of psittacosis caused by Chlamydophila psittaci. J Med Microbiol 2008;57:1296-8. [Crossref] [PubMed]
- Spoorenberg SM, Bos WJ, van Hannen EJ, et al. Chlamydia psittaci: a relevant cause of community-acquired pneumonia in two Dutch hospitals. Neth J Med 2016;74:75-81. [PubMed]
- Charles PG, Whitby M, Fuller AJ, et al. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis 2008;46:1513-21. [Crossref] [PubMed]
- Longbottom D, Coulter LJ. Animal chlamydioses and zoonotic implications. J Comp Pathol 2003;128:217-44. [Crossref] [PubMed]
- Gosbell IB, Ross AD, Turner IB. Chlamydia psittaci infection and reinfection in a veterinarian. Aust Vet J 1999;77:511-3. [Crossref] [PubMed]
- Rybarczyk J, Versteele C, Lernout T, et al. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg 2020;75:42-8. [Crossref] [PubMed]
- Forbes JD, Knox NC, Peterson CL, et al. Highlighting Clinical Metagenomics for Enhanced Diagnostic Decision-making: A Step Towards Wider Implementation. Comput Struct Biotechnol J 2018;16:108-20. [Crossref] [PubMed]
- Ménard A, Clerc M, Subtil A, et al. Development of a real-time PCR for the detection of Chlamydia psittaci. J Med Microbiol 2006;55:471-3. [Crossref] [PubMed]
- de Gier B, Hogerwerf L, Dijkstra F, et al. Disease burden of psittacosis in the Netherlands. Epidemiol Infect 2018;146:303-5. [Crossref] [PubMed]
- Wolff BJ, Morrison SS, Winchell JM. Development of a multiplex TaqMan real-time PCR assay for the detection of Chlamydia psittaci and Chlamydia pneumoniae in human clinical specimens. Diagn Microbiol Infect Dis 2018;90:167-70. [Crossref] [PubMed]
- Schlaberg R, Chiu CY, Miller S, et al. Validation of Metagenomic Next-Generation Sequencing Tests for Universal Pathogen Detection. Arch Pathol Lab Med 2017;141:776-86. [Crossref] [PubMed]
- Guan H, Shen A, Lv X, et al. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J Neurovirol 2016;22:240-5. [Crossref] [PubMed]
- Indelli PF, Ghirardelli S, Violante B, et al. Next generation sequencing for pathogen detection in periprosthetic joint infections. EFORT Open Rev 2021;6:236-44. [Crossref] [PubMed]
- Langelier C, Kalantar KL, Moazed F, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci U S A 2018;115:E12353-62. [Crossref] [PubMed]
- Wen W, Gu L, Zhao LW, et al. Diagnosis and treatment of Chlamydia psittaci pneumonia: experiences of 8 cases. Zhonghua Jie He He Hu Xi Za Zhi 2021;44:531-6. [PubMed]
- Gu W, Deng X, Lee M, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med 2021;27:115-24. [Crossref] [PubMed]
- Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet 2019;20:341-55. [Crossref] [PubMed]
- Simner PJ, Miller S, Carroll KC. Understanding the Promises and Hurdles of Metagenomic Next-Generation Sequencing as a Diagnostic Tool for Infectious Diseases. Clin Infect Dis 2018;66:778-88. [Crossref] [PubMed]
- Cillóniz C, Torres A, Niederman M, et al. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med 2016;42:1374-86. [Crossref] [PubMed]
- Smith KA, Bradley KK, Stobierski MG, et al. Compendium of measures to control Chlamydophila psittaci (formerly Chlamydia psittaci) infection among humans (psittacosis) and pet birds, 2005. J Am Vet Med Assoc 2005;226:532-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


